Received: September 5, 2013
Accepted: March 19, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014025, DOI 10.4084/MJHID.2014.025
This article is available on PDF format at:

Ashraf Soliman1, Mohamed Yassin2, Fawzia Al Yafei3, Lolwa Al-Naimi3, Noora Almarri3, Aml Sabt3 and Vincenzo De Sanctis4
1
Department of Pediatrics, Alexandria University Children’s Hospital ,
Alexandria, Egypt
2 Departments of Pediatrics, Hamad Medical
Center (HMC), Doha – Qatar
3 Departments of Hematology, Hamad Medical
Center (HMC), Doha – Qatar
4 Pediatric and Adolescent Outpatient Clinic,
Quisisana Hospital, 44121 Ferrara , Italy.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract By
performing regular blood transfusion and iron chelation therapy, most
patients with beta thalassemia major (BTM) now survive beyond the third
decade of life. Liver disease is becoming an important cause of
morbidity and mortality in these patients. Chronic hepatitis and/or
severe iron overload are both important causes of liver pathology. Iron
chelation with desferrioxamine (DFO) reduces excessive body iron, but
its efficacy is limited by poor compliance and dose related toxicity.
The recent use of Deferasirox (DFX), an oral single dose therapy, has
improved the compliance to chelation.
Aims: To study the long-term liver functions in BMT patients, seronegative for liver infections before versus after DFX treatment in relation to ferritin level. Methods: Only BTM patients with hepatitis negative screening (checked every year) and on treatment with DFO for at least five years and with DFX for four years were enrolled. Liver function tests including serum bilirubin, alanine transferase (ALT), aspartate transferase (AST), albumin, insulin-like growth factor – I (IGF-I) and serum ferritin concentrations were followed every six months in 40 patients with BTM. Results: DFX treatment (20 mg/kg/day) significantly decreased serum ferritin level in patients with BTM; this was associated with a significant decrease in serum ALT, AST, ALP and increase in IGF-I concentrations. Albumin concentrations did not change after DFX treatment. ALT and AST levels were correlated significantly with serum ferritin concentrations (r = 0.45 and 0.33 respectively , p < 0.05) . IGF-I concentrations were correlated significantly with serum ALT (r= 0.26, p = 0.05) but not with AST, ALP, bilirubin or albumin levels. The negative correlation between serum ferritin concentrations and ALT suggests that the impairment of hepatic function negatively affect IGF-I synthesis in these patients due to iron toxicity, even in the absence of hepatitis. Conclusions: Some impairment of liver function can occur in hepatitis negative thalassemic patients with iron overload. The use of DFX was associated with mild but significant reduction of ALT, AST and ALP and increase in IGF-I levels. The negative correlation between IGF-I and ALT concentrations suggest that preventing hepatic dysfunction may improve the growth potential in these patients. |
Introduction
The
β and α thalassaemias are the most common inherited single-gene
disorders in the world. Iron overload is a consequence of chronic
transfusion therapy that adversely affects the function of the heart,
liver and endocrine glands. Even with the administration of effective
subcutaneous (s.c.) iron chelation therapy with desferrioxamine (DFO),
over 50% of patients die before the age of 35 years, mainly because of
poor compliance with s.c. chelation regimens.[1]
A high prevalence of hepatic hemosiderosis (grades 3-4) has been
recorded in many studies.[2-4]
Hepatic fibrosis is also still not
uncommon in patients with β thalassemia major (BTM) despite the use of
chelation therapy. This could reflect the rather unsatisfactory
compliance rate with DFO treatment observed in many of BTM patients.
Thus, early and accurate diagnosis of liver disease followed by prompt
intervention may prevent liver disease progression.[2-4]
The liver is the primary site of iron storage and the only site for
synthesis of transferrin and ferritin. Free ferrous iron is highly
toxic and normally is protein-bound within the liver. Unbound, iron
catalyzes the production of free radicals, which have been implicated
in lipid peroxidation and hepatotoxicity. Lipid peroxidation may be the
primary event causing hepatocellular injury secondary to iron
overload.[5-8]
Significant correlation between ferritin iron concentration and
individual liver iron concentration, measured non-invasively by
superconducting quantum interference device biomagnetometry (SQUID) has
been reported in patients with BTM and hemochromatosis. However, the
relation between serum ferritin concentration and liver iron improves
when serum ferritin is lower than 2500 µg/ and in the absence of
hepatitis.[5-8] In a large cohort
of patients on chronic transfusion, a
strong statistical correlation has been found between liver histology,
serum ferritin and liver iron content (LIC).[9]
In addition, patients with thalassemia have a high prevalence of
hepatitis B and C infections.[10-12]
HCV infected patients had
significantly higher enzymes than non- infected.[13]
Chronic hepatitis
C virus infection has been associated with liver iron loading. The
cause of elevated serum iron indices in some HCV-infected individuals
is not clear. The concomitant increase of in serum alanine
aminotransferase (ALT) levels suggests that iron and ferritin be
released from damaged hepatocytes as a result of hepatic
necro-inflammation.[14] In
addition, increased iron has been shown to
enhance HCV replication in vitro.[15]
Furthermore, hyperferritinemia
and increased iron stores have been associated with the severity of
liver damage in non-alcoholic fatty liver diseases (NAFLD), and iron
depletion reduced insulin resistance and liver enzymes. Serum ferritin
concentration is an important determinant of liver enzyme levels, and
increased serum ferritin level is an independent predictor of liver
damage in these patients, so it is useful to identify patients at risk
of steatohepatitis and advanced fibrosis.[16-21]
Histological evidence
of hepatic iron accumulation has also been associated with an increased
risk of fibrosis in large multicenter studies, in patients with NAFLD
both from Europe and the United States. The β globin mutations, the
best predictor of parenchymal iron overload in the Mediterranean area,
are associated with almost double risk of severe fibrosis.[20-24]
These data suggest that incorporation of serum ferritin level can
improve the performance of noninvasive scoring of liver damage in
patients with chronic liver disease and that iron depletion still
represents an attractive therapeutic target to prevent the progression
of liver damage in these patients.[25]
Experimental evidence suggests
that iron depletion induced by chelators induce glucose uptake and
utilization in hepatocytes in vitro and in vivo liver, increasing
insulin receptor binding activity and signaling.[26,27]
Randomized and controlled trials have established that the oral
deferasirox (DFX) efficacy is comparable to the standard iron
chelator, DFO administered as a parenteral infusion, in reducing liver
iron concentration and serum ferritin levels. However, DFX may be more
effective than DFO in actual clinical practice owing to the improvement
in quality of life and, hence, increased compliance associated with the
oral route of administration.[28-30]
We investigated and reviewed the liver function in 40 BMT patients
attending the Hematology Clinic of Hamad Medical Center, Doha (Qatar)
during follow-up of 10 years, in order to ascertain the relationship,
between serum ferritin concentrations and different liver functions,
before and after DFX therapy.
Patients and Methods
The study was designed on the basis of observational study. Subjects
were randomly recruited from the hematology and endocrinology clinics
of HMC Doha (Qatar) and analyzed in the Biochemistry Laboratory of HMC.
A detailed history including the age at diagnosis of BMT and clinical
presentation and transfusion and chelation data was taken from the
patient, mother or the attendant. The ethical committee of Hamad
Medical Center has approved the study protocol as a part of protocol
for studying the endocrine and biochemical functions in thalassemic
patients. Waiver of informed consent was taken for accessing data of
patients before inclusion in the study. The diagnosis of BMT was
confirmed by Hb-electrophoresis in all the patients.
The patients who fulfilled the inclusion criteria were incorporated in
the study. All the planned information were obtained and recorded in
the data collecting sheet properly. A total of 45 subjects were
included in the study. All but five patients did not complete the study
(three left the city and two had splenectomy).
Inclusion and Exclusion Criteria
Patients with a confirmed diagnosis of BTM above the age of 5 years
were randomly selected. All the subjects were on regular blood
transfusions and iron chelation using subcutaneous pump infusion of DFO
five days per week. Exclusion criteria included: (1) Thalassemia trait
or intermedia type, (2) History of jaundice due to viral hepatitis (3)
History of splenectomy, (4) Positive screening test for hepatitis C or
B.
Only patients with hepatitis negative screening (checked every year),
and on treatment with DFO for at least five years or on treatment with
DFX for four years or more were enrolled.
Liver function tests including serum bilirubin, ALT, aspartate
transferase (AST), albumin, insulin-like growth factor – I (IGF-I) and
serum ferritin concentrations were followed every six months in all
these patients.
Statistical Analysis
Variables including age, serum ferritin, bilirubin, ALT, AST, ALP, and
IGF-I concentrations are expressed as mean +/- standard deviation.
Comparison of variables before versus after DFX treatment was performed
using Student’s t test or analysis of variance as appropriate.
The possible associations between serum ferritin and different liver
functions are tested using linear regression equation. The level of
significance was set at 0.05 in the analyses, and all the statistical
testing was two sides.
Results
Forty patients with BTM were evaluated longitudinally every six months
from age of eight to 18 years. Their mean age at the beginning of the
study was 6.8 +/- 1.2 years and at the end of the study was 18.4 +/-
1.7 years.
They started iron chelation therapy with DFO at the age of 3.8 +/- 0.9
years. They were shifted to oral DFX (20 mg/kg/day) at the age of 13.8
+/- 1.5 years.
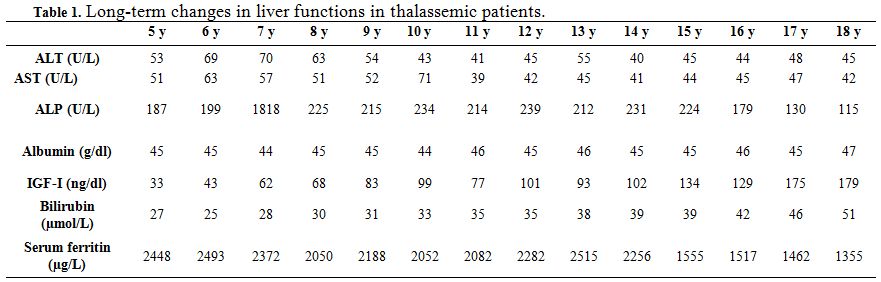 | Table 1. Long-term changes in liver functions in thalassemic patients. |
Liver functions followed longitudinally are presented in Table 1. After initiation of oral DFX, the serum ferritin level significantly decreased in all BTM patients (p < 0.001). This was associated with mild, but significant, decrease in serum ALT, AST and ALP concentrations and increase in IGF-I concentrations (p<0.01) (Figure 1-6). Albumin concentrations did not change after treatment. There was a mild significant increase in serum bilirubin concentrations.
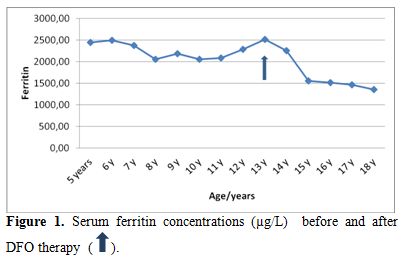 | Figure 1. Serum ferritin concentrations (µg/L) before and after DFO therapy. |
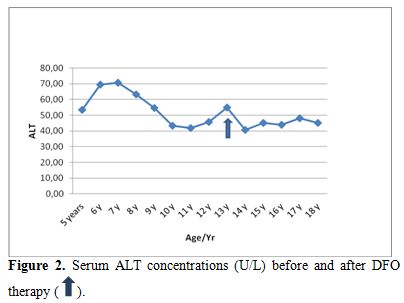 | Figure 2. Serum ALT concentrations (U/L) before and after DFO therapy. |
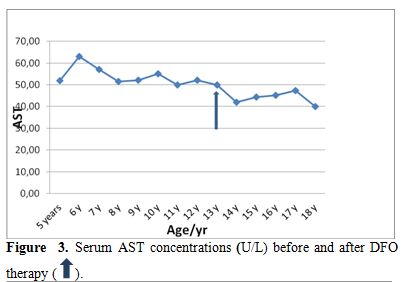 | Figure 3. Serum AST concentrations (U/L) before and after DFO therapy. |
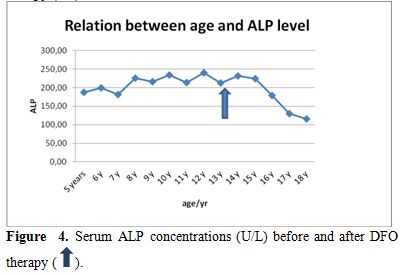 |
Figure 4. Serum ALP concentrations (U/L) before and after
DFO therapy. |
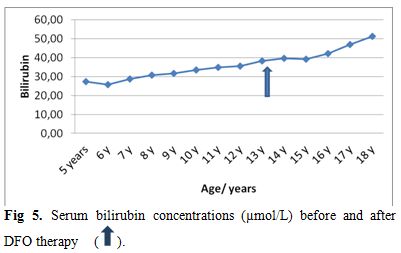 | Figure 5. Serum bilirubin concentrations (µmol/L) before and after DFO therapy. |
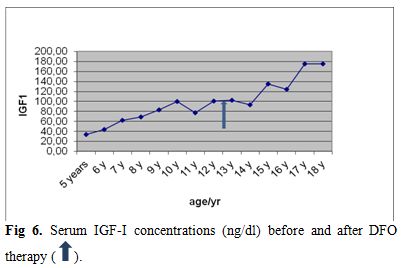 | Figure 6. Serum IGF-I concentrations (ng/dl) before and after DFO therapy. |
ALT and AST levels were correlated significantly with serum ferritin concentrations (r = 0.45 and 0.33 respectively, p < 0.05) (Figure 7). IGF-I concentrations were correlated significantly with serum ALT (r = - 0.26, p = 0.05) and serum ferritin (r = -0.29, p = 0.02) concentrations. IGF-I concentrations were not correlated with AST, ALP, bilirubin or albumin levels (p > 0.05). (Table 2)
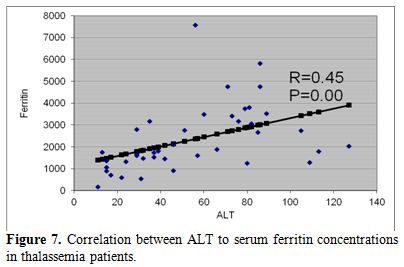 | Figure 7. Correlation between ALT to serum ferritin concentrations in thalassemia patients. |
 | Table 2. Correlation between liver functions and ferritin concentrations in thalassemic patients. |
In our BMT patients
with negative hepatitis screening, DFO treatment
given at a younger age was not associated with significant hepatic
dysfunction. However, the DFX treatment significantly induced a
decrease of ALT, AST and ALP concentrations.
The negative correlation between serum ferritin concentrations and ALT
suggests that the impairment of hepatic function negatively affect
IGF-I synthesis in these patients due to iron overload, even in the
absence of hepatitis.
Discussion
In thalassemia, abnormal liver function appears to be related to the
high ferritin levels and the age when transfusions was initiated.[5-9]
Iron-induced liver disease is often aggravated by viral infection.
Hepatic siderosis, portal fibrosis and even cirrhosis may develop
despite iron chelation therapy.[13-15]
Elevated serum ALT levels should
alert the clinician about the possibility of hepatitis due to multiple
blood transfusions.[31,32]
In this longitudinal study liver, function tests and serum ferritin
levels were observed for more than 10 years in patients with BTM with
negative screening for hepatitis C and B in order to exclude the
possible effects of hepatitis in the production determination of liver
dysfunction. They started iron chelation therapy with DFO at the age of
3.8 +/- 0.9 years then were shifted to oral DFX (20 mg/kg/day) at the
age of 13.8 +/- 1.5 years.
Liver functions, followed longitudinally, showed that the DFX treatment
decreased serum ferritin level significantly in all patients with BTM.
This effect was maintained during the five years of treatment (p
<
0.001). These findings support the concept that DFX may be even more
effective than DFO in improving liver function because of better
compliance to treatment.[28,30]
Reduction of serum ferritin concentration was associated with a
significant decrease in serum ALT, AST and ALP concentrations and
increase in IGF-I levels. The significant correlations between serum
ferritin concentrations and ALT and AST levels (p < 0.01)
suggest
that the reduction of hepatic iron load and LIC reduce the hepatic
cellular derangement and cell damage and improve liver functions. In
support to this view, histological changes of liver biopsy specimens
have been shown to correlate significantly with ALT and serum ferritin
level.[33-34]
Measurement of fibrosis not only helps to stage the severity of
disease, it allows serial determination of disease progression.
Unfortunately, neither measuring liver enzymes nor IGF-1 can
determinate the extent of hepatic fibrosis progression in BMT patients
and different outcomes of liver disease may be expected in these
patients. A non-invasive method for fibrosis determination (e.g.
transient elastography) could add some information about liver damage
at the end of follow-up, allowing a useful comparison with the
evolution of laboratory data and the efficacy of treatment.[35]
In a study on 40 thalassemic children, using the Knodell histological
activity index (HAI), 28 children (70%) had 3-4 grade hemosiderosis, 24
(60%) had HAI score between 13/22 to 18/22 and 18 patients (45%)
developed cirrhotic changes.[36]
The study done by Li et al. revealed
that 30% cases showed HAI stage three and 44% patients showed grade 3-4
hemosiderosis in transfusion dependent BMT children.[8]
Another study
by Jean et al. including histological evaluation of liver biopsy in 86
children with thalassemia indicated that some patients developed
cirrhosis as early as 7-8 years of age.[3]
IGF-I concentrations were significantly decreased in our BMT patients
compared to age and sex published standards. IGF-I concentrations were
correlated significantly with serum ALT (r = - 0.26; p = 0.05)
suggesting that impaired liver function (increased ALT) may be an
important cause of decreased synthesis of hepatic IGF-I in these
patients. Negative associations between aspartate aminotransferase
(AST) and γ- GT and IGF-1 levels, as well as between AST activity and
IGF-1 levels have been detected in patients with chronic liver
diseases.[37]
Physiologically, GH stimulates liver IGF-I synthesis and secretion.
Therefore, individuals with GH sufficiency should have normal serum
IGF-1 level. In thalassemia major, the prevalence of low serum IGF-1
was much higher than that of the GH deficiency.[38,39]
The IGF-I
response and the linear growth after exogenous administration of GH
were less than that seen in GH deficient children treated with
GH.[39,40] These data suggest that
thalassemic patients had some degree
of GH insensitivity. Many factors, other than GH, could control hepatic
IGF-I synthesis and circulating IGF-1 level. Patients with chronic
liver disease could have low serum IGF-1 despite sufficient GH
secretion.[39-41]
Hepatic stellate cells are stimulated by insulin-like growth factor 1
(IGF-I) and high IGF-1 levels attenuate fibrogenesis and accelerate
liver regeneration. This effect is mainly mediated by up-regulation of
hepatic growth factor and down-regulation of transforming growth factor
β 1.[42] Therefore, decreased
IGF-1 levels in BMT patients may impair
the regeneration and increase the risk for deterioration of function
and chronicity. In our study, a significant increase in IGF-I levels as
a consequence of decreased ferritin levels after the use of DFX therapy
points out to a possible better prognosis of hepatic regeneration
and/or improvement of synthetic hepatic functions in these patients.
Conclusions
In hepatitis-seronegative BMT patients, DFO treatment given at a
younger age was associated with mild significant hepatic dysfunction.
However, DFX treatment significantly decreased serum ALT, AST and ALP
and increased IGF-I concentrations. The positive correlation between
serum ferritin and ALT concentrations and the negative correlation
between IGF-I concentrations and ferritin and ALT suggest that hepatic
iron overload impairs in these patients the hepatic functions and
decreases IGF-I synthesis, even in the absence of hepatitis. [1]
References







































[TOP]