Received: January 7, 2015
Accepted: February 6, 2015
Meditter J Hematol Infect Dis 2015, 7(1): e2015016,, DOI 10.4084/MJHID.2015.016
This article is available on PDF format at:

Vincenzo De Sanctis1, Ashraf T Soliman2, Giancarlo Candini3, Christos Kattamis4, Giuseppe Raiola5 and Heba Elsedfy6
1 Pediatric
and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy
2 Department of Pediatrics, Division of
Endocrinology, Alexandria University Children’s Hospital, Alexandria
3 Department of Medical Physics, St. Anna
Hospital, Ferrara, Italy
4 First Department of Paediatrics,
University of Athens, Athens, Greece
5 Department of Paediatrics,
Pugliese-Ciaccio Hospital, Catanzaro, Italy
6 Department of Pediatrics, Ain Shams
University, Cairo, Egypt

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
Dear Sir
BIn the postnatal period, the liver remains the main source of
circulating insulin-like growth factor 1 (IGF 1) and its synthesis is
mainly under the effect of growth hormone (GH). Secretion of IGF-1 is
also related to age, gender, genetic factors, nutrition, insulin, and
disease conditions[1]. IGF-1 produced in the liver exerts mainly
endocrine activity while IGF-1 synthesized by other tissues acts in a
para- and/or autocrine manner. IGF-1 is an anabolic hormone that causes
a decrease in proteolysis and an increased stimulation of protein
production, followed by an increment in muscular mass [1]. The most
significant expression of IGF-1 deficiency exists in Laron-type
dwarfism [1]. In thalassaemia major (TM), IGF-1 deficiency
has been
attributed to chronic anemia and hypoxia, chronic liver disease, iron
overload and other associated endocrinopathies, e.g. GH deficiency
[2,3].
In a previous report, we found that IGF-1 levels were 2SDs
below average values for healthy individuals in 60 out of 120
thalassemic patients (50%) (33 males and 27 females). In these patients
endocrine complications and elevation of aminotransferases (ALT) were
more common compared to TM patients with IGF-1 > -2SDs
[2].
However, the influence of liver iron overload and the severity of
chronic liver disease in terms of both grade and stage were not fully
elucidated [2]. Therefore, we reviewed the available data in
85 β TM
patients followed in the last two decades at the Thalassaemia Centre
and Quisisana Pediatric and Adolescent Outpatient Clinic of Ferrara.
The present report is a part of an ongoing retrospective study
identifying the factors responsible of IGF-1 deficiency in
TM.
The
following clinical and laboratory data were registered: age at first
transfusion, height, weight, body mass index (BMI), pubertal status,
serum ferritin, creatinine, alanine aminotransferase (ALT), gamma
glutamyl transferase (γGT), alkaline phosphatase (ALP), total and
direct bilirubin, albumin, prothrombin time (PT), international
normalized ratio (INR) and serologic screening assays for hepatitis C
virus sero-positivity (HCVab and HCV-RNA). Patients with thalassaemia
intermedia, cardiac or renal failure, malnutrition and HIV positivity
were excluded from the study.
A total of 20 TM patients underwent
liver biopsy at mean age of 26 years (range 19 to 32 years) for
persistent increase of liver enzymes (6 months or more). Based on liver
ultrasound tests and α-fetoprotein (AFP) levels, neoplastic growth
(HCC) was not suspected in any of the patients.
Histopathological
findings were analyzed following the classical haematoxylin–eosin
staining employing a numerical scoring system for the grading (G=0-3)
and the stage of fibrosis (S=0-4) according to the METAVIR Cooperative
Study Group [4]. The degree and cellular distribution of iron stores
was assessed using Perls’ Prussian blue stain. The liver iron
concentration (LIC) was assayed by atomic absorption spectrophotometry
and expressed as mg/g dry weight (dw) [5].
Quantitative
estimation of LIC was done in 65 TM patients, between the
ages of 15
to 48 years, by Superconducting Quantum Interference Device (SQUID)
susceptometry [6]. Based on data from the literature in
normal people
LIC is between 0.4 and 2 mg/g of liver dry weight, while in subjects
with iron overload is classified as mild, 2-7, moderate, 7-15 and
severe > 15mg Fe/gr dry wt. Patients with LIC level > 15
mg/g
have increased liver enzyme levels, progression to liver fibrosis and
increased risk of premature death.[7]
Serum ferritin was measured
at six monthly intervals and the mean serum ferritin in the year before
evaluation was recorded. Serum ALT levels were routinely measured prior
to monthly transfusions and the annual levels before the evaluation
were recorded. IGF-1 was measured using commercial automated
immunoassay following the manufacturer’s instructions. The reported
analytic sensitivity of this assay was from 6 to 25 ng/ml. Ranges of
normal values set at the 2.5th-97.5th percentile in 547
non-hypopituitary, non-acromegalic healthy subjects of both sexes in
Italy in three age ranges were: 95.6-366.7 ng/ml for ages 25
to 39 yr,
60.8-297.7 ng/ml for 40 to 59 yr and 34.5-219.8 ng/ml for subjects aged
60 and above [8].
Characteristics of the studied patients are
reported as mean, median, number and range. Fisher' exact test was used
to calculate the probability value for the relationship between two
dichotomous variables. A p value < 0.05 was considered
significant.
A
software program used for the statistical analysis was developed by Dr.
Candini (Department of Medical Physics, St. Anna Hospital, Ferrara,
Italy) and validated according to Alder and Roesser [9].
Forty-two
females and 43 males TM patients were included in our retrospective
survey (mean age at the last observation: 36.6 years; age range:
15.1-53.1 years). Eleven (12.9%; 3 females) had insulin dependent
diabetes mellitus. The body mass index (BMI) ranged between 17.4 to
30.8 kg/m2. An abnormal ALT value
(>40 U/L) was observed
in 30 patients (35.2 %; 12 females). ALT values above 80 U/L were found
in 13 patients (15.2 %; 4 females). Hepatitis C virus seropositivity
(HCVab and HCV-RNA) was present respectively in 91% and 45.6% of TM
patients (Table
1).
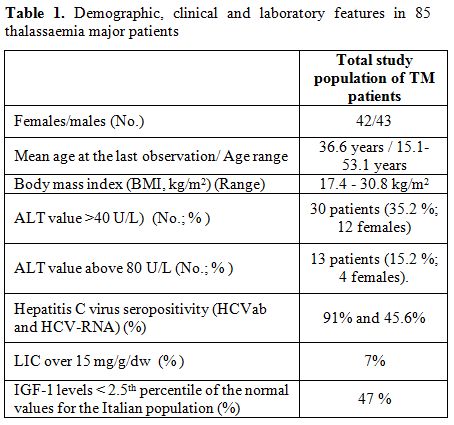 |
Table 1. Demographic, clinical and laboratory features in 85 thalassaemia major patients |
All the liver specimens showed at least grade
1 haemosiderosis; grades 3 and 4 occurred in seven out of 20 (35 %)
with LIC levels from 16.5 to 21.3 mg/g /dw.
The median LIC was 2.4
mg/g dry weight (range: 0.1 – 24.6 mg/g dry weight). Six samples (7 %)
had LIC over 15 mg/g/dw, a concentration associated with a high risk
for cardiac disease[9].
Total LIC levels correlated
significantly with serum ferritin concentrations both in males and
females (r: 0.724 and 0.65 respectively, p <0.01).
Forty TM
patients (47%) had IGF-1 levels below the 2.5th percentile of the
normal values for the Italian population 8. No correlation
was
observed, in males and females, between IGF 1 values and LIC levels (r:
0.01, p: ns; r: 0.162, p: ns, respectively). Significantly lower IGF-1
levels were observed among those with liver cirrhosis (1 male patient)
and severe stage of fibrosis (S=3-4, in 4 TM patients) according to the
METAVIR Cooperative Study Group [4].
Seven TM patients (8.2%) with
serum ferritin below 1500 ng/ml and LIC between 0.8 - 3.1 mg/g dry
weight had very low IGF-1 levels (< 30 ng/ml). Three out of 7
had
insulin dependent diabetes mellitus. Unfortunately, we have no data on
growth hormone (GH)- IGF -1 axis in these patients.
There was also a
positive correlation between serum ALT concentrations and LIC levels in
males (r: 0.316; p < 0.05) and between serum ϒGT concentrations
and
LIC levels in females (r: 0.315; p < 0.05).
We acknowledge some
limitation to our analysis. The relative small number of patients
submitted to liver histology, the absence of data regarding the
assessment of GH-IGF-1 axis and the effects of other associated
endocrine complications, and the absence of information on
the
influence of other non-hepatic factors (smoking, alcohol intake),
although they seem to be not relevant in our patients.
In summary,
in the present study a significant correlation was observed between LIC
and serum ferritin in all patients as well as between LIC and serum ALT
concentrations in males and serum ϒGT concentrations in females.
Furthermore, an association between severity of liver dysfunction and
low IGF-1 levels was observed.
The gold standard for assessing
liver iron stores, in the absence of cirrhosis, is the hepatic iron
content determined by liver biopsy and quantitation with atomic
absorption spectrophotometry [10]. However, the use of biopsy-measured
LIC as a marker of iron overload is limited by the small but finite
risk of complications of liver biopsy, lack of reproducibility of
quantitative assays, and sampling error [5]. Non-invasive methods
include blood tests (serum ferritin and iron saturation) and imaging
techniques (MRI) or SQUID. Although there was a correlation between
serum ferritin and LIC in this study, Li et al have shown that this
correlation is less reliable at ferritin concentrations above 2500
ng/ml [10]. MRI has been validated as a reliable non-invasive mean to
assess iron stores in the liver, and the heart and SQUID has shown
significant correlation with hepatic iron content as measured by biopsy
[11,12].
In conclusion, we believe that our data contribute
further to the understanding of serum IGF-1 levels in TM patients and
may represent a starting point for future studies for investigating the
correlations between IGF-1, liver iron stores and severity of liver
histology findings.
References











[TOP]