Mary Theresa Sylvia1, Biswajit Dey1, Debdatta Basu1, Sajini Elizabeth Jacob1, Rakhee Kar1 and Biswajit Dubashi2
1 Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India
2 Department of Medical Oncology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India
Corresponding
author:Dr. Biswajit Dey. Department of
Pathology, Jawaharlal Institute of Postgraduate Medical Education and
Research, Pondicherry, India-605006. Mobile no. +919932289757. E-mail:
drbish25@rediffmail.com
Published: November 1, 2016
Received: August 29, 2016
Accepted: October 17, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016060, DOI
10.4084/MJHID.2016.060
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction: Follicular lymphoma (FL) is an
indolent lymphoproliferative disorder of B-cells with variable clinical behavior.
It is the second most common subtype of Non-Hodgkin lymphoma in western
countries but reported to have a lower incidence in Asia.
Materials and methods: Cases of FL diagnosed in the
Department of Pathology of our Institute from January 2009 to June 2015 were
included in the study. The clinicopathological parameters including staging,
histological details, and immunohistochemical markers CD20, CD10 and BCL-2 were
recorded in all the cases.
Results: Of the 497 cases of Non-Hodgkin Lymphoma
reported during the study period, 36 (7.2%) cases were follicular lymphoma. The
mean age was 50 years with male to female ratio of 3.2:1. Grade 1/2 was seen in
70% cases. 22 % cases had low grade with high proliferation index (Ki67 >
40%). Granulomatous response was seen in two cases. Diffuse large cell lymphoma
component was present in four cases. Bone marrow involvement and peripheral
blood spill were seen in 12 (37.5%) and six cases (18.8%) respectively. 72%
cases were in stage 3 or 4.
Conclusion: The incidence of FL was lower in our study
than other Indian studies. FL presented in the elderly, with male predominance
and disseminated stage. The study highlights features of low grade with high
proliferation index, granulomatous response, leukemic involvement, and
transformation to high grade lymphoma.
|
Introduction
Follicular
lymphoma (FL) is an indolent B-cell lymphoma of germinal center origin.
The translocation t(14,18) causes up regulation of BCL-2 gene which
leads to the inhibition of apoptosis of the germinal center cells.
Therapy ranges from observation to chemotherapy. It is important to
identify the high risk cases among this heterogeneous group with
variable clinical behavior which requires intensive treatment unlike
the low risk group. Recently grade 3b,[1] low grade with high
proliferation index[2] and follicular lymphoma with granulomatous
response[3] are considered to behave similar to large cell lymphoma and
thereby warrants intensive therapeutic regimens. [4]
Materials and Methods
Cases
of follicular lymphoma were collected from the archives of the
Department of Pathology, of our Institute over a period of six years
six months (January 2009 to June 2015). A total 36 cases of FL was
retrieved during this period of six years six months. The age, gender,
presenting complaints, fine needle aspiration cytology (FNAC),
histological features along with grade, proliferation index, bone
marrow, and peripheral blood involvement were noted. Grading was done
using the world health organization (WHO) criteria. A panel of
immunohistochemical markers CD10, CD20, BCL-2, CYCLIN D1 and CD5 was
done in all the cases. One of the cases was negative for BCL-2, so
BCL-6 was done in that case. CD23 was performed in 11 cases and Ki-67
in 23 cases. Proliferation index was calculated as a percentage of
positive cells in 10 random fields using the MIB-1(Ki-67) antibody more
than 40% Ki-67 index was taken as the cut-off for high proliferation
index. The staging was done according to the modified Ann-Arbor staging
system. Clinical details, treatment history and follow-up as
available were also collected.
Results
Clinical features:
Follicular lymphoma constituted 7.2% (36 cases) of the 497 cases of
Non-Hodgkin Lymphoma (NHL) reported during six years six months
(January 2009 to June 2015). The mean age of patients with follicular
lymphoma was 50 (Range 30-75 years). There was male predominance with
M: F ratio of 3.2:1. Lymph node involvement was present in 35
cases, and one case had an extranodal (intestine) presentation. The
clinical presentation is summarized in Table 1.
Fine needle aspiration cytology was available for six cases which were
reported as reactive in three cases, NHL (one case), large cell
lymphoma (one case) and small lymphocytic lymphoma (one case). Staging
done for 32 cases showed six cases (19%) to be stage 1, three (9%)
stage 2, nine (28%) stage 3 and 14 cases (44%) stage 4. Post-mortem
biopsies of one patient who had expired during evaluation showed
infiltration of the liver and lung by follicular lymphoma cells.
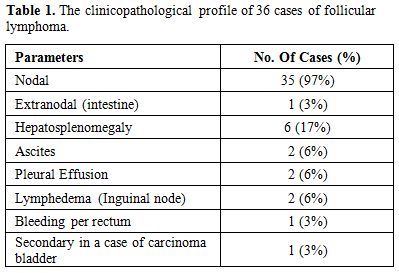 |
Table
1. The clinicopathological profile of 36 cases of follicular lymphoma. |
Histopathology:
All the cases had effacement of the lymph node by follicular
architecture with follicles of similar size present in the cortex and
medulla (Figure 1a). Tangible body macrophages were absent. High power view showed an admixture of centrocytes and centroblasts (Figure 1b).
The percentage of centroblasts varied according to the grade. Grading
was done in 31 cases. According to the WHO criteria for grading, eight
cases (26%) were grade 1, 14 cases (45%) grade 2, six cases (19%) grade
3a and three cases (10%) grade 3b. The granulomatous response was seen
in two cases. Follicular lymphoma with Diffuse large B cell lymphoma
(DLBCL) arising in follicular lymphoma was observed in four cases (Figure 2).
Composite histology was evident in two cases, and one was a relapse
with partial transformation after two years. Clinicopathologic
features are summarized in Table 2.
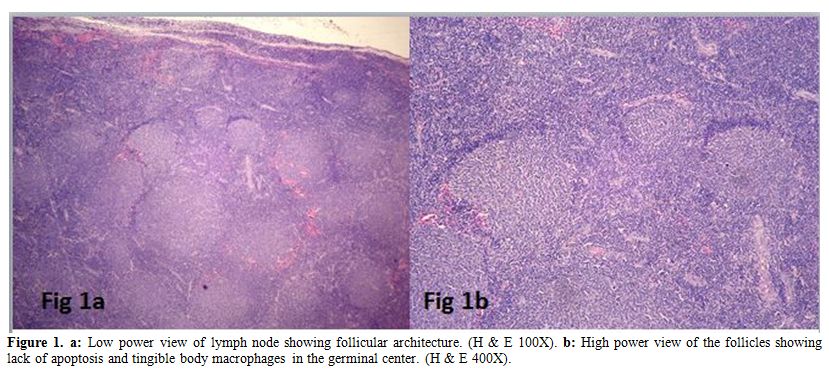 |
Figure 1. a: Low power view of lymph node showing follicular architecture. (H & E 100X). b: High
power view of the follicles showing lack of apoptosis and tingible body
macrophages in the germinal center. (H & E 400X). |
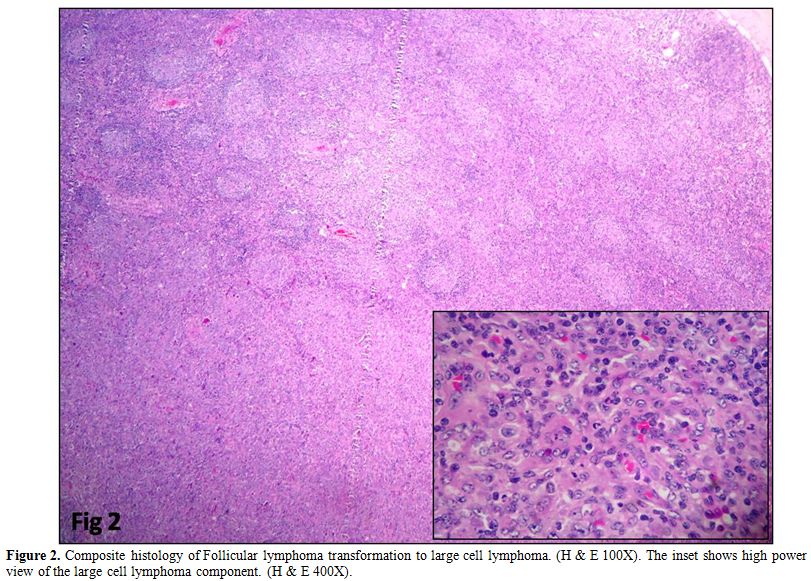 |
Figure 2. Composite histology of
Follicular lymphoma transformation to large cell lymphoma. (H & E
100X). The inset shows high power view of the large cell lymphoma
component. (H & E 400X). |
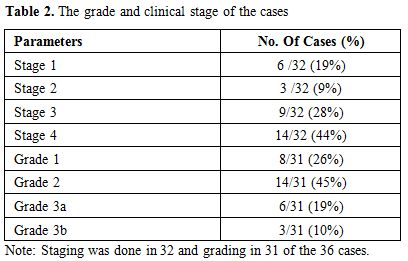 |
Table 2. The grade and clinical stage of the cases |
Bone marrow involvement:
Bone marrow was available for 32 cases. Bone marrow was involved in 12
cases (37.5%) and six cases (18.8%) had peripheral blood spill (Figure 3).
Bone marrow biopsy detected three cases with focal involvement by FL
which was missed on aspirate smears. All the 12 cases showed focal
paratrabecular structure either as a single pattern or mixed. The
diffuse pattern of bone marrow involvement was seen in five cases and,
the nodular and interstitial patterns were seen in four cases along
with the focal paratrabecular localization. Single centrally located
reactive lymphoid nodule was present in one patient. Bone marrow
involvement is summarized in Table 3.
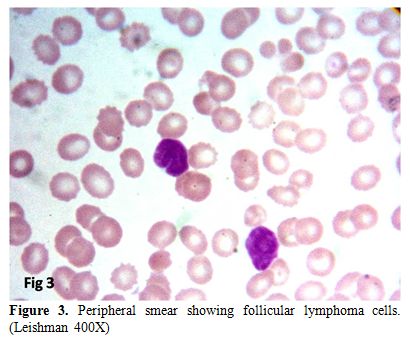 |
Figure 3. Peripheral smear showing follicular lymphoma cells. (Leishman 400X) |
 |
Table 3. Pattern of bone marrow involvement in 12 cases. |
Immunohistochemistry:
All the cases were positive for CD10 and CD20; 35 cases for BCL-2,
one case, BCL-2 negative was positive for BCL-6; all cases were
negative for CYCLIN D1 and CD5. CD23 was made in 11 cases of which
three were diffusely positive, and three had stained the follicular
dendritic cells, and five were negative. The proliferation index Ki-67
was performed in 23 cases. The range was from 10-60%. Low grade with
high proliferation index was observed in five cases (22%) (Figure 4). Proliferation index is summarized in Table 4.
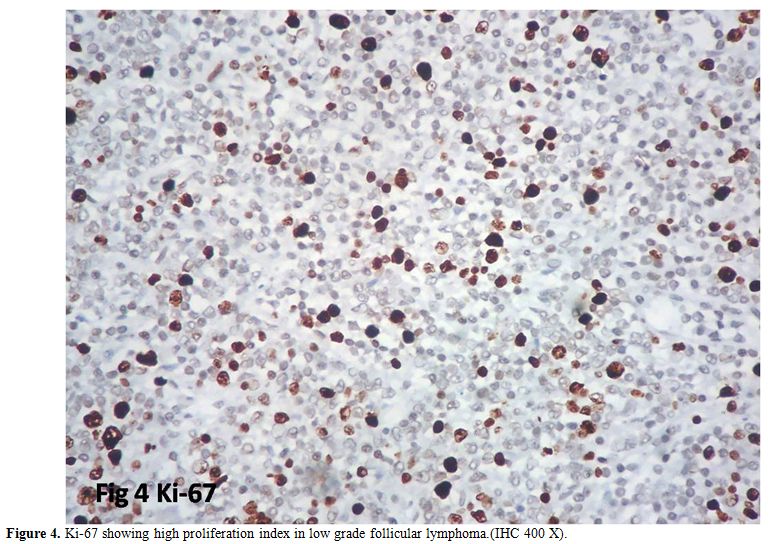 |
Figure 4. Proliferation index (Ki-67) in 23 cases at diagnosis. |
 |
Table 4. Ki-67 showing high proliferation index in low grade follicular lymphoma.(IHC 400 X). |
FLIPI (Follicular lymphoma international prognostic index):
The FLIPI scoring could be done for 12 cases. The score was low in
three cases (25%), intermediate in five (42%) and four (33%) were in
the high risk category. The scoring is shown in Table 5.
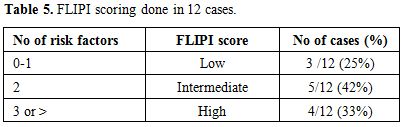 |
Table 5. FLIPI scoring done in 12 cases. |
Follow up:
Follow up was available for 12 cases. Relapse was seen in six cases and
five cases were in remission. The median relapse free time was 1.5
years. Two patients developed sensory neuropathy secondary to therapy.
Leukemic infiltration of liver, lung, and spleen occurred in one
patient who expired (Figure 5).
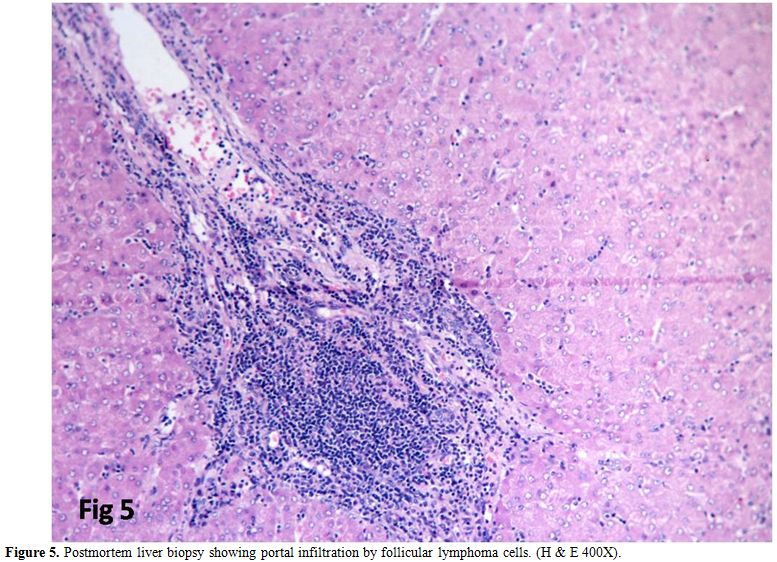 |
Figure 5. Postmortem liver biopsy showing portal infiltration by follicular lymphoma cells. (H & E 400X). |
Discussion
Epidemiology:
Follicular lymphomas are a heterogeneous group with regional variations
in incidence. It is the second most common subtype of Non Hodgkin
lymphoma in western countries constituting 20-25% but reported to have
a lower rate in Asia.[5] Our incidence is lower (7.2%) compared to the
previous Indian studies by Naresh et al., who had reported an
incidence of 12.6%[6] and Mondal et al. reported it to be 19.3% in
eastern India.[7] The average age of occurrence was 50 years in the
present study. Literature quotes the median age of follicular to be a
decade higher (59-60 years) in the developed countries.[8,9] Among the
Indian studies, Mondal et el had reported similarmean age of 51 years
for follicular lymphoma from eastern India,[7] Bharadwaj et al found
the incidence to be a decade lower (41 years) in Uttarakand, India[10]
and Sharma et al had reported a bimodal occurrence (31-40 years and
51-60 years) in northern part of India.[11] Western studies have
documented female predominance[8,9] but in our study, there was male
predominance similar to the previous India studies. It is indolent and
higher proportion of patients manifest in disseminated stage (3 &
4)[8,9] as in our series.
Histopathology:
FL with granulomas. The epithelioid granulomatous response was seen in
two cases. Kojima et al. analyzed 50 cases of follicular and large cell
lymphomas with granulomatous response and concluded it to be a separate
entity presenting at an older age, bulkier disease and prognosis
similar to DLBCL and inferior to follicular lymphoma. Hence he
concluded this to be a separate entity of centroblastic/centrocytic
lymphoma with epithelioid cell response.[3] In contrast both our cases
presented at an average age of 50 years not higher than the other
cases, histological grade 2 and low proliferation index. One of them
had bone marrow involvement. However, the number of cases is small to
draw any conclusion.
Transformation:
Histological transformation of follicular lymphoma to aggressive
variants most commonly DLBCL and rarely Burkitt or lymphoblastic
lymphoma carries a poor prognosis. The frequency of transformation
ranges from 10 to 60% in the literature.[1] It is in the lower
range (11.1%) in our study. The current gold standard definition
of transformation is the diffuse effacement of follicular architecture
by an increase in large cells, histologically proven and clonally
confirmed. Some authors require six months interval between the initial
diagnosis of FL and DLBCL to establish transformation. The simultaneous
presence of both features suggests but does not establish the
diagnosis.[1] Composite histology of DLBCL and FL at the initial
diagnosis was present in two cases, and one case was a relapse of FL
after two years with transformation to DLBCL. It is important to
differentiate DLBCL from follicular lymphoma with diffuse architecture
and grade 3b FL. FL with diffuse structure shows a mixture of
centrocytes and centroblasts and lacks sheets of large cells. FL grade
3b has solid sheets of centroblasts (>15 centroblasts / 0.159 mm2)
whereas DLBCL with FL component has diffuse area with solid sheets of
centroblasts outside histologically or immunophenotypically (CD21,
CD23+ FDC) recognizable follicles. DLBCL rising in FL is usually of
germinal center phenotype[1] as in our study.
The risk of
transformation was higher in patients presenting with the bulkier
disease, advanced stage, B symptoms and high FLIPI scores.[1] We do not
have data regarding the initial presentation in the relapse case.
The
clinical features at transformation include old age i.e. more than 60
years, a discordant growth of lymph nodes, increase in the stage, the
appearance of B symptoms and extranodal involvement.[1] The average age
of our four cases was 60 years, the advanced stage frequent, in one
patient with primitive extranodal localization and the relapse of the
disease was in the lymph nodes.
Bone marrow:
Bone marrow involvement in our study was 37.5%. Three cases were
missed on aspirate and diagnosed on biopsy reinforcing the need for
biopsy for staging. All cases had concordant involvement. Bone marrow
involvement has been included as one of the prognostic factors in the
FLIPI scoring system. Paratrabecular localization has been described as
most common and characteristic of follicular lymphomas.[12] We found it
in all the 12 (100%) cases. Single centrally located reactive lymphoid
nodule was seen in one case causing a diagnostic difficulty.
Differentiation of reactive nodule from the follicular pattern of
involvement by FL requires a synopsis of criteria. The topographical
location (central perivascular in reactive, paratrabecular in FL),
peripheral infiltration “Indian file” like (well defined border in
reactive) and dense reticulin fibres help the morphological
differentiation in FL. Use of immunohistochemistry is also critical.
Both can have a mixture of T and B cells and can express BCL-2.[13]
Bone
marrow involvement was more common in grade 1 and 2 rather than grade 3
cases. Hence bone marrow involvement appears early. This is explained
by Kluin et al. as a bidirectional migration of the tumor cells both in
the lymph nodes and marrow at the beginning of the disease process.[14]
Peripheral blood involvement:
Peripheral blood involvement was seen in 18.8 % of our patients, which
is higher than the previous studies.[15] Circulating cells of
follicular lymphoma are seen in many cases, but leukemic involvement
ranges from 4-23%.[16] Four of our cases had a detectable leukemic
phase at the time of diagnosis. Few circulating lymphoma cells do not
affect the prognosis, but leukemic spread and high total counts have an
adverse prognosis. Sarkozy et al. reported 7.4% of their cases to have
leukemic involvement and had shorter progression free and overall
survival compared to a group of follicular lymphoma without a leukemic
spill. More than 4x109/L
circulating lymphoma cells was found to have a bad prognosis.[17] In
the present study out of the four patients in leukemic phase one
patient expired, the second one had an initial response to
chemotherapy, and the other two were lost to follow up.
Proliferation index:
The proliferation rate of lymphomas is assessed using the MIB 1
antibody (Ki-67). Xin He et al. had done a meta-analysis of Ki-67 index
in lymphomas and found higher proliferation to be associated with
inferior overall survival and disease free survival rate.[18] But the
study has not highlighted the importance in follicular lymphomas. Low
grade follicular lymphomas (grade 1 & 2) with high proliferation
index (Ki-67) have been reported recently.[2] Ki-67 index of more than
40% is considered as high proliferation index.[2] This subgroup has
been found to behave as higher grade lymphomas/DLBCL and requires high
dose chemotherapy. When estimating the Ki-67 index, it is important to
avoid areas of normal germinal centers in a partially effaced node and
areas of transformation to high grade lymphoma which usually have high
proliferation index. In the present study, 22% of the cases were of low
grade with high proliferation index as compared to the western
literature of 18%.[18] The higher percentage of our cases may be due to
racial and geographical variation. Wang et al. had also proved high
proliferation index as a poor prognostic marker in follicular
lymphoma.[19] Hence it is important to identify this subgroup which
needs intensive therapy. All our five cases were in an advanced stage;
one had progressive disease and one in remission. Other 3 cases were
lost to follow-up.
CD 23:
CD 23 is a low affinity receptor for IgE, present in follicular
dendritic cells (FDC) and promoting survival of germinal center B
cells. It is positive in chronic lymphocytic leukemia. Recently CD23
positivity in neoplastic cells has been described in follicular
lymphomas. Olteanu et al. have reported 70% of their cases of
follicular lymphomas to be positive for CD23. It was associated with
inguinal lymphadenopathy, lower grade, and a better prognosis compared
to the negative group.[20] In our series CD23 was done in 11 cases of
which the three positive cases were of lower grade. However one of the
cases of the inguinal lymph node was negative in contrast to previous
studies which have reported an association with inguinal lymph
nodes.[20] The concept of tumor microenvironment in FL has gained
importance. The FDC and T regulatory cells play a significant role in
FL in the lymph node as well as bone marrow colonisation.[21] Hence it is
mandatory to study it in detail.
BCL-6:
BCL-6 located in 3q27 functions as a transcriptional repressor of
germinal center B cells. It is highly expressed in FL, but
translocation is present in 6.4% to 14.3% of FL.[22] It coexists with
BCL-2 translocation in half of the cases. Akasaka et al. have reported
BCL-6 positivity as a marker of genomic instability and early
transformation.[22] One of our cases was negative for BCL-2, so we did
BCL-6 which was positive, but not routinely performed in all cases.
Prognostic scoring: The FLIPI scoring distribution is comparable with the previous study by Solal-Celigny et al. (2004)[23] as shown in Table 5. However, the numbers of cases are inadequate for further survival analysis.
Conclusion
The
incidence of follicular lymphomas is lower in Southern India as
compared to western countries and other parts of India and, has a male
predominance. Features like grades 3b, follicular lymphomas with the
granulomatous response, low grade with high Ki-67 index are
highlighted. Involvement of bone marrow and peripheral blood is high in
follicular lymphomas. It is mandatory to do Ki-67, CD 23 and BCL-6 in
all cases of follicular lymphomas, and P53 in transformed cases. Hence
it is important to identify the high risk cases in this low grade group
which can be treated with high dose regimens or experimental therapies.
References
- Lossos IS, Gascoyne RD. Transformation of Follicular Lymphoma. Best Pract Res Clin Haematol 2011; 24:147-163. http://dx.doi.org/10.1016/j.beha.2011.02.006 PMid:21658615 PMCid:PMC3112479

- Das
S, Basu D, Dubashi B, Jain A. Low grade follicular lymphoma with high
proliferation index; diagnostic and management issues. Indian J Pathol
Microbiol 2012;55:516-518. http://dx.doi.org/10.4103/0377-4929.107795 PMid:23455792

- Kojima
M, Nakamura S, Ichimura K, Suzuki R, Kagami Y, Kondo E et al.
Centroblastic and centroblastic/centrocytic lymphoma associated with a
prominent epitheliod granulomatous response: A clinicopathologic study
of 50 cases. Mod Pathol 2002;15:750-758. http://dx.doi.org/10.1097/01.MP.0000018980.83088.D3 PMid:12118113

- Prochazka
V, Papajik T, Jarosova M, Indrak K. Prognostic factors in follicular
lymphoma in the rituximab era: How to identify a high-risk patient?
Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:99-108. http://dx.doi.org/10.5507/bp.2011.015 PMid:21804618

- Salles
GA. Clinical features, prognosis and treatment of follicular lymphoma.
Hematology Am Soc Hematol Educ Program 2007:216-225. http://dx.doi.org/10.1182/asheducation-2007.1.216 PMid:18024633

- Naresh
KN, Srinivas V, Soman CS. Distribution of various subtypes of
non-Hodgkin's lymphoma in India: A study of 2773 lymphomas using
R.E.A.L. and WHO classifications. Ann Oncol 2000;11:S63-S67. http://dx.doi.org/10.1093/annonc/11.suppl_1.S63

- Mondal
SK, Mandal PK, Samanta TK, Chakaborty S, Roy SD, Roy S. Malignant
lymphoma in Eastern India: A retrospective analysis of 455 cases
according to World Health Organization classification. Indian J Med
Paediatr Oncol 2013;34:242-246. http://dx.doi.org/10.4103/0971-5851.125235 PMid:24604951 PMCid:PMC3932589

- Relander
T, Johnson NA, Farinha P, Connors JM, Sehn LH, Gascoyne RD. Prognostic
factors in follicular lymphoma. J Clin Oncol 2010; 28:2902-2913. http://dx.doi.org/10.1200/JCO.2009.26.1693 PMid:20385990

- Gaman
A M. Follicular non-Hodgkins lymphoma: correlation between histology,
pathophysiology, cytogenetic, prognostic factors, treatment, survival.
Rom J Morphol Embryol 2013; 54:71-76. PMid:23529311

- Bhardwaj
A, Kishore S, Kusum A. Comparative Analysis of the Distribution of
Various Subtypes of Lymphoid Malignancies in Uttarakhand with other
Regions in India. Ind Med Gaz 2012: 147-152.

- Sharma
M, Mannan R, Madhukar M, Navani S, Manjari M, Bhasin TS et al.
Immunohistochemical (IHC) analysis of Non-Hodgkins lymphoma (NHL)
spectrum according to WHO/REAL classification: A single centre
experience from Punjab, India. J Clin Diagn Res 2014;8:46-49.
PMid:24596721 PMCid:PMC3939585

- Torlakovic
E, Torlakovic G, Brunning R D. Follicular pattern of bone marrow
involvement by follicular lymphoma. Am J Clin Pathol 2002;118:780-786. http://dx.doi.org/10.1309/EG2M-YHB9-WEFW-7H1R PMid:12428800

- Thiele
J, Zirbes T K, Kvasnicka H M, Fischer R. Focal lymphoid aggregates
(nodules) in bone marrow biopsies: differentiation between benign
hyperplasia and malignant lymphoma-a practical guideline. J Clin Pathol
1999;52:294-300. http://dx.doi.org/10.1136/jcp.52.4.294 PMid:10474523 PMCid:PMC501336

- Kluin P M. Origin and migration of follicular lymphoma cells. Haematologica 2013;98:1331-1333. http://dx.doi.org/10.3324/haematol.2013.091546 PMid:24006404 PMCid:PMC3762086

- Catovsky
D. Chronic lymphocytic leukaemia and other B-cell disorders.In:
Hoffbrand AV, Catovsky D, Tuddenham EGD, eds. Postgraduate Hematology.
Victoria, Australia. Blackwell Publishing Asia Pty Ltd. 2005;619-643. http://dx.doi.org/10.1002/9780470987056.ch38

- Beltran
BE, Castillo JJ, Quinones P, Morales D, Alva JC, Miranda RN et al.
Follicular lymphoma with leukemic phase at diagnosis: An aggressive
disease. Report of seven cases and review of the literature. Leuk Res
2013; 37: 1116-1119. http://dx.doi.org/10.1016/j.leukres.2013.05.016 PMid:23790442 PMCid:PMC4038155

- Sarkozy
C, Baseggio L, Feugier P, Callet-Bauchu E, Karlin L, Seymour JF et al.
Peripheral blood involvement in patients with follicular lymphoma: a
rare disease manifestation associated with poor prognosis. Br J Hematol
2014;164:659-67. http://dx.doi.org/10.1111/bjh.12675 PMid:24274024

- Xin
He, Zhigang Chen, Tao Fu, Xueli Jin, Teng Yu, Yun Liang et al. Ki-67 is
a valuable prognostic predictor of lymphoma but its utility varies in
lymphoma subtypes: evidence from a systematic meta-analysis. BMC Cancer
2014,14:153. http://dx.doi.org/10.1186/1471-2407-14-153 PMid:24597851 PMCid:PMC3995999

- Wang
SA, Wang L, Hochberg EP, Muzikansky A, Harris NL, Hasserjian RP et al.
Low histologic grade follicular lymphoma with high proliferation index:
morphologic and clinical features. Am J Surg Pathol 2005;29:1490-6. http://dx.doi.org/10.1097/01.pas.0000172191.87176.3b PMid:16224216

- Olteanu
H, Fenske TS, Harrington AM, Szabo A, He P, Kroft SH. CD23 expression
in follicular lymphoma clinicopathologic correlations. Am J Clin Pathol
2011;135:46-53. http://dx.doi.org/10.1309/AJCP27YWLIQRAJPW PMid:21173123

- Kridel R, Sehn L H, Gascoyne R D. Pathogenesis of follicular lymphoma. J Clin Invest. 2012; 122:3424-3431. http://dx.doi.org/10.1172/JCI63186 PMid:23023713 PMCid:PMC3461914

- Akasaka
T, Lossos IZ, Levy R. BCL6 gene translocation in follicular lymphoma: a
harbinger of eventual transformation to diffuse aggressive lymphoma.
Blood 2003; 102:1443-1447. http://dx.doi.org/10.1182/blood-2002-08-2482 PMid:12738680

- Solal-Celigny
P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R et al.
Follicular lymphoma international prognostic index. Blood
2004;104:1258-1265. http://dx.doi.org/10.1182/blood-2003-12-4434 PMid:15126323

[TOP]

































