Does Insulin Like Growth Factor-1 (IGF-1) Deficiency Have a
“Protective” Role in the Development of Diabetic Retinopathy in
Thalassamia Major Patients?
Vincenzo De Sanctis1, Carlo Incorvaia2, Ashraf T Soliman3, Giancarlo Candini4, Alessia Pepe5, Christos Kattamis6, Nada A. Soliman7, Heba Elsedfy8 and Mohamed El Kholy8
1 Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy
2 Department of Ophthalmology, University of Ferrara, Ferrara, Italy.
3 Department of Pediatrics, Division of Endocrinology, Alexandria University Children’s Hospital, Alexandria
4 Medical Physicists, Honorary Member of Italian Association of Medical Physics ( AIFM ) , Ferrara, Italy
5 Cardiovascular MR Unit, Fondazione G. Monasterio CNR-Regione Toscana and Institute of Clinical Physiology, Pisa, Italy
6 First Department of Paediatrics, University of Athens, Athens, Greece
7 Ministry of Health , Alexandria, Egypt
8 Department of Pediatrics, Ain Shams University, Cairo, Egypt
Corresponding author: Vincenzo De Sanctis MD,
Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, 44100
Ferrara, Italy; Tel: 39 0532 770243; E-mail:
vdesanctis@libero.it
Published: May 20, 2015
Received: March 23, 2015
Accepted: May 8, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015038, DOI
10.4084/MJHID.2015.038
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Rationale: Both insulin and
IGF-1 have been implicated in the control of retinal endothelial cell
growth, neovascularization and diabetic retinopathy. Recent findings
have established an essential role for IGF-1 in angiogenesis and
demonstrated a new target for control of retinopathy that explains why
diabetic retinopathy initially increases with the onset of insulin
treatment
Objective: This cross-sectional study was
designed to give insights into relationship between
Insulin-Growth-Factor 1 (IGF-1) levels and diabetic retinopathy (DR) in
a sample of thalassemia major (TM) patients with insulin dependent
diabetes mellitus (IDDM). Τhis relation was not previously evaluated,
despite the fact that both diseases co-exist in the same patient. The
study also describes the clinical and biochemical profile of the
associated complications in TM patients with and without IDDM.
Design: A population-based cross-sectional study.
Participants: The
study includes 19 consecutive TM patients with IDDM and 31 age- and
sex-matched TM patients without IDDM who visited our out-patient
clinics for an endocrine assessment
Methods: An extensive
medical history, with data on associated complications and current
medications, was obtained. Blood samples were drawn in the morning
after an overnight fast to measure the serum concentrations of IGF-1,
glucose, fructosamine, free thyroxine (FT4), thyrotropin (TSH) and
biochemical analysis. Serologic screening assays for hepatitis C virus
seropositivity (HCVab and HCV-RNA) were also evaluated; applying
routine laboratory methods. Plasma total IGF-1 was measured by a
chemiluminescent immunometric assay (CLIA) method. Ophthalmology
evaluation was done by the same researcher using stereoscopic fundus
biomicroscopy through dilated pupils. DR was graded using the scale
developed by the Global Diabetic Retinopathy Group. Iron stores were
assessed by direct and indirect methods.
Results: Eighteen
TM patients with IDDM (94.7 %) and ten non-diabetic patients (32.2 %)
had IGF-1 levels below the 2.5th percentile of the normal values for
the Italian population. The mean serum IGF-1 concentrations were
significantly lower in the diabetic versus the non-diabetic TM groups
(p < 0.001). DR was present in 4 (21 %) of 19 TM patients with IDDM
and was associated with the main classical risk factors, namely
inefficient glycemic control and duration of the disease but not
hypertension. Using the scale developed by the Global Diabetic
Retinopathy Group, the DR in our patients was classified as non
proliferative diabetic retinopathy (NPDR). Only a few numbers of
microaneurysms [1-3] were detected. Our data also confirm the strong
association of IDDM in TM patients with other endocrine and
non-endocrine complications.
Conclusions: These results
although on a small number of patients, suggest a possible ‘protective’
role of low IGF-1 in the development of DR in TM patients. |
Introduction
Insulin dependent diabetes (IDDM) and impaired glucose tolerance
(IGT) are relatively common complications in thalassaemia major (ΤΜ)
patients with iron overload and sub-optimal chelation therapy. The
prevalence of IDDM and IGT in adolescents and young adults with TM
mainly treated with desferrioxamine mesylate (DFO) varies in different
studies from 0 to 21% and from 9.3 to 24.3 %, respectively.[1,2]
A
substantial aim in diabetes care is the prevention, early detection and
proper management of complications, including microvascular (diabetic
retinopathy, nephropathy and neuropathy) and macrovascular
complications (cardiovascular disease, cerebrovascular disease and
peripheral vascular disease). Prevalence of diabetic retinopathy (DR)
depends on various factors including age, sex (male), ethnicity, type
of diabetes, pregnancy, hypertension, state of metabolic control and
diabetes duration.[3,4]
The prevalence of DR was
examined in a population-based study in the Veneto region of North East
Italy. Of 1321 diabetic patients selected, the prevalence of DR was
26.2% (24.4% background and 1.8% proliferative). The prevalence of DR
was significantly related to the duration of diabetes (17.3% for less
than five years; 60.8% for greater than 20 years). Proliferative
retinopathy was much more prevalent after 20 years of diabetes. No
significant differences were found in the prevalence of total or
proliferative retinopathy between males and females.[4]
Numerous
pathways have been implicated in the pathogenesis of DR. Hypoxia is one
of the most important initiating factors. It is responsible for the
activation of transcription factors such as hypoxia- inducible factor
(HIF)-1 α and HIF-1 β; these factors, finally, bind to the hypoxia
response elements of the vascular endothelial growth factor (VEGF)
promoter. Another known modifier of VEGF expression is IGF-1.[5]
Studies
on transgenic mouse models have shown the presence of retinal
neovascularization associated with VEGF expression mediated by
increased induction of IGF-1 in retinal glial cells.[6]
IGF-1
is known to trigger a critical cascade of molecular events that
initiate retinal angiogenesis. Increased vitreous IGF-1 levels have
been correlated with the severity of ischemia-associated diabetic
retinal neovascularization.[7-11] The action of IGF-1
may also depend on genetic factors and/or metabolic changes in the
retinal epithelium affecting oxygenation, VEGF and P44/42 protein
kinase activity.[7-11]
Considering that IGF-1
administration to patients with diabetes improves diabetes control, by
increasing insulin sensitivity and decreasing secondary GH resistance,
Laron and Weinberger have speculated that IGF-1 has a ‘permissive’
mediating or even a ‘protective’ role in the development of diabetic
retinopathy.[12]
This cross-sectional study was
designed to give insights into the relationship between IGF-1 levels
and DR in a sample of TM patients with IDDM. In none of the previous
studies, this relation was evaluated, despite the fact that both
diseases (DR and IDDM) co-exist in the same patient. Furthermore, the
study intends to describe the clinical and biochemical profile of the
associated complications in TM patients with and without IDDM.
Patients and Methods
Setting and study design.
The study was started at the beginning of 2009 by VDS, Coordinator of
International Network of Clinicians for Endocrinopathies in Thalassemia
and Adolescent Medicine (ICET-A)[13] at the
Thalassaemia Centre of Ferrara and was completed by the end of 2014 at
the Quisisana Pediatric and Adolescent Outpatient Clinic of Ferrara.
The
study included 19 consecutive TM patients with IDDM and 31 age- and
sex-matched TM patients without evidence of IDDM, who visited our
out-patient clinics for an endocrine assessment. The duration of
diabetes was defined as the interval between diagnosis of IDDM and the
time of enrollment in this study. Insulin usage was recorded for each
diabetic patient.
Exclusion criteria.
Exclusion criteria were: 1) duration of diabetes less than 10 years; 2)
previous or current treatment with drugs known to interfere with
glucose or lipid metabolism or to influence blood pressure; 3) previous
treatment with corticosteroids for longer than 2 weeks; 4) smoking of
more than 15 cigarettes/day and alcohol abuse (more than three glasses
of wine/day); 5) presence of factors that could interfere with
fructosamine determination (such as low serum albumin concentration,
dyslipidemia and hyperbilirubinemia).[14]
Research design.
An extensive medical history, including data on associated
complications and current medications, was obtained and a physical
examination including anthropometry (weight, height, BMI), vital signs
(blood pressure, heart rate) and gonadal and menstrual status was
performed. Body mass index (BMI) was calculated as the body weight
divided by the height squared (Kg/m2). A subject was considered overweight when the BMI was between 25 and 29.9 and obese when the BMI was above 30.
The
following clinical data were also recorded: age at first transfusion,
duration, type and compliance of iron chelation therapy, compliance
with treatment of diabetes, duration of diabetes and associated
endocrine complications, as previously described.[15]
Subjects were considered to have a macrovascular disease if they had
ever been diagnosed with a myocardial infarction or other vascular
accident.
Definitions.
For the screening, diagnosis and treatment of growth disorders and
endocrine complications we used the criteria as previously described.[15]
Blood sampling and methods.
Blood samples were drawn in the morning after an overnight fast to
measure the serum concentrations of IGF-1, glucose, fructosamine, free
thyroxine (FT4), thyrotropin (TSH), urea, creatinine, electrolytes
(including calcium and phosphate) and total proteins.
The last
insulin injection on the day before blood sampling was administered at
22.00 h in IDDM subjects receiving intensive insulin therapy (four
daily injections) and at 18.00 h in IDDM subjects receiving two daily
injections of a mixture of short- and medium-acting insulins. TM
patients were invited to abstain from ascorbic acid supplements for a
minimum of 24 hours prior to sample collection.
In order to
exclude severe liver injury and dysfunction, serum concentrations of
alanine aminotransferase (ALT), gamma glutamyl transferase (γGT),
alkaline phosphatase (ALP), total and direct bilirubin, albumin,
prothrombin time (PT) and international normalization ratio (INR) were
measured. Serologic screening assays for hepatitis C virus
seropositivity (HCVab and HCV-RNA) were also obtained applying routine
laboratory methods.
Plasma total IGF-1 was measured by a
chemiluminescent immunometric assay (CLIA) method (Nichols Institute
Diagnostics, San Juan, CA). The assay was performed after separation of
IGF-1 from binding proteins by Liaison® autoanalyzer (DiaSorin SpA,
Saluggia, Italy).The sensitivity of the test was six ng/ml, whereas the
intra- and interassay coefficients of variation (CVs) of our in-house
pooled serum control sample were 4.8% and 6.7 %, respectively.
The reported analytic sensitivity of this assay was from 6 to 25 ng/ml. Ranges of normal values set at the 2.5th-97.5th
percentile in 547 non-hypopituitary, non-acromegalic healthy subjects
of both sexes in Italy in three age ranges were: 95.6-366.7 ng/ml for
ages 25 to 39 yrs, 60.8-297.7 ng/ml for 40 to 59 yrs and 34.5-219.8
ng/ml for subjects aged 60 and above.[16] For the
diagnosis of microalbuminuria, a first-morning urine sample was
analyzed by immunoturbidimetry (MAU; normal range: 20-200 mg/l).[17]
Assessment of iron overload.
Iron overload was assessed by direct and indirect methods. At the
beginning of the study it was assessed by serum ferritin level and was
arbitrarily categorized as mild (<1000ng/ml), moderate (1000-2000
ng/ml and severe (>2000ng/ml).[18]
In 10 TM
patients with IDDM and 21 patients without IDMM, cardiac iron
concentration (CIC) was available. Magnetic resonance imaging (MRI) was
performed using a 1.5 T scanner (GE Signa/Excite HD, Milwaukee, WI,
USA) within the Myocardial Iron Overload in Thalassemia (MIOT) network,
where MRI scans are performed using homogeneous, standardized and
validated procedures.[19] A conservative cut off value of T2* > 20 ms was considered normal.[20]
The
liver iron concentration (LIC) was assayed in 11 TM patients with IDDM
and in 14 patients without IDDM by atomic absorption spectrophotometry
and expressed as mg/g dry weight (dw)[21] or by MRI[22]
or Superconducting Quantum Interference Device (SQUID) susceptometry.
Liver T2* values were converted into LIC values by using the
calibration curve introduced by Wood et al.[23] Based
on data from the literature the normal LIC is considered between 0.4
and 2 mg/g of liver dry weight while iron overload is classified as
mild: 2-7, moderate: 7-15 and severe > 15 mg Fe/gr dry wt.[23]
Assessment of diabetic retinopathy (DR) and metabolic control.
Nowadays, different standards for DR have been published, morphological
features of lesions are commonly mentioned parameters for disease
severity grading.[24-26] Ophthalmology evaluation was
done by the same researcher (CI) using stereoscopic fundus
biomicroscopy through dilated pupils. DR was graded using the scale
developed by the Global Diabetic Retinopathy Group (Table 1).[25]
When the diabetic retinopathy was asymmetric, the subject was assigned
to the group corresponding to the eye with the worse retinopathy
findings.
In each case, metabolic glucose control was assessed
by the concentration of fasting blood glucose, the serum concentration
of fructosamine and the results of glycosuria and ketonuria monitoring
at home. Poor control was arbitrarily defined as the presence of a
fasting blood glucose concentration >11.1 mmol/l and/or a serum
concentration of fructosamine > 350 μmol/l.
Ethical aspects. The study protocol was conducted in accordance with the ethical guidelines of the 1996 Declaration of Helsinki.[27] Informed consent was obtained from all patients.
Statistical Analysis.
Characteristics of the studied patients are reported as total number
and mean ± standard deviation (SD). Statistical significance of the
differences between variables was assessed using the unpaired
two-tailed Student’s t test. Fisher's Exact test was used to calculate
the probability value for the relationship between two dichotomous
variables. A p value < 0.05 was considered as significant. A
software program used for the statistical analysis was developed by Dr.
Candini (Department of Medical Physics, St. Anna Hospital, Ferrara,
Italy) and validated according to Alder and Roesser.[28]
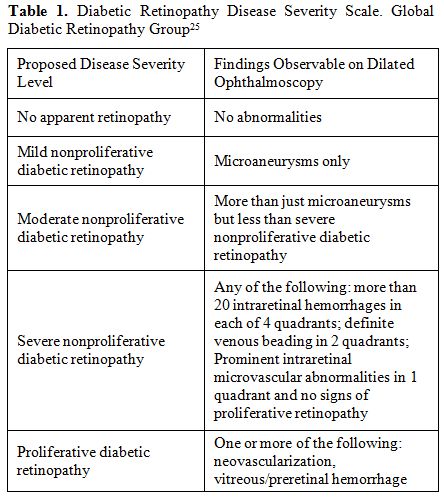 |
Table 1. Diabetic Retinopathy Disease Severity Scale. Global Diabetic Retinopathy Group[25] |
Results
Patients’ characteristics.
All patients were on regular transfusions (mean haemoglobin level 11.5
g/dl) and iron chelation therapy with deferoxamine (43 patients: 30-45
mg/kg body weight, 4-6 days a week by slow subcutaneous infusion by
pump, starting in 1977-1978), or oral deferiprone (26 patients: 75
mg/kg body weight daily), or deferiprone plus deferoxamine (12
patients; 75 mg/kg body weight daily and 40 mg/kg body weight, 3 days a
week, by slow subcutaneous pump infusion) or oral deferasirox (6
patients: 20-30 mg/kg body weight daily). Chelation therapy has changed
over time. Treatment with intramuscular deferoxamine has been available
for most patients since 1969. Regular subcutaneous infusion of
deferoxamine was started in 1978. Since 1995 and 2007 the new oral
chelating agents, deferiprone or deferasirox, have been given to some
patients who were unable or unwilling to receive deferoxamine.
Deferiprone plus deferoxamine was given to few selected patients with
severe iron overload.
The baseline clinical characteristics of 19 IDDM subjects with TM (7 women and 12 men) are shown in Table 2.
All TM patients with IDDM were on an insulin dose schedule of 2–4 times
daily, using combinations of short-acting and intermediate-acting
insulin. Age, gender distribution and BMI did not differ between
diabetic and non-diabetic patients. One diabetic patient was
overweight, and one was obese. All patients had normal systolic and
diastolic blood pressures.
A significant percentage of TM patients with IDDM had other endocrinopathies (Table 1).
Hypogonadotropic hypogonadism was present in 89.5% of diabetic versus
54.8% of non diabetic patients (p <0.05). All but five were on
hormone replacement therapy with sex steroids. Hypothyroidism and
hypoparathyroidism were significantly more prevalent in diabetic versus
non-diabetic patients, but statistically different only for
hypothyroidism (p <0.05). All hypothyroid and hypoparathyroid TM
patients were taking thyroxine or calcitriol respectively. No adrenal
insufficiency was documented. Growth hormone status was not assessed.
An
abnormal ALT value (> 80 U/L) was observed in 4 female TM patients
with IDDM (21 %) and in 2 (1 male and 1 female) TM patients without
IDDM (6.4 %). A statistical difference was observed for serum γGT in
the two groups (p< 0.05).Hepatitis C antibodies were present in 100
% in both groups (Table 1). The percentage of HCV-RNA seropositivity did not differ in diabetic versus non-diabetic patients (57.9% versus 51.6%).
Serum
lipids were not significantly altered in the two groups of patients
compared to the normal ranges of our central laboratory service (<
180 mg/dL for total cholesterol; < 159 mg/dL for LDL-C and 150 mg/dL
for triglycerides). Only one male TM patient with DR had a borderline
total cholesterol level (179 mg/dL). Two patients had triglyceride
levels higher than 130 mg/dL. Ten of our patients with DR had low HDL-C
levels (<40 mg/dL for men and <50 mg/dL for women) and only one
patient had LDL-C level higher than 159 mg/dL (168 mg/dL).
Assessment of iron overload.
Mean serum ferritin level was significantly higher in diabetic versus
non-diabetic TM subjects (p <0.05). Serum ferritin level >2000
ng/ml (severe iron overload) was present in 15.8% of diabetic versus
3.2% of non-diabetic TM patients.
A liver iron concentration >
7 mg/g dry weight was present in 15.8 % of diabetic TM patients and
none of the non-diabetic TM patients. A 36 year old female patient with
IDDM had a LIC concentration of 15.9 mg/g/dw (a concentration
associated with a high risk for cardiac disease). Although the
percentage of TM patients with T2* < 20 msec was higher in IDDM
compared to those without diabetes, the difference was not
statistically significant (Table 2).
Insulin-like growth factor 1 (IGF-1) analysis. Eighteen TM patients with IDDM (94.7 %) and 10 non-diabetic patients (32.2 %) had IGF-1 levels below the 2.5th percentile of the normal values for the Italian population.[16] The mean serum IGF-1 concentrations were significantly lower in the diabetic versus the non-diabetic TM groups (p < 0.001; Table 2).
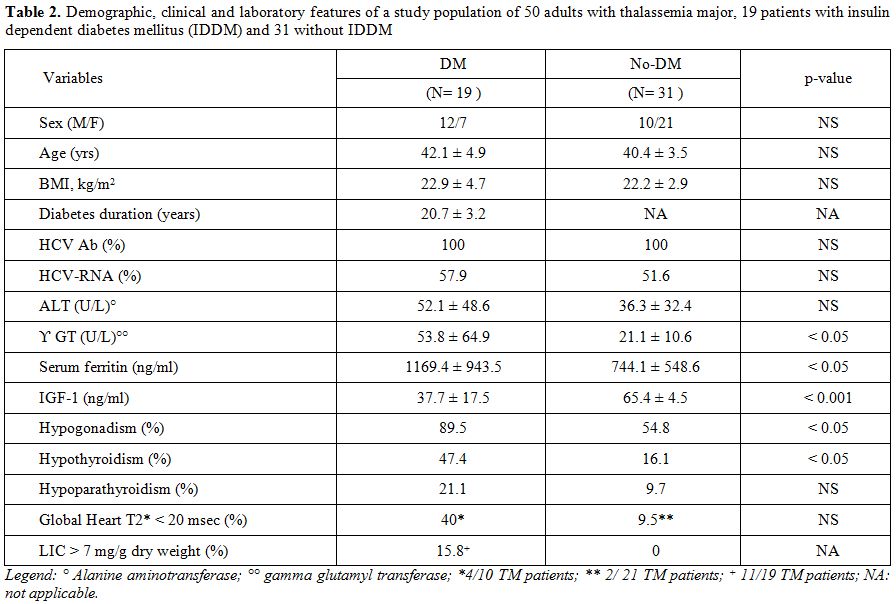 |
Table 2. Demographic, clinical and
laboratory features of a study population of 50 adults with thalassemia
major, 19 patients with insulin dependent diabetes mellitus (IDDM) and
31 without IDDM . |
A significant negative correlation was observed between IGF-1 and fructosamine levels in diabetic and non diabetic TM patients (Figure 1; r: - 0.5516.; p: < 0.05).
In
our study, DR was present in a low, percentage in 4 (21 %) of 19 TM
patients with IDDM and was associated with the main classical risk
factors, of inefficient glycemic control and duration of disease but
not of hypertension (Table 3). Using the scale developed by the Global Diabetic Retinopathy Group,[25]
the DR in our patients was classified as non proliferative diabetic
retinopathy (NPDR). Only a few numbers of microaneurysms (1-3) were
detected. Certain recognized ocular and systemic factors which are
known to protect a diabetic patient against the development of DR, such
as high myopia, raised intraocular pressure and moderate carotid
stenosis were not present or reported in our TM patients.
In eight
diabetic patients inefficient metabolic glucose control, arbitrarily
defined as the presence of a fasting blood glucose concentration >
11.1 mmol/l and/or a serum concentration of fructosamine > 350
μmol/l, was found (Figure 1). Microalbuminuria was present in five patients with IDDM (26.3%).
In
two patients with fair (no.1) and good (no.3) compliance to combined
iron chelation therapy, the LIC (mg Fe/gr dry wt) was normal (Table 3).
|
|
Figure 1. Correlation between fructosamine (μmol/l) and
IGF-1 (ng/ml). Normal IGF-1 values: 95.6-366.7 ng/ml for ages 25 to 39
yrs, 60.8-297.7 ng/ml for 40 to 59 yrs and 34.5-219.8 ng/ml.[16] |
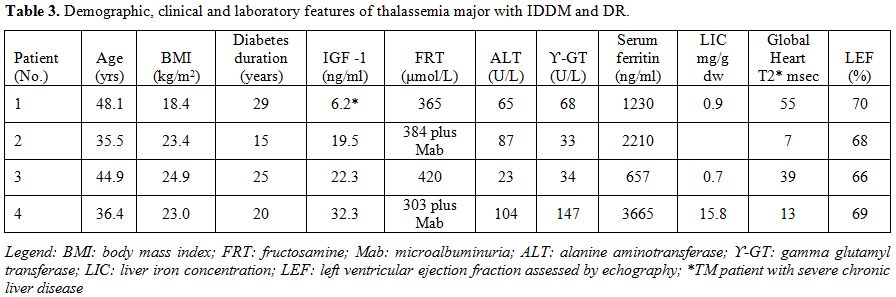 |
Table 3.Demographic, clinical and laboratory features of thalassemia major with IDDM and DR. |
Discussion
IDDM
is a complex disease with many end organ complications. However, good
control of the disease can prevent the onset or delay the progression
of the various complications, including diabetic retinopathy (DR). The
prevalence of DR worldwide ranges from 6.8 to 44.4% in patients with
diabetes mellitus.[29-31]A
systematic literature review was conducted by Yau et al. to identify
all population-based studies in general populations or individuals with
diabetes. A total of 35 studies (1980–2008) provided data from 22,896
individuals with diabetes. The overall prevalence was 34.6% for any DR,
6.96% for proliferative DR, 6.81% for diabetic macular edema, and 10.2%
for vision-threatening diabetic retinopathy (VTDR). All DR prevalence
end points increased with diabetes duration, hemoglobin A1c, and blood pressure levels were higher in people with type 1 compared with type 2 diabetes.[29] Similar results were also reported by other Researchers.[30-32]Our
screening modality of DR was in accordance with the UK National
Institute for Clinical Excellence (NICE) recommendations regarding the
sensitivity, specificity and technical failure rate.[33]
In our study, DR was present in a relatively small percentage (21%) of
diabetic TM patients with duration of diabetes above ten years. IDDM
was associated with the main classical risk factors of inefficient
glycemic control and duration of the disease but not of hypertension (Table 3). Using the scale developed by the Global Diabetic Retinopathy Group,[25]
DR in our patients was classified as non proliferative diabetic
retinopathy (NPDR). A small number of microaneurysms (from 1 to 3) were
detected by an expert ophthalmologist using a stereoscopic fundus
biomicroscopy through dilated pupils.The
major risk factors for DR are hyperglycemia, hypertension, duration of
diabetes and dyslipidemia. Genetic and growth factors have also been
implicated in the development of DR. On the other hand, there are
certain recognized systemic and ocular factors which are known to
protect a diabetic patient against the development of DR, e.g. low
lipid levels, hypogonadism, growth hormone deficiency, reduced IGF-1
levels, high myopia, raised intraocular pressure and moderate carotid
stenosis.[34-41]In
our TM patients serum lipids were within the defined normal range (<
180 mg/dL for total cholesterol; < 159 mg/dL for LDL-C and 150 mg/dL
for triglyceride).Several
mechanisms may play a role in determining low serum cholesterol and
triglyceride levels in TM patients including plasma dilution resulting
from anemia, increased cholesterol requirement associated with
erythroid hyperplasia, macrophage system activation with cytokine
release, increased cholesterol uptake by the reticuloendothelial
system, iron overload and oxidative stress. In the light of these data,
it would be speculated that the low prevalence of DR in TM patients
compared to patients with diabetes mellitus might be a consequence of a
better lipid profile. All
male TM patients but one with hypogonadism were on hormone replacement
therapy with long acting sex steroids. Their serum free testosterone
levels, however, were in the subnormal or below the adult normal range
because of the presence of a high serum SHBG level secondary to chronic
liver disease and iron overload. Therefore, a mild degree of androgen
insufficiency could be an additional factor in reducing the risk of DR
in TM patients.Unfortunately,
growth hormone was not assessed routinely in our patients because the
majority of them were followed in an outpatient endocrine clinic.
Nevertheless, data from the literature report a prevalence of GHD and
/or IGF-I deficiency in TM patients from 8% to 44 % in different
centers.[15-18] It has been reported that IGF-1 is a
potent stimulator of retinal endothelial cell growth and play a major
role in the development of diabetic retinopathy.A significant number of our TM patients with and without IDDM had an IGF-1 deficiency (< 2.5th
percentile of age- and sex- matched Italian population). However, the
mean IGF-1 value was particularly lower in diabetic versus non-diabetic
patients (p < 0.001). Decreased IGF-1 secretion occurs in the
majority of TM patients especially those with growth and pubertal
delay. Many factors contribute to this decreased synthesis of IGF-1
including under-nutrition, insufficient blood transfusion with
significant periods of anemia, inadequate iron chelation with iron
overload in the pituitary gland (GH, LH, FSH, TSH deficiencies), liver
(systemic IGF-1 deficiency) and the co-occurrence of other endocrine
disorders, such as hypothyroidism and diabetes mellitus.[42-44]
IDDM group was significantly more iron overloaded than nondiabetic
group as indicated by the higher serum ferritin and LIC values. Our
results call into question whether very low serum IGF-1 contributes to
the pathogenesis of DR, inhibiting the molecular events that initiate
retinal angiogenesis. The pathogenesis of diabetic retinopathy is
complex. The major causative factor for the development of diabetic
retinopathy is hyperglycemia, which leads to increased
vasopermeability, endothelial cell proliferation, and
neovascularisation. While hyperglycemia is a major factor, diabetes is
associated with changes in insulin, IGF-1 and many other hormones and
metabolites, including free fatty acid, amino acids, advanced glycation
end products, and components of the oxidative stress pathway. IGF-1 is
known to trigger a critical cascade of molecular events that initiate
retinal angiogenesis.[45] The
proliferative effect of hyperglycemia on vascular endothelial cells is
thought to be mediated by vascular endothelial growth factor (VEGF).[46]
IGF-1 receptor regulation of VEGF action is mediated at least in part
through control of VEGF activation of p44/42 mitogen-activated protein
kinase, establishing a hierarchical relationship between IGF-1 and VEGF
receptors. Therefore, these findings establish an essential role for
IGF-1 in angiogenesis and demonstrate a new target for control of
retinopathy and also explain why diabetic retinopathy initially
increases with the onset of insulin treatment.[47-49]Nevertheless,
while experimental and clinical evidence suggests that serum IGF-1
concentrations may be involved in the development of diabetic
retinopathy the relationship is still controversial. Several
studies have reported that higher serum IGF-1 levels may be a risk
factor for the development of severe diabetic retinopathy; on the other
hand few studies have shown no association between serum IGF-1 levels and the development or progression of diabetic retinopathy.In
our patients, a significant correlation was observed between
fructosamine levels and IGF-1 values. It has been reported by Chantelau
that the reduction of hyperglycaemia from >16 mmol/l (equivalent to
HbA1c >11%) to <10 mmol/l (HbA1c <8%) increase the serum IGF-1 levels by 70-220%, within 5 months.[45]
While proteinuria and symptomatic neuropathy regressed in his patients,
retinopathy progressed from the mild to the severe non-proliferative
stage with maculopathy (n=4), and to the proliferative stage (n=1). The
biological mechanisms underlying this phenomenon remain unknown.
Insulin signalling in endothelial cells has been shown to regulate the
expression of some potential mediators of neovascularization, including
VEGF, eNOS and endothelin-1.[35-41]These
findings are probably less relevant in TM patients because the IGF-1
levels are < -2SDs in 50% of patients compared to healthy
individuals[44] and the prevalence of DR remains low
for a long duration of diabetes, however, we cannot ignore this point
because it suggests that another particular risk factor for the
progression of DR is represented by the upregulation of serum IGF-1.[46-47]We
used fructosamine as an index of metabolic control because a number of
clinically significant haemoglobin disorders alter haemoglobin,
structurally or chemically, thereby affecting the reliability of the
A1c test.[50] As the mean half‐life of plasma
proteins is approximately 2–3 weeks, fructosamine provides a shorter
term representation of glycaemic control than HbA1c. However, it should
be noted that a recent clinical trial in patients with TM has shown a
direct correlation between fructosamine and fasting blood glucose
values.[51]Our
data confirm the strong association of IDDM in TM patients with other
endocrine and non-endocrine complications related to severe iron
overload. Therefore, early recognition of these complications,
establishment of appropriate chelation therapy and adequate treatment
of each complication are the main keys to successful management of
these patients. Conclusions
This study although not fully representative of the general
population suggests a possible ‘protective’ role of low IGF-1 in the
development of diabetic retinopathy. Therefore, it could be interesting
to study further possible systemic, local and genetic factors in
relation to the development and degree of DR in a larger cohort of
patients with diabetes versus diabetic TM patients. Although specific
guidelines have been prepared and published by the ICET-A Network for
the treatment of endocrine complications in thalassemic patients,[52,53]
there is still an urgent need to consider specific worldwide treatment
protocols for managing patients with multiple/complex complications.
References
- Cunningham MJ, Macklin EA, Neufeld EJ, Cohen AR.
Thalassemia Clinical Research Network. Complications of
beta-thalassemia major in North America. Blood 2004;104:34-39 http://dx.doi.org/10.1182/blood-2003-09-3167 PMid:14988152

- De
Sanctis V, Soliman AT, Yassin M. Iron overload and glucose metabolism
in subjects with ß-thalassaemia major: an overview. Curr Diab Rev.2013;
9, 1573-3998/13

- Dodson
PM. Management of diabetic retinopathy: could lipid-lowering be a
worthwhile treatment modality? Eye (Lond). 2009;23: 997-1003 http://dx.doi.org/10.1038/eye.2008.428 PMid:19169236

- Segato
T, Midena E, Grigoletto F, Zucchetto M, Fedele D, Piermarocchi S,
Crepaldi G.The epidemiology and prevalence of diabetic retinopathy in
the Veneto region of north east Italy. Veneto Group for Diabetic
Retinopathy. Diabet Med. 1991;8 Spec No:S11-16. http://dx.doi.org/10.1111/j.1464-5491.1991.tb02149.x PMid:1825948

- Poulaki
V, Joussen AM, Mitsiades N, Mitsiades CS, Iliaki EF, Adamis AP.
Insulin-like growth factor-I plays a pathogenetic role in diabetic
retinopathy. Am J Pathol. 2004; 165:457–469 http://dx.doi.org/10.1016/S0002-9440(10)63311-1

- Ruberte
J, Ayuso E, Navarro M, Carretero A, Nacher V, Haurigot V, George M,
Llombart C, Casellas A, Costa C, Bosch A, Bosch F- Increased ocular
levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J
Clin Invest.2004; 113: 1149–1157. http://dx.doi.org/10.1172/JCI19478 PMid:15085194 PMCid:PMC385397

- Uthra
S, Raman R, Mukesh BN, Rajkumar SA, Kumari R P, Agarwal S, Paul PG,
Lakshmipathy P, Gnanamoorthy P, Sharma T, McCarty CA, Kumaramanickavel
G. Diabetic retinopathy and IGF-1 gene polymorphic cytosine-adenine
repeats in a Southern Indian cohort. Ophthalmic Res. 2007;39: 294-9. http://dx.doi.org/10.1159/000108124 PMid:17851271

- Janssen
JAMJL, Lamberts SWJ. Circulating IGF-I and its protective role in the
pathogenesis of diabetic angiopathy. Clin. Endocrinol. 2000; 51: 1–9. http://dx.doi.org/10.1046/j.1365-2265.2000.00922.x

- Kondo
T, Vicent D, Suzuma K, Yanagisawa M, King GL, Holzenberger M, Kahn CR.
Knockout of insulin and IGF-1 receptors on vascular endothelial cells
protects against retinal neovascularisation. J Clin Invest. 2003
;111:1835-1842. http://dx.doi.org/10.1172/JCI200317455 PMid:12813019 PMCid:PMC161423

- Simo
R, Hernandez C, Segura RM, Garcia-Arumi J, Sararols L, Burgos R, Ana
Canto N,Mesa J. Free insulin-like growth factor 1 in the vitreous fluid
of diabetic patients with proliferative diabetic retinopathy: a case
control study. Clin Sci.2003; 104: 223–230. http://dx.doi.org/10.1042/CS20020278 PMid:12605576

- Chantelau
E. Evidence that up regulation of serum IGF-1 concentration can trigger
acceleration of diabetic retinopathy. Br J Ophthalmol.1998; 82:
725–730. http://dx.doi.org/10.1136/bjo.82.7.725 PMid:9924360 PMCid:PMC1722687

- Laron
Z, Weinberger D. Diabetic retinopathy, nephropathy and cardiovascular
disease in a patient with GH gene deletion. Clin Endocrinol (Oxf).2005;
63:699-700. http://dx.doi.org/10.1111/j.1365-2265.2005.02402.x PMid:16343109

- De
Sanctis V, Soliman AT, Angastiniotis M, Eleftheriou A, Kattamis Ch,
Karimi M, El Kholy M, Elsedfy H, Yassin MA, El Awwa A, Stoeva I,
Skordis N, Raiola G, Fiscina B. International network on endocrine
complications in thalassaemia (I-CET): an opportunity to grow.Georgian
Med News. 2012; 205:52- 57 PMid:22665732

- Austin
GE, Mullins RH, Morin LG. Non-enzymatic glycation of individual plasma
proteins in normoglycemic and hyperglycemic patients. Clin Chem
1987;33:2220-2224 PMid:3690840

- De
Sanctis V, Soliman AT, Elsedfy H, Skordis N, Kattamis C, Angastiniotis
M, Karimi M, Yassin MA, El Awwa A, Stoeva I, Raiola G, Galati MC,
Bedair EM, Fiscina B, El Kholy M. Growth and endocrine disorders in
talassemia. The international network on endocrine complications in
thalassemia (I-CET) position statement and guidelines. Indian J
Endocrinol Metab. 2013;17:8-18 http://dx.doi.org/10.4103/2230-8210.107808 PMid:23776848 PMCid:PMC3659911

- Aimaretti
G, Boschetti M, Corneli G, Gasco V, Valle D, Borsotti M, Rossi A,
Barreca A, Fazzuoli L, Ferone D, Ghigo E, Minuto F.Normal age-dependent
values of serum insulin growth factor-I: results from a healthy Italian
population. J Endocrinol Invest. 2008;31:445-449. http://dx.doi.org/10.1007/BF03346389 PMid:18560263

- Gross
JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T.
Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes
Care. 2005;28:164-176. http://dx.doi.org/10.2337/diacare.28.1.164 PMid:15616252

- Soliman
A, De Sanctis V, Elsedfy H, Yassin M, Skordis N, Karimi M, Sobti P,
Raiola G, El Kholy M. Growth hormone deficiency in adults with
thalassemia: an overview and the I-CET recommendations. Georgian Med
News. 2013;222 :79-88. PMid:24099819

- Ramazzotti
A, Pepe A, Positano V, Rossi G, De Marchi D, Brizi MG, Luciani A,
Midiri M, Sallustio G, Valeri G, Caruso V, Centra M, Cianciulli P, De
Sanctis V, Maggio A, Lombardi MMulticenter validation of the magnetic
resonance T2* technique for segmental and global quantification of
myocardial iron. J Magn Reson Imaging. 2009l;30:62-68. http://dx.doi.org/10.1002/jmri.21781 PMid:19557847

- Positano
V, Pepe A, Santarelli MF, Scattini B, De Marchi D, Ramazzotti A, Forni
G, Borgna-Pignatti C, Lai ME, Midiri M, Maggio A, Lombardi M, Landini
L.Standardized T2* map of normal human heart in vivo to correct T2*
segmental artefacts. NMR Biomed. 2007;20:578-590. http://dx.doi.org/10.1002/nbm.1121 PMid:17205488

- Ramazzotti
A, Pepe A, Positano V, Rossi G, De Marchi D, Brizi MG, Luciani A,
Midiri M, Sallustio G, Valeri G, et al. Multicenter validation of the
magnetic resonance T2* technique for segmental and global
quantification of myocardial iron. J Magn Reson Imaging. 2009;
30:62–68. http://dx.doi.org/10.1002/jmri.21781 PMid:19557847

- Meloni
A, Luciani A, Positano V, De Marchi D, Valeri G, Restaino G, Cracolici
E, Caruso V, Dell'amico MC, Favilli B, Lombardi M, Pepe A.Single region
of interest versus multislice T2* MRI approach for the quantification
of hepatic iron overload. J Magn Reson Imaging. 2011;33:348-355. http://dx.doi.org/10.1002/jmri.22417 PMid:21274976

- Meloni
A, Rienhoff HY Jr, Jones A, Pepe A, Lombardi M, Wood JC. The use of
appropriate calibration curves corrects for systematic differences in
liver R2* values measured using different software packages.Br J
Haematol. 2013;161:888-891 http://dx.doi.org/10.1111/bjh.12296 PMid:23496418 PMCid:PMC3672338

- Chen
Z, Zhang SS, Zhu HM. Analysis of international clinical diabetic
retinopathy disease severity scale. Int J Opthalmol 2011, 11:1394–1401.

- Wilkinson
CP1, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D,
Kampik A, Pararajasegaram R, Verdaguer JT; Global Diabetic Retinopathy
Project Group. Proposed international clinical diabetic retinopathy and
diabetic macular edema disease severity scales. Ophthalmology.
2003;110:1677-1682. http://dx.doi.org/10.1016/S0161-6420(03)00475-5

- Kohner
EM: The lesions and natural history of diabetic retinopathy. In: Pickup
JC, Williams G (Eds): Chronic Complications of Diabetes. Blackwell,
Oxford, 1994, p. 63-76
- World Medical Organization: Declaration of Helsinki. BMJ 1996; 313: 1448–1449. http://dx.doi.org/10.1136/bmj.313.7070.1448a

- Alder
R, Roesser EB.Introduction to probability and statistics. WH Freeman
and Company Eds. Sixth Edition.San Francisco (USA), 1975
- Yau
JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ,
Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK,
Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP,
Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H,
Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X,
Mitchell P, Wong TY; Meta-Analysis for Eye Disease (META-EYE) Study
Group.Global prevalence and major risk factors of diabetic
retinopathy.Diabetes Care. 2012;35:556-564 http://dx.doi.org/10.2337/dc11-1909 PMid:22301125 PMCid:PMC3322721

- Esteves
JF, Kramer CK, Azevedo MJ, Stolz AP, Roggia MF, Larangeira A, Miozzo
SA, Rosa C, Lambert JH, Pecis M, Rodrigues TC, Canani LH. Prevalence of
diabetic retinopathy in patients with type 1 diabetes mellitus. Rev
Assoc Med Bras. 2009;55:268-273 http://dx.doi.org/10.1590/S0104-42302009000300017 PMid:19629344

- Bek
T, Lund-Andersen H, Hansen AB, Johnsen KB, Sandbaek A, Lauritzen T. The
prevalence of diabetic retinopathy in patients with screen-detected
type 2 diabetes in Denmark: the ADDITION study. Acta Ophthalmol.
2009;87:270-274 http://dx.doi.org/10.1111/j.1755-3768.2008.01207.x PMid:18823287

- Lim
MC, Lee SY, Cheng BC, Wong DW, Ong SG, Ang CL, Yeo IY. Diabetic
retinopathy in diabetics referred to a tertiary centre from a
nationwide screening programme. Ann Acad Med Singapore.
2008;37:753-759. PMid:18989491

- National
Institute for Clinical Excellence. Management of Type 2 diabetes.
Retinopathy -screening and early management. London: NICE; 2002
- Raman
R, Gupta A. Absence of diabetic retinopathy in a patient who has had
diabetes mellitus for 69 years, and inadequate glycemic control: case
presentation: response.Diabetol Metab Syndr. 2010 Mar 25;2:20. doi:
10.1186/1758-5996-2-20 http://dx.doi.org/10.1186/1758-5996-2-20

- Poulaki
V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD,
Adamis AP. Acute intensive insulin therapy exacerbates diabetic
blood-retinal barrier breakdown via hypoxia-inducible factor-1 alpha
and VEGF. J. Clin. Invest. 2002; 109:805–815. http://dx.doi.org/10.1172/JCI0213776

- Józkowicz
A, Pankiewicz J, Dulak J, Partyka L, Wybranska I, Huk I, Dembinska-Kiec
A. Nitric oxide mediates the mitogenic effects of insulin and vascular
endothelial growth factor but not of leptin in endothelial cells. Acta
Biochim. Pol. 1999; 46:703–715. PMid:10698278

- DingY,
Vaziri ND, Coulson R, Kamanna VS, Roh DD. Effects of simulated
hyperglycemia, insulin, and glucagon on endothelial nitric oxide
synthase expression. Am. J. Physiol. Endocrinol. Metab. 2000;
279:E11–E17. PMid:10893317

- Kuboki
K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP,
Herbert TP, Rhodes CJ, King GL.Regulation of endothelial constitutive
nitric oxide synthase gene expression in endothelial cells and in vivo:
a specific vascular action of insulin. Circulation. 2000;101:676–681. http://dx.doi.org/10.1161/01.CIR.101.6.676 PMid:10673261

- Oliver
FJ, de la Rubia G, Feener EP, Lee ME, Loeken MR, Shiba T, Quertermous
T, King GL. Stimulation of endothelin-1 gene expression by insulin in
endothelial cells. J. Biol. Chem. 1991;266:23251–23256.
PMid:1744120

- Desideri
G, Ferri C, Bellini C, De Mattia G, Santucci A. Effects of ACE
inhibition on spontaneous and insulin-stimulated endothelin-1
secretion: in vitro and in vivo studies. Diabetes.1997; 46:81–86. http://dx.doi.org/10.2337/diab.46.1.81 PMid:8971086

- Cardillo
C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, Panza JA.
Insulin stimulates both endothelin and nitric oxide activity in the
human forearm. Circulation. 1999;100:820–825. http://dx.doi.org/10.1161/01.CIR.100.8.820 PMid:10458717

- Soliman
AT, Sanctis VD, Elalaily R, Yassin M. Insulin-like growth factor- I and
factors affecting it in thalassemia major. Indian J Endocr Metab
2015;19:245-251 http://dx.doi.org/10.4103/2230-8210.131750 PMid:25729686 PMCid:PMC4319264

- De
Sanctis V, Soliman AT, Candini G, Elsedfy H. The recommendation of the
International Network of Clinicians for Endocrinopathies in Thalassemia
and Adolescent Medicine for the assessment of growth hormone secretion
in thalassemia. Indian J Endocr Metab 2015;19:306-307 http://dx.doi.org/10.4103/2230-8210.149331 PMid:25729702 PMCid:PMC4319280

- De
Sanctis V, Soliman AT, Candini G, Yassin M, Raiola G, Galati MC,
Elalaily R, Elsedfy H, Skordis N, Garofalo P, Anastasi S, Campisi S,
Karimi M, Kattamis C, Canatan D, Kilinc Y, Sobti P, Fiscina B, El Kholy
M. nsulin-like Growth Factor-1 (IGF-1): Demographic, Clinical and
Laboratory Data in 120 Consecutive Adult Patients with Thalassaemia
Major. Mediterr J Hematol Infect Dis. 2014 Nov 1;6(1):e2014074. http://dx.doi.org/10.4084/mjhid.2014.074

- Chantelau
E. Evidence that upregulation of serum IGF-1 concentration can trigger
acceleration of diabetic retinopathy. Br J Ophthalmol. 1998; 82:
725–730. http://dx.doi.org/10.1136/bjo.82.7.725 PMid:9924360 PMCid:PMC1722687

- Wilson
SH, Davis MI, Caballero S, Grant MB. Modulation of retinal endothelial
cell behaviour by insulin-like growth factor I and somatostatin
analogues: implications for diabetic retinopathy. Growth Horm IGF Res.
2001;11 (Suppl A):S53-59 http://dx.doi.org/10.1016/S1096-6374(01)80009-5

- Smith
LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S,
Bischoff J, Zhang B, Schaeffer JM, Senger DR. Regulation of vascular
endothelial growth factor-dependent retinal neovascularization by
insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390-1395 http://dx.doi.org/10.1038/70963 PMid:10581081

- Lauritzen
T, Frost-Larsen K, Larsen HW, Deckert T. Two-year experience with
continuous subcutaneous insulin infusion in relation to retinopathy and
neuropathy. Diabetes. 1985;34 (Suppl 3):74-79. http://dx.doi.org/10.2337/diab.34.3.S74 PMid:4018423

- Dahl-Jørgensen
K, Brinchmann-Hansen O, Hanssen KF, Sandvik L, Aagenaes O. Rapid
tightening of blood glucose control leads to transient deterioration of
retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br
Med J (Clin Res Ed). 1985;290:811-815. http://dx.doi.org/10.1136/bmj.290.6471.811

- Smaldone A. Glycemic control and hemoglobinopathy: when A1C may not be reliable. Diabetes Spectrum. 2008;21:46-49 http://dx.doi.org/10.2337/diaspect.21.1.46

- Kosaryan
M, Mahdavi MR, Aliasgharian A,Mousav M, Roshan P. Credibility of
measurement of fructosamine and hemoglobin A1C in estimating blood
glucose level and diabetic patients with thalassemia major. Open J
Hematol. 2012;3:1-7. http://dx.doi.org/10.13055/ojhmt_3_1_4.121107

- De
Sanctis V, Soliman AT, Elsedfy H, Skordis N, Kattamis C, Angastiniotis
M, Karimi M, Yassin MA, El Awwa A, Stoeva I, Raiola G, Galati MC,
Bedair EM, Fiscina B, El Kholy M. Growth and endocrine disorders in
thalassemia: The international network on endocrine complications in
thalassemia (I-CET) position statement and guidelines. Indian J
Endocrinol Metab. 2013 ;17:8-18 http://dx.doi.org/10.4103/2230-8210.107808 PMid:23776848 PMCid:PMC3659911

- De
Sanctis V, Soliman AT, Angastiniotis M, Eleftheriou A, Kattamis Ch,
Karimi M, El Kholy M, Elsedfy H, Yassin MA, El Awwa A, Stoeva I,
Skordis N, Raiola G, Fiscina B. International network on endocrine
complications in thalassaemia (I-CET): an opportunity to grow.Georgian
Med News. 2012;205:52-57 PMid:22665732

[TOP]






















































