Received: November 26, 2015
Accepted: February 17, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016018, DOI 10.4084/MJHID.2016.018
This article is available on PDF format at:


| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background and Objectives:
Candida-associated bloodstream infections are frequent and potentially
life-threatening conditions in hematology patients. The aim of this
study is to evaluate the characteristics, risk factors, and outcome of
Candida-associated bloodstream infections in children with
hematological diseases. Methods: The medical records of the patients with hematological diseases and hematopoietic stem cell transplantation (HSCT) recipients who were diagnosed as Candida-associated bloodstream infection between February 2010 and February 2014 were reviewed retrospectively. Results: Thirty episodes of candidemia involving 26 patients (38% female, and 62% male) with a median age of 7-year (range; 1 to 17) were noted. The incidence of candidemia in our study was 5.2 per 1000 hospital admissions. Infections with non-albicans Candida spp. occurred more frequently (63%) and C. krusei was the predominant microorganism among non-albicans Candida spp. (37%). Candida albicans was isolated from 11 of the 30 episodes (37%). Twenty-six of the episodes (88%) patients had a central venous catheter (CVC) prior to candidemia, and they were removed in 16 (62%). Thirty-day mortality rate was 20%. Isolated Candida spp, underlying disease and its status, presence of mucositis, neutropenia, using of broad spectrum antibiotics, corticosteroids or total parenteral nutrition were not identified as predictors of outcome. Multivariate analysis revealed that CVCs kept in place was the only significant factor associated with mortality (OR, 0.07; 95% CI, 0.006-0.716). Conclusions: Candida-associated bloodstream infections were common in children with hematological diseases and HSCT recipients, particularly in patients with CVCs. In addition to appropriate antifungal therapy, CVC removal improves the outcome of candidemia in children with hematological disease. |
Introduction
Candida-associated bloodstream infections have high morbidity and
mortality in immune-compromised children like leukemia, aplastic anemia
or undergoing hematopoietic stem cell transplantation (HSCT).[1-3] Most
of these infections are related to central venous catheter (CVC), and
candidemia represents 10% of hospital-acquired CVC-related bloodstream
infections.[4,5] Furthermore, treatment with wide spectrum antibiotics,
corticosteroids or chemotherapeutic drugs, invasive procedures, and
prolonged neutropenia often predispose children to the fungal
infections.[3] In Turkey, nosocomial infection rate due to Candida spp.
was reported as 3.22 per 1000 patient-days, and 42.9% of them were
bloodstream infections.[6] Celebi et al. also reported that 4.5% of the
catheter related bloodstream infections were due to C. albicans in children with malignant disease.[7]
Candida species (spp.)
commonly present in the gastrointestinal tract, may produce invasive
infections in the immune compromised host when mucosal barrier is
disrupted, or normal gastrointestinal flora is abrogated by
antimicrobial treatment.[5] Candida spp.
may also colonize to form biofilm formation at CVCs that results in
compromised antifungal treatment and cause recurrence of fungal
infections after cessation of antifungal therapy [3]. The Infectious
Diseases Society of America (IDSA), Fourth European Conference on
Infections on Leukemia (ECIL-4), and European Society of Clinical
Microbiology and Infectious Diseases guidelines (ESCMID) recommend
systemic antifungal therapy and CVC removal for bloodstream infections
associated with Candida.[8,9,10] Even though many new antifungal drugs
have been discovered, the candidiasis-related mortality rate remains
high, ranging from 7.7% to 26%.[11]
The aim of our study is to
evaluate the characteristics, risk factors, and outcome of
Candida-associated bloodstream infections in children with
hematological diseases.
Materials and Methods
This retrospective study included children with a hematological
disease such as leukemia, severe aplastic anemia and HSCT recipients
who had a persistent fever and were diagnosed as “Candida-associated
bloodstream infection-candidemia” at the Hematology Department of
Ankara Children’s Hematology and Oncology Hospital between February
2010 and February 2014. Ankara Children’s Hematology and Oncology
Hospital is a large tertiary pediatric hospital with
hematology-oncology research and treatment center, and has 35
hematology and 10 HSCT beds, and it has around 1450 hematology
inpatient admissions per-year. Eligible children were reviewed from
their electronic medical records and microbiology laboratory reports
for blood and CVC cultures, which yielded Candida spp.
Demographic and clinical data of the patients including age, gender,
type and stage of primary disease, presence and type of CVC, time from
the end of last chemotherapy to the positive culture, presence and
duration of neutropenia, history of HSCT and graft-versus-host disease
(GvHD), presence of mucositis, administration of total parenteral
nutrition (TPN), use of broad spectrum antibiotics, administration of
corticosteroids or cyclosporine within 14 days of positive blood
culture were recorded.
As a policy, in our department patients
with relapsed acute myeloblastic leukemia (AML) and acquired aplastic
anemia receive fluconazole prophylaxis during relapse protocol and
immunosuppressive therapy. Hematopoietic SCT recipients receive
antifungal prophylaxis with fluconazole for 30 days after
transplantation. Patients with febrile neutropenia first receive
empiric intravenous extended spectrum penicillin with an
aminoglycoside, and if fever persists for 3-5 days glycopeptides and
empiric/preemptive antifungal therapy is added. Liposomal amphotericin
B is the most common antifungal drug that is used empirically;
caspofungin or voriconazole are the other antifungal drugs which are
used for either suspected or proven fungal infections.
Blood
specimens were cultured in BACTEC Blood Culture System (Becton
Dickinson Diagnostic Instrument Systems, Towson, MD, USA). Candidemia
was defined as positivity of blood culture obtained from either
peripheral vein and/or CVC, associated with clinical symptoms of
bloodstream infection such as fever or hypotension. All positive CVC
cultures were also assessed for the interval between the isolation of Candida spp. and CVC removal.
Mortality
attributable to candidemia was defined as death within 30 days after
the first positive blood or catheter culture in the absence of any
apparent cause for death.
Statistics:
Analysis of data was primarily descriptive, using standard deviations,
ranges, and mean and median values. Categorical variables were analyzed
using the chi-square test. After doing univariate analysis, all
variables reaching a 95% level of significance were included in a
binary logistic regression analysis. Survival rates were calculated by
Kaplan-Meier analysis and the log rank test. All analyses were
performed using SPSS 18 for Windows (SPSS Inc., Chicago, IL, USA). p≤
0.05 was considered statistically significant.
Results
Thirty episodes of candidemia involving 26 patients (38% female, and 62% male) were detected with a median age of 7-year (range; 1 to 17 years). Recurrent episodes of candidemia occurred in two patients with leukemia, one patient with severe acquired aplastic anemia, and one with severe combined immunodeficiency disease (SCID) undergoing HSCT. The incidence of candidemia was 5.2 per 1000 hospital admissions. The demographic characteristics of the patients with candidemia were shown in Table 1. Acute leukemia was the most common underlying disease (17 patients; 65%), and 6 (35%) of these leukemic patients were at induction phase of chemotherapy, and another 6 (35%) patients were at relapse treatment. Nine patients developed candidemia after allogeneic HSCT, and four patients had severe acquired aplastic anemia. During the study period, the overall incidence of candidemia episodes in patients with ALL, AML, AA, and HSCT were 5.4%, 3%, 30%, and 6.9%, respectively.
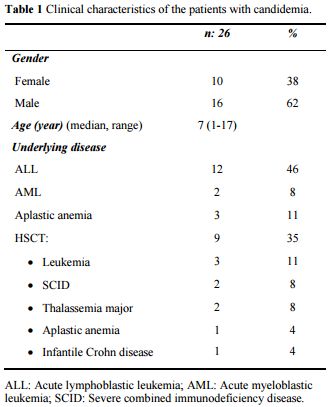 |
Table 1. Clinical characteristics of the patients with candidemia. |
Risk factors associated with outcome were presented in Table 2. Eighty three percent of the patients had received broad-spectrum antibiotics with a median of 14 days (range, 1-180 days), and 83% of the patients had received chemotherapy one month prior to candidemia. Candidemia occurred during severe neutropenia in 73% of patients, lasting a median of 21 days (range, 7-110 days). Furthermore, 47%, 30% and 36% of the patients developed candidemia during the corticosteroid, total parenteral nutrition, and cyclosporine treatment, respectively. In 12 of the episodes (40%), patients had a history of fluconazole prophylaxis.
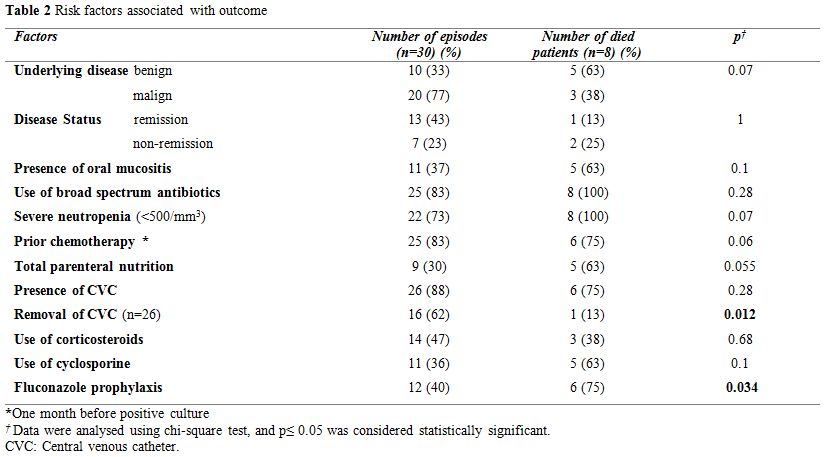 |
Table 2. Risk factors associated with outcome. |
Isolated Candida species were as follows: C. albicans (n=11), C. krusei (n=7), C. parapsilosis (n=2), C. tropicalis (n=2), C. cifferii (n=1), C. glabrata (n=1), C. dubliensis (n=1), C. lusitanae (n=1), C. kefyr (n=1), and non-albicans Candida that could not be classified (n=3). Distribution of isolated Candida spp. and the isolation sites were shown in Table 3.
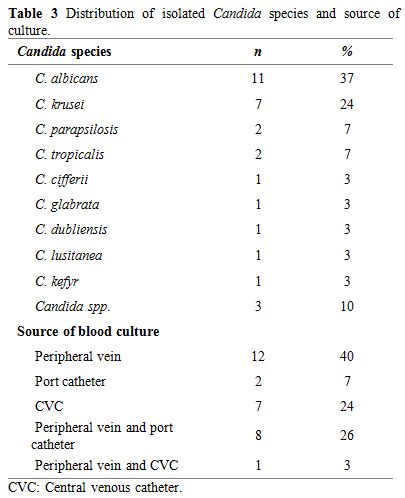 |
Table 3. Distribution of isolated Candida species and source of culture. |
Twenty-three of the 26 patients (88%) had CVC prior to candidemia. Hickman double lumen catheters had been inserted in six out of nine patients before HSCT, and nontunnelled jugular catheters had been inserted in another three patients. Patients with leukemia had port catheters. Central VCs were removed in 16 of the 26 episodes (62%) and were remained in place in 11, because of patient’s poor clinical condition or coagulopathy. The median duration between the isolation of Candida spp. to CVC removal was 7 days (range, 2-22 days). Only one of the CVC tip cultures was positive, and C. krusei was isolated. Twelve patients (40%) were on fluconazole prophylaxis, and nine of them (75%) had non-albicans Candida infection (Table 4). We found no association between fluconazole prophylaxis and isolated Candida spp. We also found no difference between isolated Candida spp. (albicans or non-albicans) in children who underwent HSCT.
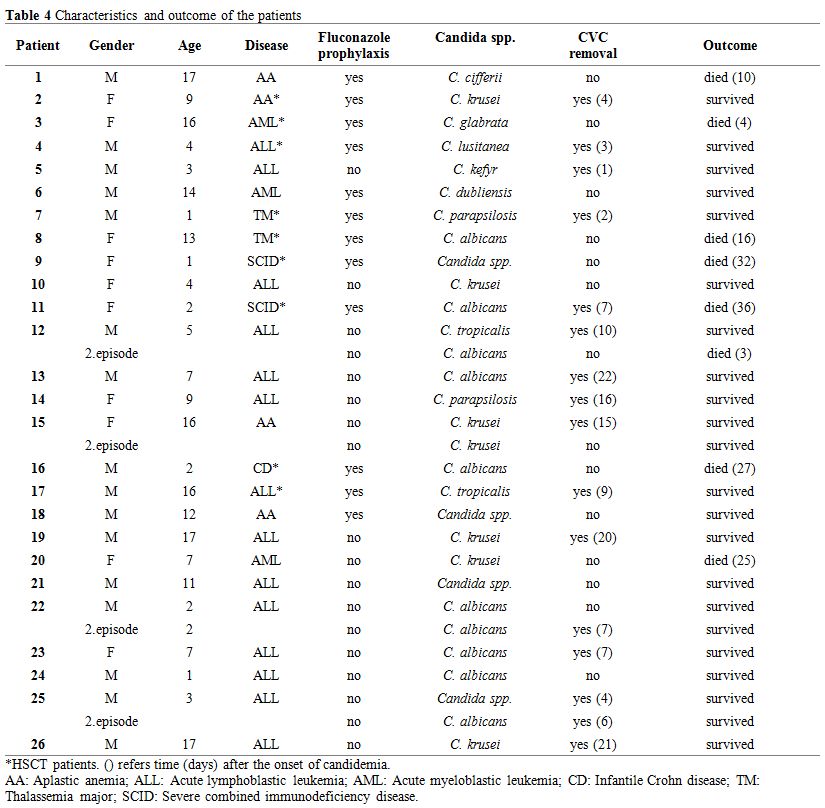 |
Table 4. Characteristics and outcome of the patients |
Overall 8 of the 26 patients (27%) died with Candida-related
septicemia in 20 days (range, 1-34 days) from the positive culture. All
patients who died from candidemia had CVC except one. In univariate
analysis, underlying disease and is status (remission or
non-remission), the presence of mucositis, presence of neutropenia,
using of wide spectrum antibiotics, corticosteroids, cyclosporine, TPN
or isolated Candida spp. were
not identified as significant predictors of outcome. However,
fluconazole prophylaxis before candidemia and removal of CVC were
important factors for a better outcome (p=0.034, and p=0.012,
respectively, Table 2). Multivariate analysis showed only CVCs kept in
place was associated with mortality (p=0.03; OR, 0.07; 95% CI,
0.006-0.716). Kaplan Meier analysis revealed that the three months
estimated survival of patients with CVCs kept in place was 80% [SE
0.179], whereas this figure was 86.6% [SE 0.088] with CVC removal
(p=0.69) (Figure 1). All surviving patients had a last negative culture following treatment.
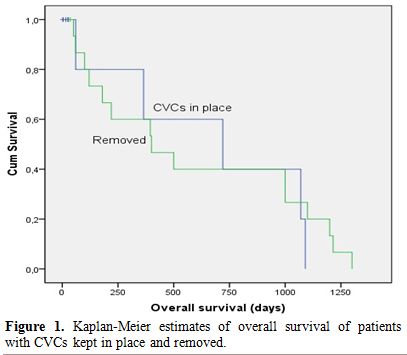 |
Figure 1. Kaplan-Meier estimates of overall survival of patients with CVCs kept in place and removed. |
Discussion
Candida infections are an important cause of morbidity in critically
ill children. However, studies on clinical and epidemiological features
of Candida-associated bloodstream infections in immune compromised
children with hematological diseases are scarce. In this study, the
incidence of candidemia was 5.2 per 1000 hospital admissions in
immune-compromised children with hematologic diseases, which was lower
than the Brasilian study[12] which reported 19.14 episodes per 1000
admission. Our study also revealed that non-albicans Candida spp. occurred more frequently (63%) and C. krusei was the predominant microorganism among non-albicans Candida
spp. (37%). It confirms the previous reports, which show the increasing
incidence of non-albicans Candida infections in patients with cancer
and hematological malignancies.[12-17] The most prominent species that
have been reported were C. parapsilosis in patients with CVC-related infections, but non-albicans Candida isolates, C. glabrata, tropicalis and krusei in patients without CVC.[14] Several risk factors have been reported to be associated with non-albicans Candida
infections including azole prophylaxis, neutropenia, type of the
disease (leukemia) and HSCT.[13,18] In the current study, we could not
identify any specific risk factor for non-albicans Candida infection. Although administration of fluconazole prophylaxis might have resulted in the occurrence of more prevalent C. krusei
infection which is inherently resistant to fluconazole therapy; but we
found no relation between fluconazole prophylaxis and isolated Candida spp. in this study.
Zaoutis
et al. reported that the presence of a CVC, a diagnosis of malignancy
and receipt of antimicrobials with activity against anaerobic bacteria
for >3 days were independently associated with the development of
candidemia.[3] History of using broad-spectrum antibiotics, prior
intensive chemotherapy and neutropenia were also observed frequently in
our patients with candidemia.
Indwelling CVC has been previously
identified to be a risk factor for candidemia in patients with
malignancy.[4,12,19] The guidelines for the treatment of candidemia
recommend the removal of CVC in the presence of bloodstream
infections.[8,9,10] However, management of infected CVC in neutropenic
patients is still controversial, because of patient’s poor clinical
condition and potential surgical complications associated with CVC
replacement. Most of our patients (88%) had CVC prior to candidemia,
and CVC could not have been removed in 38% of the episodes due to
patient’s condition. Candidemia is an independent risk factor for
predicting death in patients with nosocomial bloodstream
infections.[20] The candidemia-associated mortality rate has been
reported as 20-24%.[13,16] In the current study, the overall mortality
rate of candidemia was 27%. The mortality rate was 54% in patients with
CVCs kept in place, and it was 6% in children whom CVC had been
removed. The only factor that was significantly associated with
mortality was CVC removal. However, removal of CVC did not influence
patient’s overall survival. Removing the CVC in a patient with a poor
condition or with severe coagulopathy such as disseminated
intravascular coagulation or platelet refractoriness, may prove to be
difficult. Nevertheless, we recommend CVC removal as soon as the
patient’s condition permits.
Conclusion
The current study was limited by its retrospective design and low sample size in a single center experience. We conclude that as long as the medical condition permits, CVC removal should be considered in patients with hematological diseases and Candida-associated bloodstream infections. Further large-scale studies are warranted to evaluate the contribution of the other risk factors, such as pre-existent medical conditions, to outcome in children with candidemia.
References




















[TOP]