Received: March 3, 2016
Accepted: April 11, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016026, DOI 10.4084/MJHID.2016.026
This article is available on PDF format at:


| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
In thalassemia major (TM) patients, impairment of the
hypothalamic-pituitary-adrenal (HPA) axis secondary to hemosiderosis of
the pituitary gland and/or adrenal glands is well established. In TM,
hypocortisolism is paucisymptomatic or causes nonspecific symptoms.
Although adrenal insufficiency (AI) is rare; an acute crisis may occur
in the event of acute cardiac decompensation, stress, or sepsis.[1-3]
Furthermore, screening for adrenal insufficiency is commonly overlooked
by physicians who manage patients with thalassemia major. The Authors
report their experience in the diagnostic utility of glucagon
stimulation test (GST) for the diagnosis of central adrenal
insufficiency (CAI) and debate the cut-off cortisol level commonly used
for the diagnosis of CAI.
The pathophysiological basis of AI in TM
has not been well-defined, and there are currently no clear guidelines
on how to diagnose AI in these patients.[4]
The
diagnosis of CAI is relatively simple when glucocorticoid secretion is
profoundly depressed. However, basal cortisol level may be normal in
partial CAI and stimulation tests are then necessary to investigate the
integrity of the HPA axis and establish the diagnosis.[5]
The
standard tests for diagnosing CAI are the insulin hypoglycemia test
(ITT) and the metyrapone test (MT). ITT remains the gold standard
procedure for the diagnosis of HPA insufficiency. However, it requires
close surveillance because of inherent risks of severe hypoglycemia. It
also has specific contraindications in patients with epilepsy and heart
disease. Therefore, ITT requires close supervision and appears to be
demanding for some patients and medical staff.[5]
Moreover, for regularly accepted cut-off points, false positive results
are documented, even in normal volunteers, and reproducibility is far
from perfect.[6] A cortisol response < 18 µg/dL (< 500 nmol/l ) has been defined as an evidence of deficiency.[5]
When
ITT is contraindicated and where the compound is available the MT test
may be performed. Metyrapone inhibits 11β-hydroxylase and, hence, the
conversion of 11-DOC into cortisol. Thereby it reduces the negative
feedback and triggers ACTH release which, in turn, increases 11-DOC
production. Although TM is an excellent test, its use is limited by the
difficulty involved in obtaining the medication in many countries and
the risk of precipitating an adrenal crisis.[5] The sum of cortisol and DOC after MT should exceed 16.5 μg/dl (455 nmol/l).[5]
Recent
reports have re-evaluated the diagnostic utility of the glucagon
stimulation test (GST) which elicits an ACTH-dependent cortisol
response. Glucagon works by stimulating the release of GH and ACTH
through a hypothalamic mechanism.[5]
Little
information is available in the literature regarding a prevalence of
CAI in TM. Poomthavorn et al.6 reported a prevalence of 80.7% in TM
patients using ITT. Huang et al.[7] reported a
prevalence of 60% using GST. The prevalence was higher in males vs.
female patients (92% vs. 29%, p=0.049). Ten of 11 subjects who failed
the GST subsequently demonstrated normal ACTH and cortisol responses to
ovine corticotropin-releasing hormone (oCRH) stimulation test,
indicating a possible hypothalamic origin to their AI.[7]
.Deficient patients had lower liver iron content (LIC) and smaller
pituitary volume (p = 0.08 and 0.11, respectively) than those with
normal cortisol response.[7]
In both studies, a
peak total cortisol = or > 20 μg/dl after ITT, and = or > 18
μg/dL after GST, were considered as normal.[6,7]
Nevertheless, the optimal cut-off for the diagnosis of CAI after GST is
still a matter of debate. Recently, the sensitivity and specificity of
GST have been studied in 49 adult patients after trans-sphenoidal
surgery. ROC analysis revealed an upper cut-off of 21.7 µg/dL (599
nmol/l) with 100% sensitivity and 32% specificity for AI while the
lower cut-off (10 µg/dL; 277 nmol/l) had a specificity > 95% and a
sensitivity of 72%.[8] Similar results were also reported by Hamrahian et al.[9]
The Authors, using GST for diagnosing AI in adults, established a lower
cortisol cut-off point (9 µg/dL= 42.7 nmol/L; 92% sensitivity and 100%
specificity) after GST.[9] A cortisol response between 9 and 18 µg/dL (250 nmol/l and 500 nmol/l) to glucagon stimulation was considered a “gray zone).[9]
We
undertook the present study to evaluate the adrenal response to GST
test in 17 adult patients (8 males) with TM. Their ages ranged from 25
to 53 years (mean 35.2±7.4 yr). All patients were on regular blood
transfusions and iron chelation therapy with deferoxamine, deferiprone
or deferasirox. Three males and two females were on hormone replacement
therapy with sex steroids for hypogonadotropic hypogonadism or
secondary amenorrhea (1 patient). Three patients (2 females) received
daily levothyroxine for primary hypothyroidism. Thirteen patients were
carriers of HCV virus, and two were on treatment for cardiac disease.
Serum ferritin was measured by the electrochemiluminescence immunoassay.
Sampling
was conducted during the patients’ routine clinical care for endocrinal
evaluation. The GST was performed by intramuscular injection of 1 mg of
glucagon. Blood samples were drawn every 30 minutes from baseline to
180 minutes for glucose, cortisol and growth hormone (GH)
determinations.
Using a cut-off level of 9 μg/dL (250 nmol/l )
as evidence of CAI one female patient (5.8%), 26 years old with
a serum ferritin of 1288 ng/ml had a poor cortisol response (7.9 µg/dL
= 217.9 nmol/l ). Six patients (35.2%; 33± 4.1 yr; serum ferritin 895 ±
434 ng/ml; range: 360-1594 ng/ml) had a normal cortisol response. The
remaining 10 patients (58.8%; 37.6± 8.3 yr; serum ferritin 1157 ± 1158
ng/ml; range: 217-3064 ng/ml) a cortisol response (14.4 ± 2 µg/dL =
397.2±55.1 nmol/l) in the “gray zone” (between 9 and 18 µg/dL=250
nmol/l and 500 nmol/l) 9 and a cortisol rise of less than 6.1 µg/dL
(170 nmol/L) respect to the basal level. Using a cut-off level of 18
μg/dL (500 nmol/l) 64.7% of our TM patients had an adrenal
insufficiency (5/11- 45.4% were males).
The maximum cortisol
release, during GST, was observed after 30 minutes in 5 patients, after
60 minutes in 4 patients and after 120-180 min in the remaining 8
patients. No differences in cortisol response were observed between
males and females. The side effects reported in the majority of
patients were mild flushing, nausea, and headache. One male patient had
hypotension.
Five TM patients with a peak cortisol level between
9 and 18 µg/dL (250 nmol/l and 500 nmol/l) after GST, consented to
receive an ITT. The test was done giving 0.1 IU/kg of regular insulin
(Actrapid, Novo Nordisk) intravenously to achieve blood glucose below
50% of fasting level or less than 40 mg/dl. Blood samples for cortisol
and glucose were collected at 0,15, 30, 45, 60, 90, and 120 min. The
maximum interval between the two dynamic tests was 2 months. All
procedures were carried out between 0800 and 0830 h after overnight
fasting. Serum cortisol levels were measured with soli-phase
competitive chemiluminescent immunoassay; the inter- and intra-assay
CVs were below 6.7%.
Using ITT, 2 out of the 5 patients (1 male ad
1 female, aged 45 and 37 yr with a serum ferritin of 644 ng/ml and 1221
ng/ml, respectively) had low peak cortisol response (16.1 µg/dL=444
nmol/l and 14.4 µg/dL=397.2 nmol/l, respectively) confirming the
central origin of AI. Both patients were asymptomatic and had a basal
cortisol level between 9 and 10 µg/dL (248-275 nmol/l) before GST
and/or ITT. The adrenocorticotropin hormone (ACTH) level was not
measured in both patients.
Nine patients (52.9%); 2 with normal
cortisol response and 7 with cortisol response in the “gray zone” had a
GH peak after GST< 3.0 μg/L, a value compatible with severe growth
hormone deficiency (GHD).
Deposition of iron in the pituitary
gland leads to hypogonadotropic hypogonadism and other manifestations
of hypopituitarism, including central hypothyroidism and growth hormone
(GH) deficiency.[10-13] Therefore, it might also
reduce ACTH secretion producing secondary CAI. Although in our study no
correlation was observed between basal cortisol level and serum
ferritin (r: 0.498;p: NS).The prevalence of AI appears to be more in
patients with greater transfusion burden, poor linear growth and
wasting.[1,2,14]
In conclusion,
the identification of TM patients with subtle abnormalities of the HPA
is mandatory to avoid a potential adrenal crisis during stressful
conditions. Although GST represents an alternative to the ITT as a
screening test for CAI because of its accessibility, lack of influence
by gender and relatively few contraindications, further larger studies
are required to accurately assess the cut-off cortisol level for
diagnosing an AI. Fifty-eight percent of our TM patients had a cortisol
response in the “gray zone”, after GST (between 9 and 18 µg/dL (250
nmol/l and 500 nmol/l). Two out of the 5 patients with a gray zone
response mounted a subnormal response after ITT (CAI).
We
believe that the test of choice for diagnosing CAI requires knowledge
of the available reference assays and the vagaries of each test. A flow
chart for screening and diagnosing adrenal insufficiency in thalassemia
is given in figure 1.
Furthermore, GST should be cautiously used in TM patients with
co-morbidities, including vascular and cardiac diseases, which increase
the potential risk of GST.
Yours faithfully.
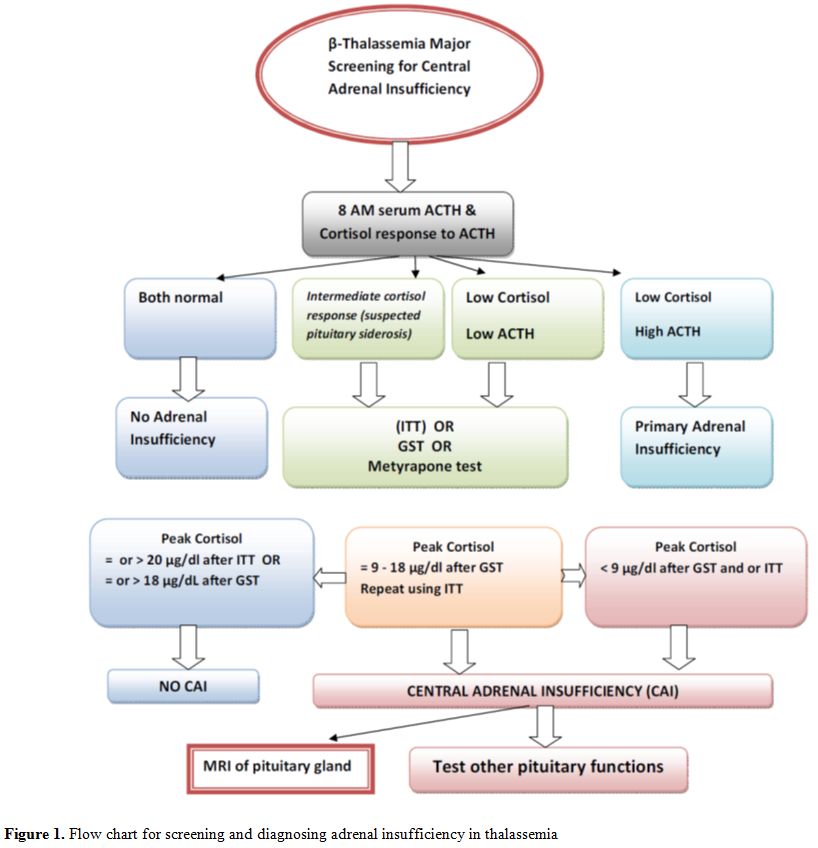 |
Figure 1. Flow chart for screening and diagnosing adrenal insufficiency in thalassemia |
References














[TOP]