Received: January 31, 2016
Accepted: March 21, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016021, DOI 10.4084/MJHID.2016.021
This article is available on PDF format at:


| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background:
Cutaneous hyperpigmentation is an often overlooked clinical sign in
megaloblastic anemia (MA) which has been sporadically reported in the
literature. Methods: We describe the bone marrow (BM) changes and clinicolaboratory characteristics of 25 of 198 adult cases (>16 years) with cutaneous hyperpigmentation who underwent BM evaluation for cytopenia (s). Results: Twenty-one of 25 cases (84%) had MA, while MA without hyperpigmentation occurred only in 12 of remainder 173 cases (P<0.001). Knuckle pad hyperpigmentation (KP) was noted in 16 (64%) cases; whereas 9 (36%) had diffuse brownish black discoloration (DP) of the palms and /or soles. Eighteen of 25 (72%) cases had pancytopenia (13 with KP) and 7 of 25 (28%) had bicytopenia (3 with KP). In addition, five cases (20%) presented with pyrexia. Of the 17 cases where data available, eleven were B12 deficient [<190 pg/ml; eight had severe deficiency (<100 pg/ml); ref.; 190-800pg/ml], while 4 had pure folate deficiency (< 4.0 ng/ml; ref.; 4-20 ng/ml); and remainder 2 had combined B12 and folate deficiency. Compared to those with diffuse pigmentation; KP group had lower Hb (69.6±24.2 vs. 86.3±33.9 g/L), higher MCV (106.1±2.6 vs. 99.2±7.6 fL), lower platelet count (50.9±29.3 vs. 69.6±36.5 x 109/L), and lower median B12 [100.0 (30.0 – 822.0) vs. 316.0 (142.0 – 1617.3) pg/ml] (P>0.05). In six cases where follow-up data were available, there was a significant reversal of hyperpigmentation at 12 weeks following parenteral cobalamin therapy. In all five cases with pyrexia, fever subsided after 24 to 72 hours following administration of parenteral cobalamin therapy. Conclusion: Cutaneous hyperpigmentation and cytopenia (s) are strongly associated with megaloblastic anemia. Knuckle pad hyperpigmentation is much more frequent than diffuse pigmentation of the palms and/or soles in such patents. A nonsignificant trend towards a greater degree of MA was found in cases with pigmentation of the knuckles. |
Introduction
Megaloblastic anemia (MA) is a heterogeneous group of reversible
bone marrow failure syndromes characterized by a variable degree of
peripheral blood cytopenia (s) in the presence of a normo or
hypercellular bone marrow. The hallmark pathophysiologic mechanism of
MA is an impairment of DNA synthesis in all nucleated cells secondary
to vitamin B12 (B12)
and/or folate deficiency, resulting in nuclear-cytoplasmic asynchrony;
distinctive megaloblastic changes, increased apoptosis, and ineffective
hematopoiesis in the bone marrow.[1] The
manifestations of MA are diverse and may range from nonspecific signs
and symptoms of anemia to gastrointestinal disturbances and potentially
fatal neuropsychiatric and cardiovascular disorders.[2]
Megaloblastic
anemia is not uncommon in the Indian subcontinent as well as other
parts of Asia with females and vegetarians being more susceptible to B12 deficiency. Various studies in the past have shown that occult B12 deficiency may be rather prevalent among Indian urban and rural population.[3-5]
Cutaneous manifestations associated with B12 deficiency
include characteristic mucocutaneous hyperpigmentation (most common),
vitiligo, angular cheilitis, and hair-nail changes, which are often
missed or overlooked in early, asymptomatic phases of the disease.[6]
In this manuscript, we describe the association of cutaneous
hyperpigmentation (CP) with bone marrow changes in a series of 25 cases
from a tertiary center in South India with a correlation of
hematological and biochemical parameters, and also present a concise
review of relevant literature. Furthermore, the association of pyrexia
in MA is also briefly highlighted.
Materials and methods
In this retrospective study the bone marrow records of all adult
cases (> 16 years), who underwent bone marrow aspiration (BMA) and
trephine biopsy (Bx) over the last five years (October 2010 to December
2015) in the Department of Pathology of our Institute, were reviewed
for the presence of CP. Informed written consent had been obtained from
each case prior to the BM procedure; and the study was approved by the
Institutional Ethics Committee.
As a part of the protocol for BM
procedure, CP was prospectively documented in the bone marrow record by
one of the authors (SP), prior to performing the procedure. Two types
of pigmentation were documented: 1) dominant brownish-black
pigmentation over dorsal aspect of hands and/or feet with accentuation
over interphalangeal joints and periungual areas [knuckle pad (KP)
group], and 2) diffuse and/or patchy, macular, dusky brownish-black
discoloration/pigmentation over palms and/or soles (diffuse
pigmentation (DP) group). Nature of BMA and gross appearance of Bx core
were also recorded in each case. Data pertaining to routine
hematological parameters such as hemoglobin (Hb), mean corpuscular
volume (MCV), total leukocyte count (TLC), total platelet count (Plt),
mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin
concentration (MCHC), and peripheral blood smear (PBS) findings were
collected from laboratory records in each case at the time of BM
procedure.
BMA smears and Bx sections of all the above cases
were retrieved from the departmental archives. Two pathologists (AR,
RGV) who were blinded to the presence and nature of the pigmentation,
reviewed the slides for the presence of definite megaloblasts. In each
case, two MGG (May Grunewald Giemsa) and one Prussian blue (Perl stain)
stained BMA smears were available for morphological study and
assessment of the iron stores, respectively. A definitive diagnosis of
MA was rendered with a constellation of PBS and BM findings such as
erythroid hyperplasia, altered myeloid to erythroid ratio (M:E),
presence of definite megaloblasts with sieve-like chromatin,
nuclear-cytoplasmic asynchrony, giant abnormal shaped metamyelocytes
and/or band forms, and/or abnormal (multinucleated) megakaryocytes with
adequate (1-3+) or increased (≥ 4+) iron stores. A diagnosis of
dimorphic marrow picture was rendered when there was admixture of both
megaloblast and micronormoblast (with reduced hemoglobinization) and
reduced (1+) or absent (0) iron store on Perl stain.[7]
When definite megaloblasts were not seen or very sparse, but dyspoietic
changes were present, a possibility of macronormoblastic erythroid
maturation with dyspoiesis was reported. Furthermore, those with
dyspoietic changes and increased iron stores (≥ 4+) were also screened
for the presence of ringed sideroblasts for a possible diagnosis of
myelodysplastic syndrome (MDS) in the appropriate clinical setting.
Bone
marrow changes were then correlated with the presence or absence and
nature of hyperpigmentation, and serum biochemical parameters such as
serum B12, folate, ferritin, and iron levels (wherever available). Serum B12
(ref.: 190 to 800 pg/ml), folate (ref.: 4 to 20 ng/ml), and ferritin
levels [ref.: 30 to 400 ng/ml (males), 15 to 150 ng/ml (females)] were
measured by electrochemiluminescence immunoassay (ECLIA) technique in
Cobas e411 automated analyzer (Roche, Germany). Serum iron [ref.: 50 to
168 μg/dl (males), 35 to 145 μg/dl (females)] was measured in a
semi-auto analyzer (Microlab 300, Merck) by using commercially
available kits. Cases with B12 level <190 pg/ml were considered as B12deficient and those with levels < 100 pg/ml were considered as severe B12 deficiency.[2]
Similarly, cases with folate levels < 4ng/ml were considered as
folate deficient. Cases with serum ferritin < 30 ng/ml were
considered as depleted iron stores; and those with < 15 ng/ml were
considered as a severe iron deficiency.
Demographic profile, place
of origin, dietary habits, history of prior illness and medications (if
any), provisional clinical diagnoses, relevant clinical,
microbiological and/or serological data were collected from the case
records. All cases where a diagnosis of MA was made received parenteral
(intramuscular) cyanocobalamin (1000 μg/day) for 7 days, followed by a
weekly dose of the same for a minimum of 10 to 12 weeks. Those with
dimorphic anemia were prescribed both parenteral and/or oral cobalamin
in addition to hematinics and multivitamins.
Statistical analysis:
Continuous variables were described by mean (± SD) and Box plot were
used to highlight the distribution of variables between two groups.
Comparison between two groups was made using Fisher’s exact method for
categorical variables, student t-test for normally distributed
continuous variables, and Mann-Whitney test for not normally
distributed continuous variables. All the tests were 2 sided; and a P
value less than 0.05 was considered as statistically significant. SPSS
software (version 20.0) was used for analyzing the data.
Results
During the study period, a total of 304 patients underwent a BM
examination, out of which 198 were personally performed by one of the
authors (SP). All of these cases had been assessed for the presence or
absence of CP at the time of the BM examination and hence were included
in the analysis. CP was documented in 25 of these 198 patients (12.6%).
The demographic and clinico-laboratory characteristics of all 25 cases are presented in Tables 1 and 2.
There were 16 males (64%) and 9 females (36%) with a mean age of 41.2 ±
16.7 years. Among the cases, 21 (84%) were dark skinned South Indians
(in and around Puducherry). Seventeen of 25 (68%) were taking a mixed
diet while only 8 (32%) were vegetarians. Seven of 16 (43.7%) males and
none of the females had a history of alcohol abuse. Fatigue was the
most common clinical presentation noted in 15/25 (60%) cases; 3/25
(12.0%) were diagnosed cases of autoimmune hepatitis on periodic
follow-up with maintenance drugs such as azathioprine and prednisolone,
3/25 (12%) had evidence of gastric atrophy (anti-intrinsic factor
antibody positive in one), 5 (20%) presented with fever, 2 (8%) had
history of diarrhoea, 1 (4%) was a case of non-alcoholic
steatohepatitis (NASH) secondary to uncontrolled type 2 diabetes
mellitus, and 1 (4%) was a case of paranoid schizophrenia on olanzapine.
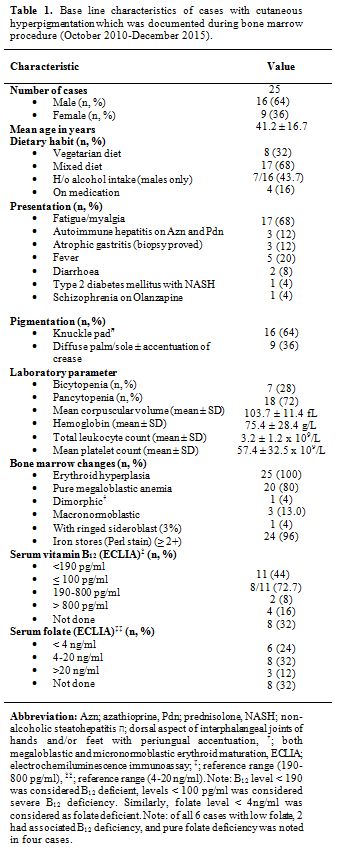 |
Table 1. Base line characteristics of cases with cutaneous hyperpigmentation which was documented during bone marrow procedure (October 2010-December 2015). |
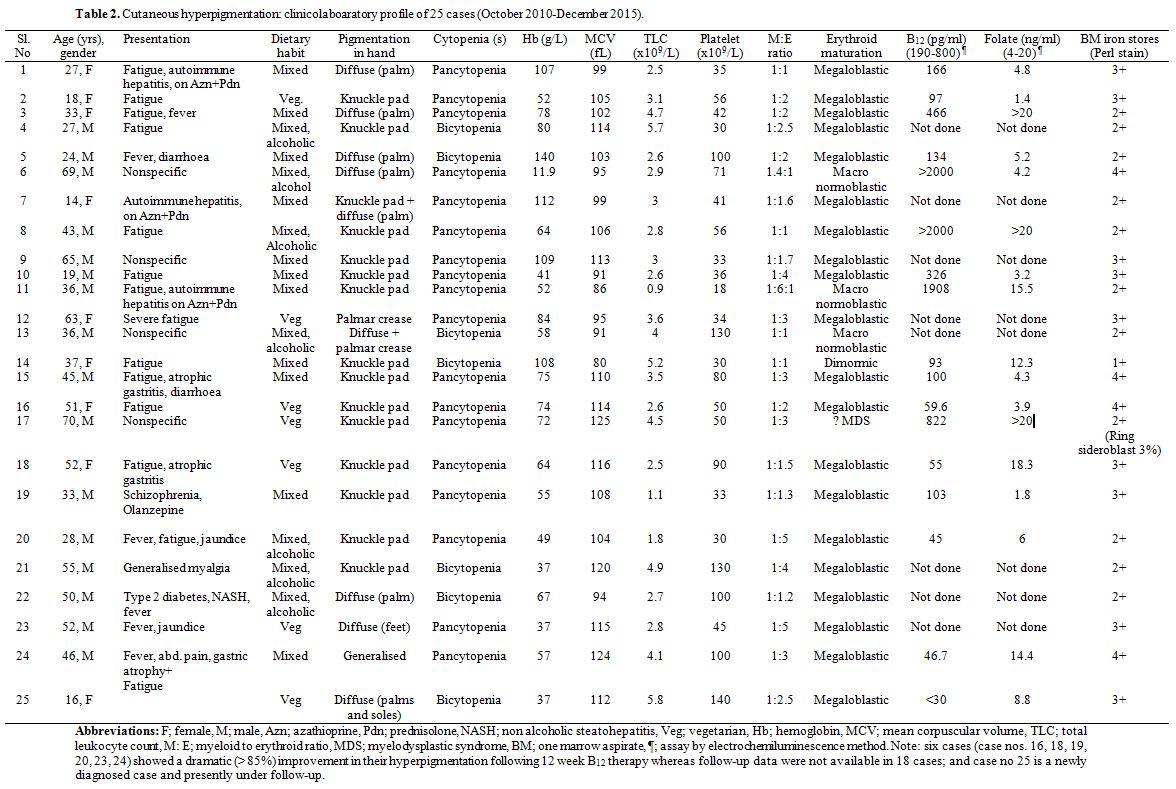 |
Table 2. Cutaneous hyperpigmentation: clinicolaboratory profile of 25 cases (October 2010-December 2015). |
The association of MA with CP was highly significant
(P<0.001) with 21 (out of 25) patients having MA [20 had pure MA and
1 had mixed MA and iron deficiency anemia (IDA)] as compared to only 12
patients with MA (5 had pure MA and 7 had mixed MA and IDA) among 173
patients without pigmentation (Table 3).
Prominent knuckle pad hyperpigmentation (KP) was documented in 16 of 25
(64%) cases whereas 9/25 (36%) cases had patchy or diffuse, dusky,
brownish-black pigmentation over palms and/or soles with accentuation
over palmar creases (DP) (Figures: 1A, 1B, 1C, 1D).
Eighteen of 25 (72%) had pancytopenia (13 with KP, 5 with DP) and 7
(28%) had bicytopenia (3 with KP, 4 with DP). The mean Hb, MCV, TLC,
and Plt were 75.4 ± 28.4 g/L, 103.7 ± 11.4 fL, 3.2 ± 1.2 x 109/L, and 57.4 ± 32.5x109/L, respectively.
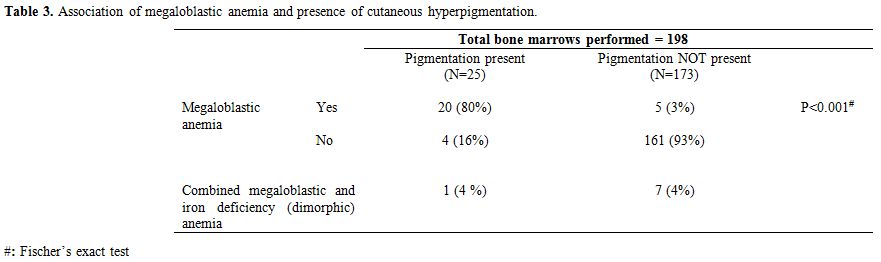 |
Table 3. Association of megaloblastic anemia and presence of cutaneous hyperpigmentation. |
The marrow findings were consistent with MA in 20/25 (80%) cases [severe in 12, moderate in 5, and mild/focal in 3); dimorphic anemia with both megaloblasts and micronormoblasts in 1 case (4%); and dyspoietic changes with ringed sideroblast (3% of erythroid nuclei) suggestive of MDS was noted in one case (4%) (Figures: 2A, 2B, 2C). Nonspecific marrow findings and dyspoietic erythroid changes in the form of either macronormoblastic erythroid maturation, binucleation, nuclear budding, internuclear bridging were described in remainder 3 (12%) cases. There was no evidence of any granulomas, necrosis, organisms, hemoparasites, or any malignancy noted in any of the cases on the bone marrow. Detailed systemic, radiological, and endocrinological examinations were unremarkable in all cases and blood and BMA culture did not reveal any growth in cases with pyrexia.
| Figure 2. Bone marrow aspirate in megaloblastic anemia with cutaneous hyperpigmentation. Note the richly particulate bone marrow aspirate (2A) obtained during bone marrow procedure in cases with cutaneous hyperpigmentation and cytopenia (s). Bone marrow aspirate smears demonstrating erythroid hyperplasia and megaloblasts with sieve-like nuclear chromatin (2B, thick arrow) and giant, abnormal shaped stab forms (2C, thin arrow). These findings were consistent with a diagnosis of megaloblastic anemia (May Grunewald Giemsa, X400). |
Serum B12 and folate levels were available in 17/25 cases. It was found that 11/17 (64.7%) were B12 deficient (<190 pg/ml) among which 8 had severe B12 deficiency (<100 pg/ml); 4/17 (23.5%) were folate deficient (< 4 ng/ml); and 2/17 (11.8%) had combined B12 and folate deficiency.
KP
group had lower Hb (69.6 ± 24.2 vs. 86.3 ± 33.9 g/L, respectively,
P=0.19), higher MCV (106.1 ± 12.6 vs. 99.2 ± 7.6 fL, respectively,
P=0.18), lower Plt (50.9 ± 29.3 vs. 69.6 ± 36.5x109/L, respectively, P=0.15) than the DP group (Table 4). Similarly, the median (50th quartile) and interquartile (25th to 75th
quartile) range for Hb and Plt were lower in the former group than the
latter [64.0 (52.0 - 80.0) vs 81.0 (60.3 - 116.0) g/L; 41.0 (30.0 -
56.0) vs. 58.0 (36.8 - 100.0) x 109/L,
respectively]. However, median and interquartile range for MCV were
higher between two groups [108.0 (99.0 - 114.0) vs. 97.0 (94.3-102.8)
fL, respectively, P>0.05]. Median and interquartile range of serum B12
values in the KP group was lesser [100.0 (30 – 822.0) pg/ml] compared
to the later group [316.0 (142.0 –1617.3) pg/ml] (P = 0.17); whereas
median serum folate levels were similar between two groups [6.0 (3.0 –
18.0) vs. 5.0 (4.3 – 17.0) ng/ml, respectively, P=0.79] (Table 4, Figures: 3A-3E).
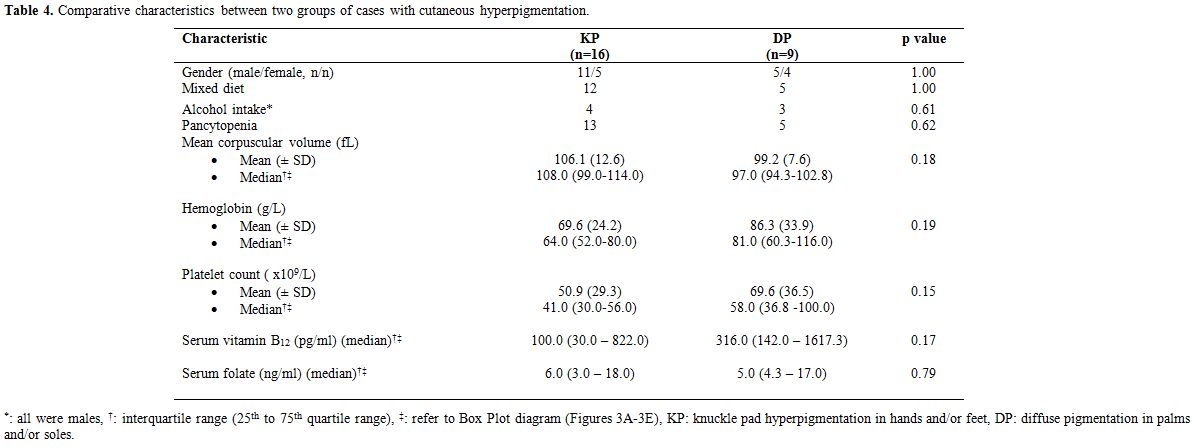 |
Table 4. Comparative characteristics between two groups of cases with cutaneous hyperpigmentation. |
| Figure 3. Box plot diagram depicting the comparison of median (50th quartile, black horizontal line) and interquartile (25th to 75th) range of mean corpuscular volume (MCV) (A), Hb (B), Platelets (C), B12 (D), and folate (E) levels among two groups of pigmentation [knuckle pad (KP) vs diffuse type (DP)]. Note that the median and interquartile range of Hb, Platelets, and serum B12 were lower in the KP group than in the DP group; whereas the median and interquartile range of MCV were higher in KP group than the DP group. Also note that the group with DP has a wider B12 value compared to the KP group (D). The median value of serum folate was similar among two groups. |
Six of 25 cases where follow-up data were available, showed
dramatic improvement (> 85%) in their hyperpigmentation following 12
weeks B12 therapy (Figures: 4A and 4B),
whereas the rest were lost to follow-up. In all the five cases who
presented with pyrexia, their fever subsided following day 3 to 4 of
starting parenteral cyanocobalamin therapy.
| Figure 4. Reversal of hyperpigmentation in the patient of figure 1A and 1B, 12 weeks after initiation of parenteral cyanocobalamin therapy. |
Discussion
Megaloblastic anemia is a multisystemic disorder where hematological
and neuropsychiatric manifestations usually dominate the clinical
picture. In 1944, Dr Bramwell Cook first observed that
hyperpigmentation of the skin was associated with a macrocytic anemia
and that both it and the anemia responded to crude liver extract.[8]
Since then, there have been sporadic case reports and small case series
with descriptions of the peculiar skin-hair-nail changes in patients
with megaloblastic anemia.[9-13] In our series, we
observed a strong association of cutaneous hyperpigmentation with
megaloblastic anemia, and knuckle pad pigmentation was much more
frequent than the diffuse type.
We also observed a good
correlation between the presence of CP and BM changes with serum
biochemical parameters. Barring one case of dimorphic anemia, none of
the cases with hyperpigmentation had depleted marrow iron stores which
in turn correlated well with the normal or increased serum ferritin
levels in 24/25 cases. A greater proportion of our cases were B12 deficient (<190 pg/ml); and eight had significant B12 deficiency (<100 pg/ml). Cases with KP were associated with a greater degree of B12
deficiency, macrocytosis, and pancytopenia; though this lacked
statistical significance. Our observation was in accordance with that
published in the literature.[8,9,11]
Though majority (68%) of our subjects were on a mixed diet, a higher
proportion of MA diagnosis suggests that factors other than poor intake
like impaired absorption might be responsible for B12
and/or folate deficiency; and alcohol abuse was a contributing factor
among our male subjects. However, the presence of hyperpigmentation in
a case of suspected MDS as well as in other 3 cases with reactive
marrow changes contradicts the above hypothesis. Furthermore, an
interesting aspect of our cohort was the fact that 5 of 21 cases
(23.8%) with MA presented with pyrexia requiring extensive work up.
However, in all cases, fever subsided within 24 to 72 hours of
initiation of parenteral cyanocobalamin therapy. This reinforces our
previous observation that florid ineffective hematopoiesis in MA, in
conjunction with other but yet unidentified mechanisms, might be the
underlying cause for such phenomenon.[14,15]
A brief comparative review of literature regarding mucocutaneous hyperpigmentation in MA and/or B12 deficiency is presented in Table 5.[8-13,15]
In 1963, Baker and colleagues described characteristic reversible
brownish-black pigmentation over dorsal aspect of interphalangeal
joints of hands and feet (KP) in a large series of 21 South Indian
subjects with MA (15 adults, 6 infants/children). Malabsorption was the
commonest cause of B12 deficiency in that series; and the mean serum B12 level among cases was very low (49 pg/ml) by using microbiological assay method.[8]
Similarly, reversible Addisonian type of mucocutaneous
hyperpigmentation and nail changes were also reported in a dermatologic
setting among nine South Indian patients with biochemical evidence of B12 deficiency.[9] Aaron et al[10]
described mucocutaneous changes as a significant physical finding in 26
of 63 patients (41%) with neurological manifestations secondary to B12
deficiency. Cutaneous hyperpigmentation was found to be the most common
(19% of cases) whereas hair changes and vitiligo were described in 9%
and 3% of cases, respectively. Hyperpigmentation did not show any
correlation with duration of symptoms, severity of megaloblastosis, and
MCV; and follow-up data of skin changes were not reported in that
series.[10] A recent prospective study from Turkey[11]
recruited 57 pediatric subjects (mean age; 12.75 ± 4.75 months) of
which 49 (86%) were exclusively breastfed. A higher proportion (63%) of
cases had a severe B12 deficiency (<100 pg/ml); and 44 of 57 mothers were also B12
deficient (<200 pg/ml). Forty-nine of 57 (86%) babies had CP and 40
(70%) had atrophic glossitis. On serial follow-up at the end of 1 week,
4 weeks, and 12 weeks, there was a dramatic improvement in
mucocutaneous changes at 12 weeks following parenteral cobalamin
therapy.[11] Similarly most of the other published
reports have also noted a dramatic improvement or near complete
reversal of the pigmentary changes following 8 to 12 weeks of
parenteral cobalamin therapy.[12,13,15]
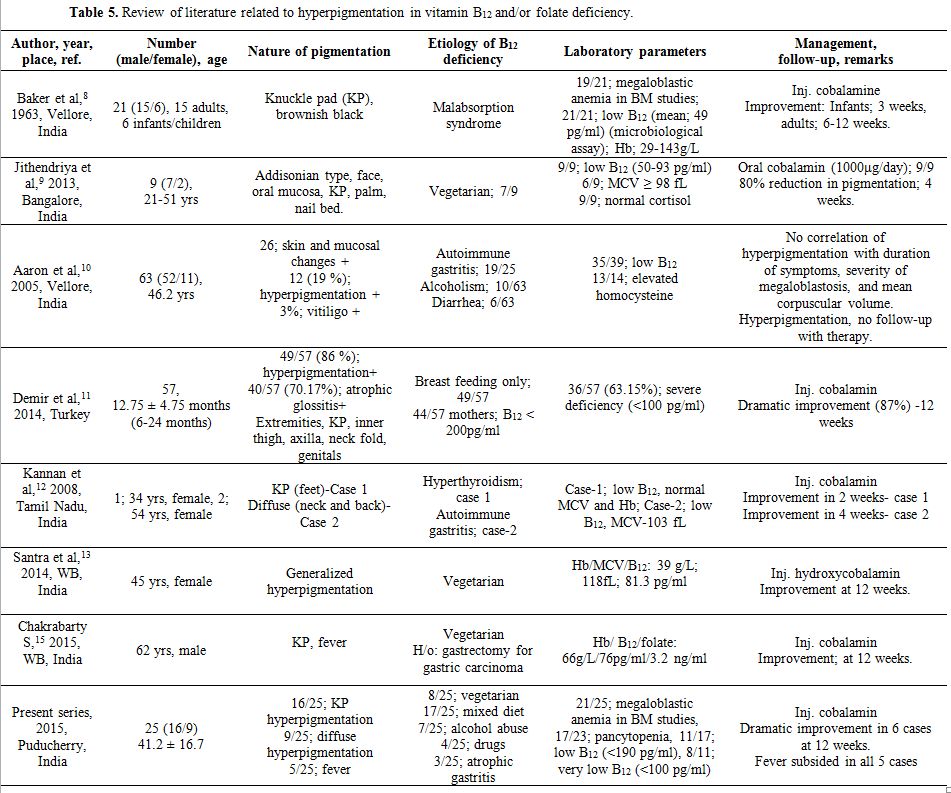 |
Table 5. Review of literature related to hyperpigmentation in vitamin B12 and/or folate deficiency. |
The pathophysiologic mechanism associated with hyperpigmentation in B12 and/or folate deficiency seems to be complex and is poorly understood.[6,16] However, the most accepted hypotheses are i) increased melanin synthesis, and ii) defective melanin transfer from melanocytes to adjacent megaloblastic keratinocytes (Figure 5).
| Figure 5. The postulated biochemical mechanism of hyperpigmentation in megaloblastic anemia.[6,16] The 4 most accepted mechanisms involved are: 1) low methylcobalamin level in melanocytes leads to reduced level of reduced glutathione (GSSH); which in turn activates Tyrosinase enzyme in melanin synthesis pathway, 2) defective DNA synthesis activates Microphthalmia-associated transcription factor (MITF); which causes activation of both Tyrosinase and Tyrosinase related protein 1 and 2 (TRP 1 and 2),[16] 3) hyperhomocysteinemia leads to accumulation of cysteine leading to increased melanin synthesis, 4) defective melanin transfer from the melanocytes to adjacent megaloblastic keratinocytes. Increased angiogenesis secondary to upregulation of dermal vascular endothelial growth factor (VEGF) may also lead to increased pigmentation.[18] Both histopathologic and ultrastructural studies have postulated that hyperpigmentation is due to increased number of basal melanocytes as well as increased melanosomes.[19] |
Reduced methylcobalamin causes a reduction in intracellular
reduced glutathione (GSSH) which in turn, activates Tyrosinase enzyme
in the L-phenylalanine - L-tyrosine - melanin pathway. Also, defective
DNA synthesis leads to activation of micro-ophthalmia associated
transcription factor (MITF), which upregulates both Tyrosinase and
Tyrosinase related proteins (TRP 1 and 2).[16] Furthermore, hyperhomocysteinemia in B12deficiency
leads to increased cysteine level augmenting melanin synthesis. Both
histopathologic and ultrastructural studies in skin biopsies have
suggested that hyperpigmentation is not due to a defect in melanin
transport but is secondary to an increase in melanin synthesis.[17,18]
Moreover, increased angiogenesis secondary to upregulation of vascular
endothelial growth factor (VEGF) has also been postulated to be
responsible for the reddish brown discoloration seen in some cases.[19]
However, the reason for the localized regional hyperpigmentation over
the knuckle regions and greater prevalence among dark skinned
individuals remains an enigma. It is open to speculation whether
genetic and racial differences are responsible for this peculiar
phenomenon.
The present study had certain limitations. 1) The
retrospective nature of the survey led to the fact that CP was not
assessed in all patients undergoing a bone marrow evaluation, resulting
in a limited sample size. Not all patients with MA diagnosed on the
basis of hematological parameters patients would have had a bone marrow
examination performed. We also do not have data as to how many patients
who presented to the hospital during this period had the typical
cutaneous hyperpigmentation. 2) A lack of correlation of MA with
biochemical parameters was found in up to 40% of cases. This can be
explained by the poor sensitivity and wider reference range of the
assay technique (ECLIA) used in our laboratory, which generally gives
higher values as compared to the more accurate microbial assay
technique.[2] 3) Lastly, lack of follow-up in the 15 of 21 cases with MA was a major limitation in our study.
Conclusions
This is the first study that has systematically evaluated cutaneous
hyperpigmentation among patients undergoing a bone marrow examination
in which a definite association with megaloblastic anemia was observed.
The present study reinforces the fact that cutaneous hyperpigmentation
and pyrexia are helpful clinical signs in megaloblastic anemia. These
in the presence of cytopenia (s) are reliable markers in the
megaloblastic anemia and clinicians should be aware of these valuable
clinical signs.
References

















[TOP]