Masafumi Taniwaki1,2, Mihoko Yoshida2, Yosuke Matsumoto2, Kazuho Shimura2, Junya Kuroda3 and Hiroto Kaneko2
1 Center for
Molecular Diagnostics and Therapeutics, Kyoto Prefectural University of
Medicine, Graduate School of Medical Science, Japan.
2 Department of Hematology and Laboratory Medicine, General Incorporated Association Aiseikai Yamashina Hospital, Japan.
3 Department of Hematology, Kyoto Prefectural University of Medicine, Graduate School of Medical Science, Japan.
Corresponding
author: Masafumi Taniwaki, MD, Ph.D.,
Center for Molecular Diagnostics and Therapeutics, Kyoto Prefectural
University of Medicine, Graduate School of Medical Science, 465
Kajii-cho, Kamigyo-ku, Kyoto, 602-8566, Japan. Tel: +81-75-25- 5659,
Fax: +81-75-251-5659; E-mail:
taniwaki@koto.kpu-m.ac.jp
Published: February 15, 2018
Received: December 26, 2017
Accepted: February 6, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018014 DOI
10.4084/MJHID.2018.014
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Elotuzumab,
targeting signaling lymphocytic activation molecule family 7 (SLAMF7),
has been approved in combination with lenalidomide and dexamethasone
(ELd) for relapsed/refractory multiple myeloma (MM) based on the
findings of the phase III randomized trial ELOQUENT-2 (NCT01239797).
Four-year follow-up analyses of ELOQUENT-2 have demonstrated that
progression-free survival was 21% in ELd versus 14% in Ld. Elotuzumab
binds a unique epitope on the membrane IgC2 domain of SLAMF7,
exhibiting a dual mechanism of action: natural killer (NK)
cell-mediated antibody-dependent cellular cytotoxicity (ADCC) and
enhancement of NK cell activity. The ADCC is mediated through
engagement between Fc portion of elotuzumab and FcγRIIIa/CD16
on NK cells. Enhanced NK cell cytotoxicity results from phosphorylation
of the immunoreceptor tyrosine-based switch motif (ITSM) that is
induced via elotuzumab binding and recruits the SLAM-associated adaptor
protein EAT-2. The coupling of EAT-2 to the phospholipase Cγ enzymes SH2 domain leads to enhanced Ca2+
influx and MAPK/Erk pathway activation, resulting in granule
polarization and enhanced exocytosis in NK cells. Elotuzumab does not
stimulate the proliferation of MM cells due to a lack of EAT-2. The
inhibitory effects of elotuzumab on MM cell growth are not induced by
the lack of CD45, even though SHP-2, SHP-1, SHIP-1, and Csk may be
recruited to phosphorylated ITSM of SLAMF7. ELd improves PFS in
patients with high-risk cytogenetics, i.e. t(4;14), del(17p), and 1q21
gain/amplification. Since the immune state is paralytic in advanced MM,
the efficacy of ELd with minimal toxicity may bring forward for
consideration of its use in the early stages of the disease.
|
Introduction
Multiple
myeloma (MM) is the second most common hematological malignancy in
Western countries with 62% of patients being older than 65 years at the
time of diagnosis.[1,2] According to the National Cancer Center in
Japan, the number of patients with MM was 6697 in 2013, and that of
deaths was 4129 in 2015; the five-year relative survival rate was 36.4%
for MM patients diagnosed between 2000 to 2008.[3] Regarding morbidity
in 2015 based on age and gender, the proportions of patients older than
65 years were 90.1% for females and 87.9% for males, while those of
patients older than 75 years were 69.1% for females and 60.9% for
males.[3] Due to its high incidence in the elderly and its
incurability, there is an urgent need to develop effective and less
toxic combination therapies for unfit or frail patients with MM.
The
treatment outcomes of MM have significantly improved in the last decade
or two due to the success of molecular targeting agents including
thalidomide, lenalidomide, and bortezomib.[4-7] According to the
findings of a number of clinical trials, triplet induction therapy
containing proteasome inhibitors (PIs) and immunomodulatory drugs
(IMiDs) is the standard care for fit patients, whereas doublet
induction therapy containing PIs or IMiD is administered to frail
patients. In addition to the development of second- and
third-generation PIs and IMiDs, monoclonal antibodies (mAb) will open a
new era of MM treatments that selectively eliminate the malignant clone
and reverse tumor-mediated immune paralysis.[8-12] Elotuzumab is the
first therapeutic mAb targeting SLAMF7 that has been approved for
relapsed or refractory (RR) MM. It induces natural killer (NK)
cell-mediated antibody-dependent cellular cytotoxicity (ADCC) and
exerts stimulatory effects on immune cells, particularly NK cells,
which are mediated by the engagement of elotuzumab with SLAMF7.[13,14]
Clinically, the combination of elotuzumab with lenalidomide and
dexamethasone (ELd) is a promising treatment for frail patients
regardless of the cytogenetic risk.[8]
In this review, we will
focus on the efficacy and safety of elotuzumab for the treatment of
RRMM. We will also discuss the biological characteristics of SLAMF7 and
SLAM-associated protein (SAP), their expression and possible functions
in normal cells and hematological malignancies, as well as the modes of
action of elotuzumab. We will then propose optimal use and future
directions for elotuzumab in the treatment of MM.
Elotuzumab for the Treatment of RRMM
Efficacy and safety of elotuzumab in combination with lenalidomide and dexamethasone:
Elotuzumab was approved in combination with lenalidomide and
dexamethasone (Ld) for patients with RRMM based on the findings of the
phase III, randomized, open-label, multicenter trial, ELOQUENT-2
(NCT01239797).[8] In ELOQUENT-2, the efficacy of elotuzumab combined
with Ld (ELd) was evaluated for patients with RRMM who previously
received one to three regimens. ELOQUENT-1 is still ongoing for
patients with newly diagnosed MM (NDMM). ELOQUENT-2, in which 646
patients were randomized into ELd or Ld, demonstrated significant
increase in overall response rate (ORR) and median PFS in ELd (Table 1).[8]
Progression-free survival (PFS) has significantly improved in patients
older than 75 years, particularly those with refractory disease and
high-risk cytogenetic abnormalities (CA), i.e. t(4;14), del(17p), and
1q21 gain/amplification. A subanalysis of the Japanese population from
ELOQUENT-2 revealed similar outcomes to the global study as well as to
the Japanese phase I study; ORR were 84% in ELd vs 86% in Ld, and PFS
rates at two years were 48% in ELd vs 18% in Ld.[15,16] A three-year
follow-up and post-hoc analyses of ELOQUENT-2 recently confirmed that
ELd provided a durable improvement in efficacy; ORR were 79% in ELd and
66% in Ld.[17] ELd reduced the risk of disease progression/death by 27%
versus Ld. Interim overall survival (OS) at 3 years was 60% with ELd
versus 53% with Ld. Serum M-protein dynamic modelling showed slower
tumor regrowth with ELd.[17] An extended four-year follow-up of
ELOQUENT-2 also demonstrated a sustained improvement in PFS in ELd
versus Ld (21% vs 14%).[18] Patients with ≥ very good partial response
(VGPR) had the greatest reduction (35%) in risk of progression/death.
Median OS was 48% in ELd versus 40% in Ld.[18] These results further
support the durable efficacy of ELd.
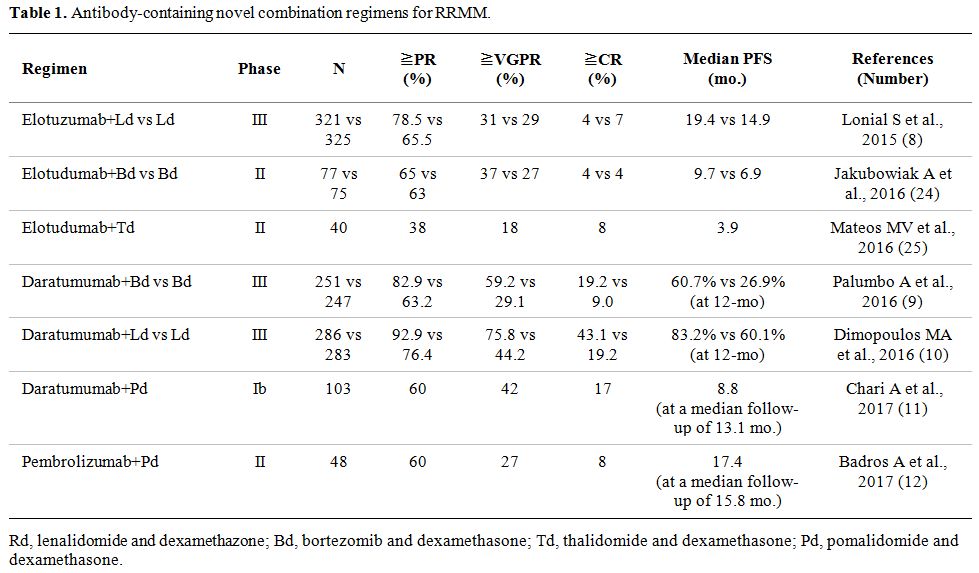 |
Table 1. Antibody-containing novel combination regimens for RRMM. |
The
safety, tolerability, and pharmacokinetics of intravenous elotuzumab
have been assessed in a Phase I study of dose-escalation monotherapy at
10-20 mg/kg, demonstrating no maximum tolerated dose and modest
activity with a best response of stable disease (SD).[19] The other two
Phase I or Phase I/II studies also reported that the safety and
tolerability of elotuzumab in combination with bortezomib or
lenalidomide were acceptable.[20-22] Severe adverse events (AEs) in
ELOQUENT-2 were 65% in ELd versus 57% in Ld; the most common grade 3/4
hematological AEs in ELd vs Ld were lymphocytopenia (77% vs 49%)
followed by neutropenia (34% vs 44%), thrombocytopenia (19% vs 20%),
and anemia (19% vs 21%).[8] Grade 3/4 hematological AEs, except for
lymphocytopenia, were less frequent with ELd than with Ld, which may be
of particular benefit for frail elderly patients. Common
non-hematological grade 3/4 AEs were fatigue (8% in both arms),
diarrhea (5% in ELd vs 4% in Ld), and pyrexia (5% and 3% in both arms).
Lymphocytopenia may develop as a result of the migration of peripheral
lymphocytes including NK cells into the involved tissue sites.[19]
Infusion reactions (IRs) appearing as pyrexia, chills, and hypertension
were very limited when compared with daratumumab, observed in 10% of
ELd versus 45.3-50% of daratumumab-containing regimens.[9-11]
Premedication with antihistamines, acetaminophen, and dexamethasone
have successfully prevented IRs, and now are standard of care as part
of the treatment with this antibody treatment. A phase II study
demonstrated that a 1-hour infusion of elotuzumab provided convenient
alternative dosing.[23]
Elotuzumab in combination with bortezomib or thalidomide: The efficacy of elotuzumab combined with bortezomib or thalidomide was also evaluated (Table 1).[24,25]
A randomized Phase II study of elotuzumab combined with bortezomib and
dexamethasone (EBd) versus bortezomib and dexamethasone (Bd), in which
152 patients with RRMM were randomized into EBd or Bd, has demonstrated
slight increase in ORR and median PFS in EBd.[24] Grade 3/4 AEs were
reported in 53 patients (71%) with EBd versus 45 patients (69%) with
Bd; the most common grade 3 or higher AEs of EBd vs Bd were infections
(21% vs 13%) and thrombocytopenia (9% vs 17%).[24] Grade 3/4 peripheral
neuropathy (9% vs 12%), paresthesia (0% vs 5%), and thrombocytopenia
were slightly less frequent in EBd than in Bd.[24] Grade 1/2 IRs were
observed in 5% of EBd; there were no grade 3 or higher IRs.
The
efficacy of 10 mg/kg elotuzumab combined with 50-200 mg thalidomide and
40 mg dexamethasone (ETd) (with or without 50 mg cyclophosphamide), was
also evaluated in a Phase II single-arm study with minimal additional
toxicity.[25] IRs were observed in 15% of ETd. This clinical trial
showed ORR of 38% in 40 RRMM patients with a median of three prior
regimens including bortezomib (98%) and lenalidomide (73%); median PFS
and OS were 3.9 months and 16.3 months, respectively.[25] These
findings suggest that the combination of elotuzumab with bortezomib or
thalidomide has potential as treatment option for patients with RRMM.
Biological Characteristics of SLAMF7 and its Adaptor Proteins.
Biological characteristics of SLAMF receptors:
SLAMF7 is one of the nine SLAMF receptors (SLAMF1-9) belonging to the
CD2 subset of the immunoglobulin superfamily. It was originally
identified as CS1 (CD2 subunit 1) by a subtractive hybridization
between naïve B cell cDNA and that of memory B cells and plasma
cells.[13] Molecular cloning revealed that CS1 is a novel human NK cell
receptor.[26] SLAMF7 may also play a growth-promoting role and be
involved in the autocrine expression of cytokines in normal B
cells,[27] whereas its function in normal plasma cells currently
remains unknown.
SLAMF receptors are type I transmembrane
glycoproteins, except a glycosylphosphatidylinositol-anchored protein
SLAMF2, which is widely expressed in hematopoietic cells but not in
other tissues (Table 2). The
genes encoding SLAMF receptors are reported to be clustered within an
approximately 350-kb region at 1q23.[3.28,29] Our fluorescence in situ
hybridization (FISH) study assigned SLAMF7 to 1q21.3 using the BAC
clone RP11-404F10 containing SLAMF2, SLAMF7, and SLAMF3 (Sakamoto N,
Taniwaki M et al., unpublished) (Figures 1A and 1B).
SLAMF7 is also included in the amplicon of chromosome 1q
gain/amplification, which is a high-risk CA frequently detected in RRMM
(Sakamoto N, Taniwaki M et al., unpublished) (Figures 1C and 1D).
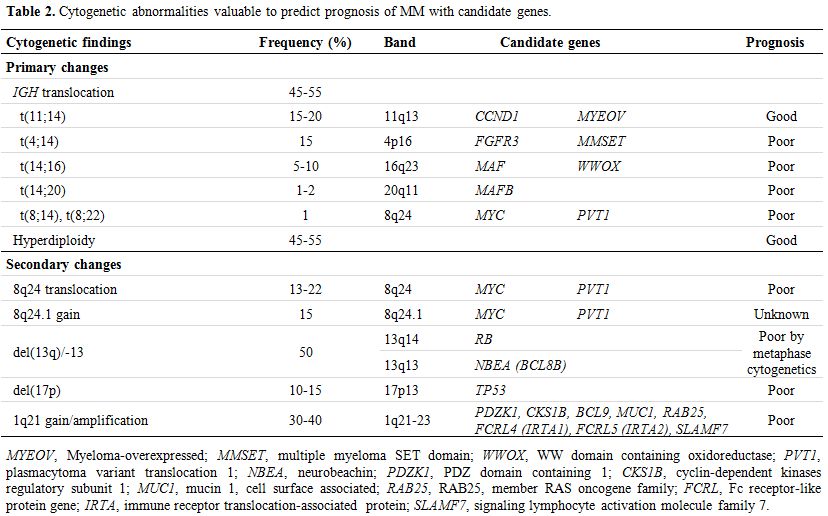 |
Table 2.
Cytogenetic abnormalities valuable to predict prognosis of MM with candidate genes. |
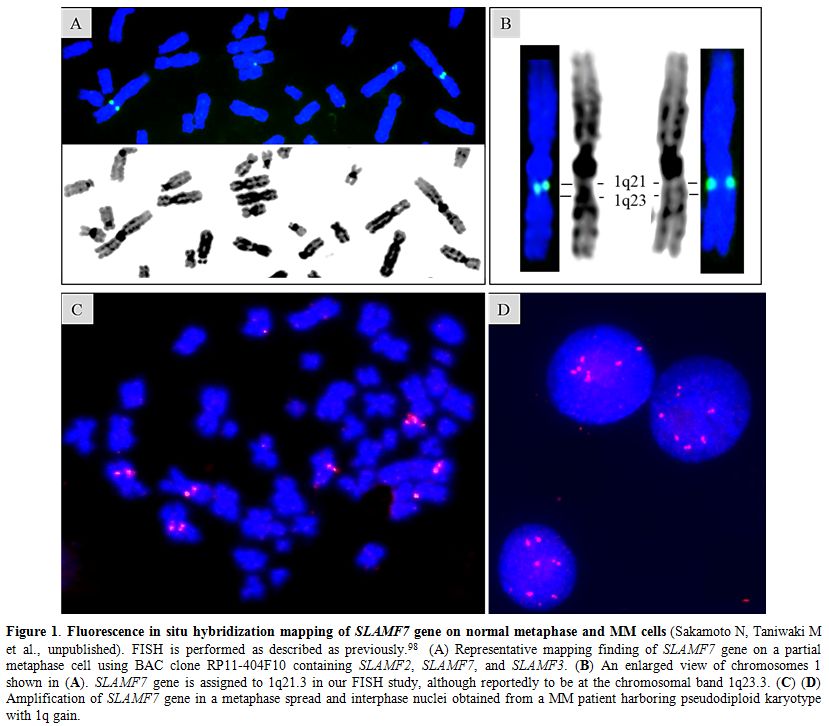 |
Figure 1. Fluorescence in situ hybridization mapping of SLAMF7 gene on normal metaphase and MM cells
(Sakamoto N, Taniwaki M et al., unpublished). FISH is performed as
described as previously.98 (A) Representative mapping finding of
SLAMF7 gene on a partial metaphase cell using BAC clone RP11-404F10
containing SLAMF2, SLAMF7, and SLAMF3. (B) An enlarged view of
chromosomes 1 shown in (A). SLAMF7 gene is assigned to 1q21.3 in our
FISH study, although reportedly to be at the chromosomal band 1q23.3.
(C) (D) Amplification of SLAMF7 gene in a metaphase spread and
interphase nuclei obtained from a MM patient harboring pseudodiploid
karyotype with 1q gain. |
SLAMF
receptors are structurally characterized by distal Ig variable-like
(IgV) and proximal C2-like (IgC2) domains within an extracellular
portion and one or more immunoreceptor tyrosine-based switch motifs
(ITSMs) within the cytoplasmic portion. The exception is that SLAMF3
has duplicated IgV-IgC2 sequences, and SLAMF8 and SLAMF9 lack tyrosine
motifs.[28,30,31] SLAMF receptors 1, 3, 5 to 7, and 9, are
“self-ligands” that recognize the same receptor molecule on another
cell as a ligand; SLAMF2 and SLAMF4 are “co-ligands” that recognize
each other.[27,28,32] Interactions between SLAMF receptors occur at
their IgV domains between identical or different types of hematopoietic
cells. The engagement of SLAMF receptors mediates regulatory effects on
immune cells in the presence of the SLAM-associated protein (SAP)
family of adaptors.[26,33,34] Two SAP family adaptors have been
identified in humans: SAP (SH2D1A) and EWS-Fli1-activated transcript-2
(EAT-2, SH2D1B), which are intracellular proteins containing the Src
homology2 (SH2) domain devoid of enzymatic activity.[28,31,35] Although
most SLAMF receptors bind SAP and EAT-2, SLAMF7 is reported to be
functionally controlled by EAT-2 only.[33,36] However, RNA interference
experiments have demonstrated that SLAMF7 may interact with SAP when
the concentration of SAP is significantly higher than that of EAT-2 in
cells.[37] Hence, the selective binding of SLAMF7 to EAT-2 is due to its
greater affinity to EAT-2 than SAP by nearly two orders of magnitude.[37]
Moreover, a recent study reported that SLAMF7 interacted with integrin
Mac-1 instead of SAP adaptors utilizing signals involving
immunoreceptor tyrosine-based activation motifs (ITAMs), which induced
the promotion of phagocytosis.[38] Further studies are needed in order to
elucidate the exact role of SLAMF7 in myeloma cell pathophysiology.
SLAM-associated adaptor proteins and downstream signal transduction:
SLAMF functions as an either inhibitory or activating receptor
depending on the availability of the SAP-related adaptor proteins, SAP
and EAT-2. SAP is expressed in T, NK, NKT, and germinal center B cells.
SAP expression has been reported in some Epstein-Barr virus
(EBV)-transformed B cells, Hodgkin’s lymphoma, and angioimmunoblastic
T-cell lymphoma.[39-41] EAT-2 is expressed in NK cells and a range of
antigen-presenting cells including monocytes.[42,43] When the SLAMF
receptor is engaged, tyrosine (Y) 281 located in ITSMs is
phosphorylated, recruiting SAP or EAT-2.[28,32] Through the SH2 domain,
SAP or EAT-2 binds SLAMF at the phosphorylated ITSMs with overlapping
specificities for activating and inhibitory binding partners. SAP
contains an arginine-based motif in the SH domain, which mediates
binding to the Src family protein Fyn, thereby stabilizing immune
synapses (Figure 2).[44] SAP
also enhances adhesion between NK and target cells. On the other hand,
EAT-2 controls NK cell function through the phospholipase Cγ
enzymes
(PLC-γ
), Ca2+
fluxes, and the MAPK/Erk pathway, leading to granule polarization and
the exocytosis of cytotoxic granules toward target cells (Figure 3).[45]
SAP and EAT-2 both prevent SLAMF receptors from interacting with
inhibitory effectors such as SH2-domain-containing tyrosine phosphatase
(SHP)-2, SHP-1, SH2 domain-containing 5’ inositol phosphatase (SHIP)-1,
or C-terminal Src kinase (Csk).[36,41] Hence, SLAMF receptors become
inhibitory in the absence of SAP-related adaptors, suppressing the
function of activating NK-cell receptors such as CD16, natural-killer
group-2 member-D (NKG2D), and DNAX accessory molecule-1 (DNAM-1).[32]
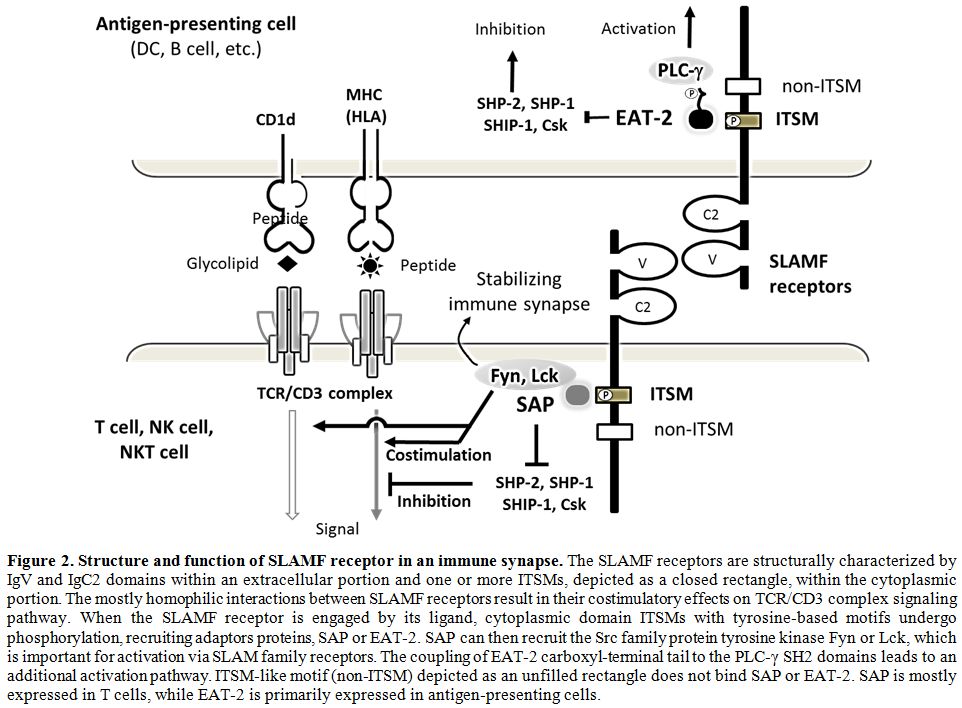 |
Figure 2.
Structure and function of SLAMF receptor in an immune synapse.
The SLAMF receptors are structurally characterized by IgV and IgC2
domains within an extracellular portion and one or more ITSMs, depicted
as a closed rectangle, within the cytoplasmic portion. The mostly
homophilic interactions between SLAMF receptors result in their
costimulatory effects on TCR/CD3 complex signaling pathway. When the
SLAMF receptor is engaged by its ligand, cytoplasmic domain ITSMs with
tyrosine-based motifs undergo phosphorylation, recruiting adaptors
proteins, SAP or EAT-2. SAP can then recruit the Src family protein
tyrosine kinase Fyn or Lck, which is important for activation via SLAM
family receptors. The coupling of EAT-2 carboxyl-terminal tail to the
PLC-γ SH2 domains leads to an additional activation pathway. ITSM-like
motif (non-ITSM) depicted as an unfilled rectangle does not bind SAP or
EAT-2. SAP is mostly expressed in T cells, while EAT-2 is primarily
expressed in antigen-presenting cells. |
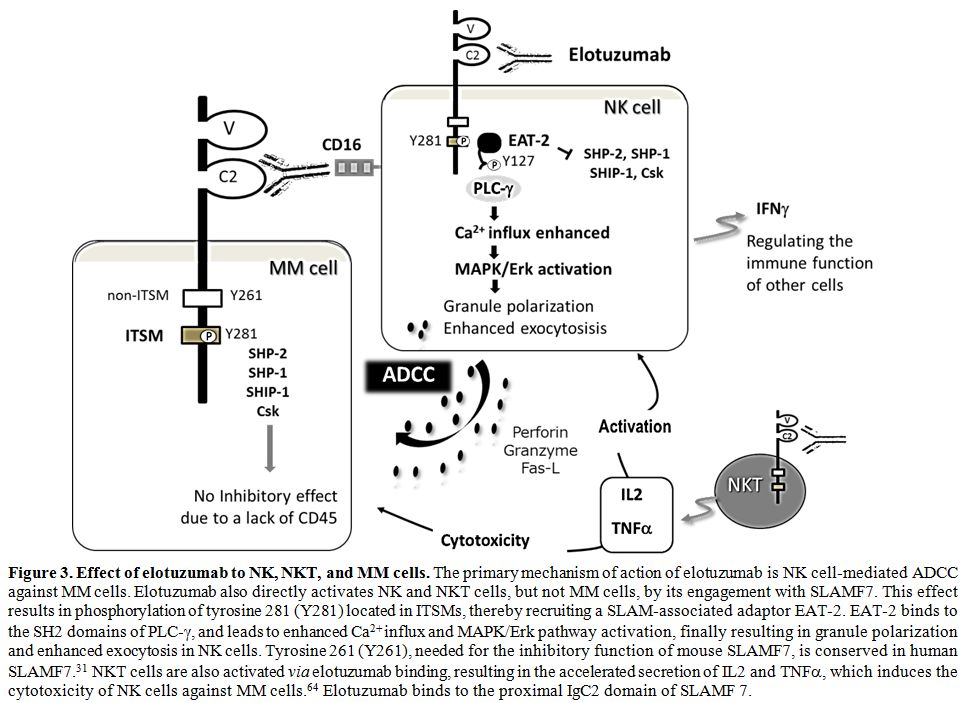 |
Figure 3. Effect of elotuzumab to NK, NKT, and MM cells. The
primary mechanism of action of elotuzumab is NK cell-mediated ADCC
against MM cells. Elotuzumab also directly activates NK and NKT cells,
but not MM cells, by its engagement with SLAMF7. This effect results in
phosphorylation of tyrosine 281 (Y281) located in ITSMs, thereby
recruiting a SLAM-associated adaptor EAT-2. EAT-2 binds to the SH2
domains of PLC-γ, and leads to enhanced Ca2+ influx and MAPK/Erk
pathway activation, finally resulting in granule polarization and
enhanced exocytosis in NK cells. Tyrosine 261 (Y261), needed for the
inhibitory function of mouse SLAMF7, is conserved in human SLAMF7.[31]
NKT cells are also activated via elotuzumab binding, resulting in the
accelerated secretion of IL2 and TNFα, which induces the cytotoxicity of NK cells against MM cells.[64] Elotuzumab binds to the proximal IgC2 domain of SLAMF 7. |
The
SAP gene located at Xq25 was identified as the causative gene altered
in X-linked lymphoproliferative syndrome (XLP).[46,47] Germline
mutations or deletions in SAP have been implicated in XLP, resulting in
aberrant functions of SLAMF1.[48,49] Aberrant functions of SLAMF1, 2,
and 6 caused by SAP mutations result in extreme sensitivity to EBV
infection in patients with XLP. EBV-specific cytotoxic CD8+ T cells in
XLP exhibit defects in the cytolysis of EBV-infected B cells. They
escape an apoptotic death, which results in the uncontrolled
proliferation of B cells and T cells, thereby causing fulminant
infectious mononucleosis (60%), lymphomas (30%), and
dysgammaglobulinemia (30%).[48,50]
Expression of SLAMF7 in Normal Cells, MM, and other Hematological Malignancies
Expression of SLAMF7 in normal cells and MM cells:
SLAMF7 is expressed on NK cells, NKT cells, a subset of cytotoxic
T-lymphocytes (CTLs) including CD8+ and CD4+ cells, mature dendritic
cells (DCs), and activated B cells, regulating T- and B-cell functions.
(Table 2).[27,31-33,51,52]
Normal plasma cells also highly express SLAMF7 at the mRNA and protein
levels.[13,14] SLAMF7 is not expressed in resting B cells, monocytes,
granulocytes, or hematopoietic stem cells.[13,14,36] On the other hand,
SLAMF7 is highly expressed in neoplastic plasma cells from more than
95% of patients with MM, plasmacytoma[13,14] and plasma cell leukemia
(PCL). It is also expressed in CD138 purified plasma cells from
patients with monoclonal gammopathy of undetermined significance (MGUS)
and smoldering MM (SMM).[14] There have been no studies describing the
higher expression of SLAMF7 in MM than in normal plasma cells. Soluble
SLAMF7 (sSLMF7) lacking transmembrane and cytoplasmic domains was
detected in patients with MM, particularly at advanced stages, but not
in those with MGUS or healthy individuals.[14] The role of sSLMF7 in
myeloma cell pathophysiology remains to be elucidated.
 |
Table 2. Cytogenetic abnormalities valuable to predict prognosis of MM with candidate genes. |
Although
SLAMF7 expression level in MM cells were independent of the cytogenetic
subtypes of MM, one of the highest expression levels was found in
t(4;14)-positive MM.[13] A recent study demonstrated that the knockdown
of SLAMF7 induced cell cycle G1 arrest or apoptosis, and also reduced
colony formation in t(4;14) MM cells.[53] Overexpressed SLAMF7 in
t(4;14)-positive MM cell lines was down-regulated by MMSET shRNAs.[53]
These findings suggest a direct effect on the transcription of SLAMF7
by the MMSET protein. Although the mechanisms underlying the
upregulation in plasma cells and MM cells currently remain unclear, a
recent study demonstrated that SLAMF7 transcription was positively
regulated by Blimp-1 (B lymphocyte-induced maturation protein-1) in NK
cells and B cells.[54] Blimp-1 is a known transcriptional repressor in
macrophages, NK cells, B cells, T cells, and skin epithelial cells.
Plasma cell function is controlled by Blimp-1 through the regulation of
immunoglobulin secretion and the unfolded protein response.[55]
Expression of SLAMF7 in other hematological malignancies:
Most of B-cell lymphomas including various histological subtypes and
Hodgkin lymphoma do not express SLAMF7, as assessed by
immunohistochemistry (IHC). Neither acute myeloid leukemias nor
lymphoblastic leukemias express SLAMF7.[13] The SLAMF7 protein was
detected in 25% of peripheral T-cell lymphomas (PTCL) at a modest level
using IHC. PTCL is a heterogeneous disease, but generally shows the
CD4-positive phenotype. Using IHC, we identified various CD4+ Th
subsets (Th1, Th2, Th17, and Treg) as possible normal counterparts of
PTCL based on the expression of master regulators such as T-bet, GATA3,
BCL6, RORγt, and FOXP3.[56] These findings suggest that some functional
subsets of CD4+ T cells expressing SLAMF7 exist. Recent studies
demonstrated the clonal expansion of CD4+ CTLs expressing SLAMF7,
granzyme A, IL-1β, and TGF-β1, at inflamed tissue sites of IgG4-related
disease.[52] Although CD4+ CTLs may develop from naïve T (Th0) and
various Th subsets, Th1 cells regulated by T-bet represent the majority
of CD4+ CTLs secreting IFN-γ
.[57]
CD4+ CTLs have been detected among peripheral blood lymphocytes under
conditions of chronic viral infections and during antitumor
responses.[58,59]
Dual Immunotherapeutic Mechanism of Elotuzumab
Elotuzumab induces NK cell-mediated ADCC: Elotuzumab is a humanized immunoglobulin G1 kappa (IgG1k)
monoclonal antibody, that binds a unique epitope on the IgC2 domain of
SLAMF7.[13,14] Human IgG1 elicits ADCC and complement-dependent
cytotoxicity (CDC) activities. However, elotuzumab and the novel
anti-SLAMF7 mAb PDL241 did not mediate CDC.[60,61] Elotuzumab-induced
ADCC is mediated through the engagement of its Fc portion with Fcγ
RIIIa/CD16
on NK cells.[14,61] On the other hand, elotuzumab is unable to directly
suppress the growth of MM cells. In MM cells lacking EAT-2, inhibitory
molecules including SHP-2, SHP-1, SHIP-1, and Csk are recruited to the
phosphorylated ITSMs of SLAMF7.[62] However, inhibitory effects are not
induced in MM cells, partly due to a lack of CD45. Elotuzumab also does
not induce the proliferation of myeloma cells (Figure 3).[45,62]
Preclinical
studies demonstrated that elotuzumab strongly induced cytotoxicity in
established MM cell lines and primary samples including
bortezomib-resistant MM cells when incubated with peripheral blood
mononuclear cells (PBMCs) or purified NK cells.[63] This anti-myeloma
effect of elotuzumab was prevented when CD16 was inhibited.[64]
Elotuzumab alone does not affect the viability of MM cells without
PBMCs or purified NK cells in vitro. SLAMF7 may also potentiate
interactions between NK and target MM cells through its homotypic
engagement recognizing the distal epitope IgV.[65] NK cells activated
by elotuzumab do not show cytotoxicity against autologous NK cells.[14]
In mice, the interaction between NK cells by the SLAMF7 engagement may
enhance their function.[36]
Elotuzumab directly stimulates NK cells:
Elotuzumab directly enhances the cytotoxic activity of NK cells in
addition to primarily inducing ADCC against MM cells, giving rise to a
dual immunotherapeutic mechanism of action.[13,14,63] NK cell
activation is mediated by the SLAMF adaptor proteins EAT-2 and SAP, the
cooperated expression of which promotes the cytotoxic activity of NK
cells. NK cell cytotoxicity is also dependent on PLCγ
1 and PLCγ
2.[66]
SAP promotes and stabilizes adhesion between NK cells and target cells
in a dual manner: one is by the coupling of SLAMF receptors to the
protein tyrosine kinase Fyn, and the other is by preventing SLAMF
receptors from coupling inhibitory signals involving SHIP and
SHP-1.[67,68] On the other hand, EAT-2 does not enhance adhesion
between NK and target cells, but controls NK cell function through PLCγ
, Ca2+
fluxes, and the MAPK/Erk pathway, leading to granule polarization and
the exocytosis of cytotoxic granules toward target cells (Figure 3).[45] NKT cells are also activated via elotuzumab binding, resulting in the accelerated secretion of IL2 and TNFα, which induces the cytotoxicity of NK cells against MM cells (Figure 3).[64] While most SLAMF receptors bind SAP and EAT-2,[35] SLAMF7 is functionally controlled by EAT-2, not SAP.[34,35]
A previous study showed that lenalidomide augmented elotuzumab-induced ADCC against MM cells in vitro.[14,64]
The enhanced NK cell function was associated with the up-regulation of
IL-2Rα expression, IL-2 production by CD3+CD56+ lymphocytes including
NKT cells, and TNFα production.[64] Augmentations in NK-cell cytotoxic
activity were also demonstrated with pomalidomide.[69,70] Low-dose
bortezomib[71] and carfilzomib[72] also augmented NK-cell cytotoxic
activity against MM cells. This effect was associated with the enhanced
expression of the activating or co-activating molecules of NK cells
including MHC class I polypeptide-related sequence A (MICA), NKG2D, and
DNAM-1 ligands (PVR and Nectin-2). These findings may provide the
rationale for combining these agents with elotuzumab. However, further
studies are needed in order to delineate which and how immune cells
other than NK cells are modulated in their function by elotuzumab.
Quantity and quality of NK cells in MM:
The quantity and quality of effector cells including NK cells are
essential for ADCC activity. Peripheral blood (PB) NK cell counts from
MM patients increased or showed no changes in the earlier stages and
decreased in the advanced stages.[73-75] Patients with MGUS also showed
no changes in PB NK cell counts form those of the controls.[74,76,77]
On the other hand, NK cell counts in bone marrow (BM) from MM patients
were reported to increase.[73,78] However, the functions of NK cells
differ among their subsets. CD56brightCD16-/dim NK cells are mainly responsible for the production of cytokines, while CD56dimCD16+ NK cells are mainly responsible for cytotoxic activities.[75] CD16+ subsets were decreased in MM patients.[79]
Regarding
the quality of NK cells in MM patients, previous studies suggested that
they were dysfunctional and showed decreased or no cytotoxicity in
advanced MM, while they remained functional in MGUS.[79-83] NK cell
dysfunction is often associated with the down-regulated expression of
activating molecules including natural cytotoxicity receptors, NKG2D,
and SLAMF4 (2B4) in BM NK-cells.[84] Other studies also demonstrated
the down-regulated expression of SLAMF4 and DNAM-1 in NK cells, and
this was associated with a reduction in NK cell cytotoxicity against
MM.[83,85] MM cells escape NK cell cytotoxicity due to the lack of a
HLA Class I loss, the shedding of surface MICA, and circulating MICA,
which result in the down-regulation of NKG2D. NK cells from MM patients
also express programmed death protein 1 (PD-1), which results in escape
from immune surveillance.[86,87] In mouse tumor models, an anti-PD-1
antibody enhances elotuzumab efficacy due to the production of
tumor-infiltrating NK and CD8+ T cell activity.[88] These findings may
provide the rationale for combination therapy of elotuzumab and PD-1
blockade.
Response to elotuzumab and the polymorphism of Fcγ
RIIIa/CD16: The Fcγ
RIIIa/CD16
genotype may provide some guidance for the administration of elotuzumab
to patients who are expected to have a favorable response. Since the
allelic variation affects the affinity of Fcγ
RIIIa
for IgG1 antibodies, differential responses to mAb have been reported
to correlate with specific polymorphisms.[89,90] The presence of a
valine (V) at position 158 of Fcγ
RIIIa
is associated with high-affinity to the Fc portion of IgG1 mAb, in
contrast to phenylalanine (F) with low affinity. The high-affinity “VV”
genotype of Fcγ
RIIIa has been associated with enhanced ADCC in rituximab treatments for patients with follicular lymphoma.[91,92]
In a randomized phase II study of EBd versus Bd for RRMM, patients homozygous for the high-affinity Fcγ
RIIIa V allele (VV) showed longer survival than those who were homozygous for the low-affinity Fcγ
RIIIa
F allele (FF).[24] A subanalysis of PFS by the CD16a genotype showed no
significant difference between VV and FF in ELOQUENT-2. A difference
was noted between VV/VF and FF in the study of elotuzumab monotherapy,
although the interpretation of this finding is limited by the small
number of patients with each genotype.[93] The incidence of the
high-affinity VV allele is 59% in the Japanese population versus 17% in
the populations of Western countries.[24,94] In Japanese patients, the
genetic Fcγ
RIIIa-V158F polymorphism may have a significant impact on myeloma cell killing by ADCC.
Optimal use of ELd for the Treatment of RRMM
Three
factors need to be considered in order to achieve better outcomes using
ELd: risk of the disease, frailty of the patients, and the quantity and
quality of effector cells. Prior to introducing elotuzumab, many
patients were treated with lenalidomide-based regimens until disease
progression as first-line therapy, and were lenalidomide refractory at
the time of first relapse. Since elotuzumab is approved in combination
with Ld for the treatment of RRMM, there are two possible conditions
under which to administer elotuzumab: starting ELd as second-line later
treatment or adding elotuzumab to Ld ongoing as first-line or later
treatment. In the case of second-line or later treatment, patients with
PR, VGPR, or CR using Ld may be the ideal candidates for the addition
of elotuzumab. This is because PFS by tumor responses between the ELd
and Ld groups was significantly better in patients who achieved PR or
better than in patients with a minor response or SD in ELOQUENT-2.[8]
According
to the ELOQUENT-2 study, elotuzumab is beneficial for patients with
high-risk CA including del(17p), 1q21 gain/amplification, and
particularly t(4;14). A direct effect on SLAMF7 transcription by the
MMSET protein has provided the rationale to use elotuzumab for
t(4;14)-positive MM patients.[53] Secondary CA may impact adversely on
treatment outcomes and survival in both NDMM and RRMM regardless of the
primary high-risk CA (Table 3).
For example, t(11;14) is not necessarily associated with a good, but
with a poor prognosis when identified concomitantly with a high-risk
secondary CA, such as 1q21 gain/amplification and del(17p) (Figure 4).[95]
In the novel agent era, chromosomal rearrangements at 8q24 is also
high-risk CA.[96,97] We previously detected 8q24 rearrangements
involving MYC or PVT1 (plasmacytoma variant translocation 1) loci in 24% of patients with MM.[98]
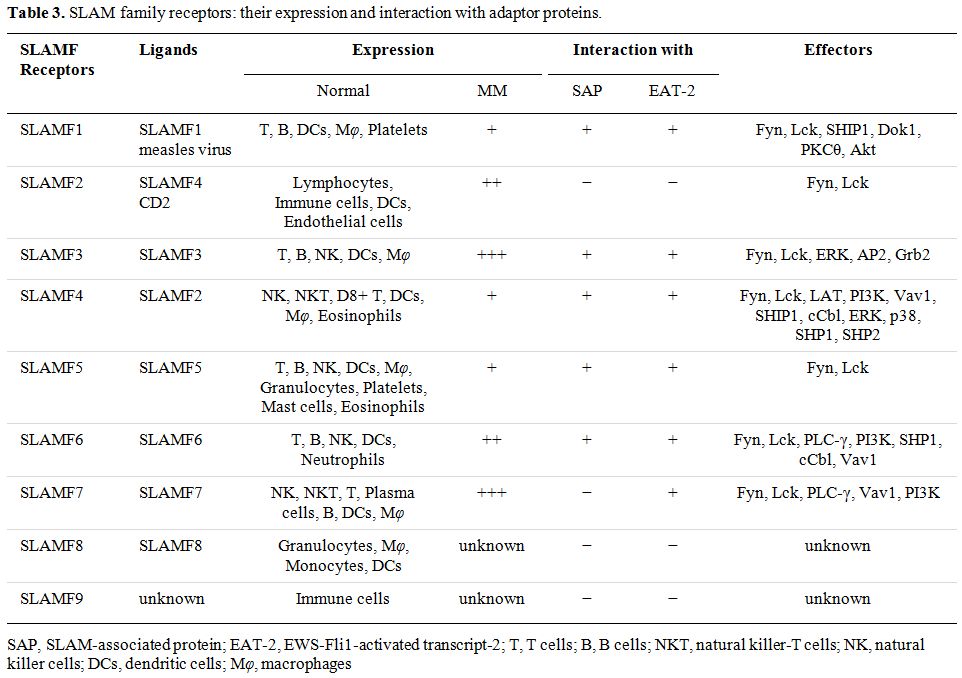 |
Table 3.
SLAM family receptors: their expression and interaction with adaptor proteins. |
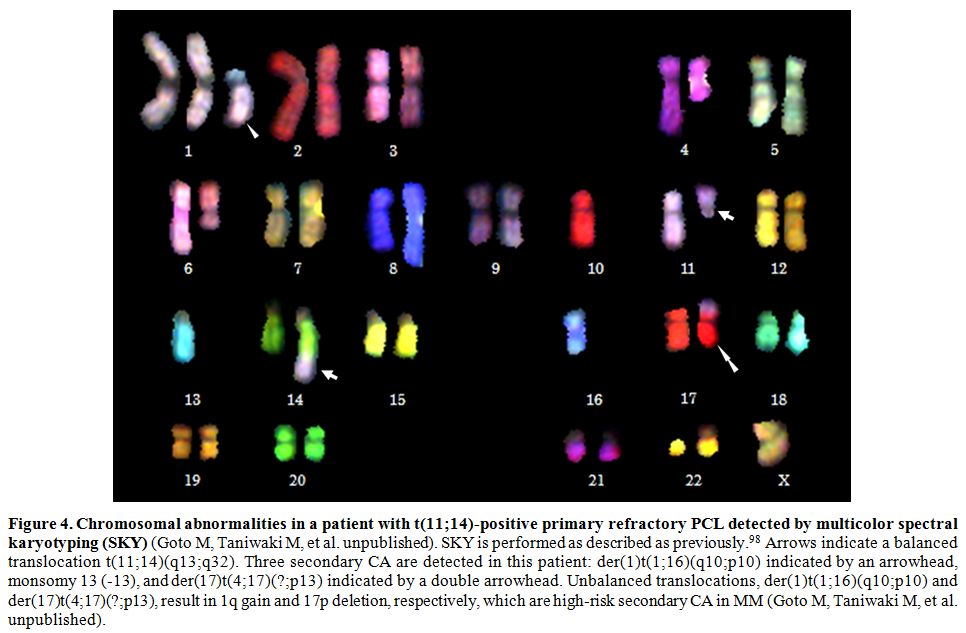 |
Figure 4. Chromosomal
abnormalities in a patient with t(11;14)-positive primary refractory
PCL detected by multicolor spectral karyotyping (SKY) (Goto M,
Taniwaki M, et al. unpublished). SKY is performed as described as
previously.[98] Arrows indicate a balanced translocation
t(11;14)(q13;q32). Three secondary CA are detected in this patient:
der(1)t(1;16)(q10;p10) indicated by an arrowhead, monsomy 13 (-13), and
der(17)t(4;17)(?;p13) indicated by a double arrowhead. Unbalanced
translocations, der(1)t(1;16)(q10;p10) and der(17)t(4;17)(?;p13),
result in 1q gain and 17p deletion, respectively, which are high-risk
secondary CA in MM (Goto M, Taniwaki M, et al. unpublished). |
Taking
the modes of action of elotuzumab into consideration, the counts and
functions of immune cells, particularly NK cells are crucial as already
mentioned. In this regard, the findings of Phase II and III trails in
patients with SMM have been encouraging. Elotuzumab monotherapy may
delay progression to MM in patients with SMM, resulting in favorable
PFS, because most patients achieved the best overall response of SD or
MR, with ≥MR in 29% including PR in 10%.[99] Early treatments with Ld
in patients with high-risk SMM provided a significant benefit over
observations in terms of time to progression.[100] Since elotuzumab is
well tolerated with minimal toxicity, elderly or frail patients who are
ineligible for PI/MiD-based triplet therapy or transplantation are
suitable candidates for ELd treatment. Moreover, the addition of
elotuzumab to bortezomib, lenalidomide, and dexamethasone (LBd) is
feasible without major additive AEs beyond what is already known about
LBd, as demonstrated in SWOGS1211 trial.[101] However, the efficacy of
elotuzumab in combination with LBd needs to be studied.
Conclusions
A
number of molecular targeting agents are currently available for MM;
therefore, risk stratification and frailty assessments are critical for
their optimal combination. Secondary CA are effective biomarkers, and
more than 50% of patients are unfit because they are older than 75
years. However, even with the use of novel agents, MM remains incurable
with recurrence and refractoriness to treatment, and frequently
develops extramedullary disease and secondary plasma cell leukemia
(sPCL) at the end stages of the disease. Although a number of clinical
trials have attempted to achieve high tumor responses in RRMM using
novel triplet therapy with second- and third-generation PIs and IMiDs,
difficulties are associated with successfully treating extramedullary
lesions and sPCL. Therefore, it is important not only to develop
treatments with high tumor responses, but also to have early
therapeutic interventions for MM. Moreover, 30-50% of MM patients are
transplant-ineligible or unable to receive PI/IMiDs based triplets
therapy.[102,103] Hence, elotuzumab is promising and beneficial for the
treatment of frail patients with MM.
The mechanisms of action of
elotuzumab and the functional role of SLAMF7 in relation to
pathophysiology of MM remain unclear. For example, what the signal
transduction pathway of engaged SLAMF7 in MM cells is involved in is
unknown, and which or how immune cells other than NK cells are
implicated in killing MM cells has yet to be elucidated by elotuzumab.
It will be beneficial for patients with RRMM to clarify whether
elotuzumab has a marked impact on the recovery of immune paralysis in
combination with other novel molecular targeting agents such as
carfilzomib and pomalidomide. In order to address these questions,
basic research is conducted to investigate the molecular mechanisms
involving SLAMF receptors and SAP-related adaptors with their
downstream molecules in the signal transduction pathway.
The
efficacy of ELd with minimal toxicity and the paralytic immune state in
advanced MM may bring forward for consideration of early therapeutic
intervention in patients with SMM. However, studies are needed in order
to clarify whether ELd is effective for patients with SMM.
Acknowledgments
This
work was supported in part by a Grant-in-Aid for Scientific Research
from The Ministry of Education, Culture, Sports, Science and Technology
of Japan (MEXT KAKENHI 16K09856) (MT); by the National Cancer Center
Research and Development Fund (29-A-3); by a grant (Practical Research
for Innovative Cancer Control) from the Japan Agency for Medical
Research and Development (AMED) (17ck0106348h0001); and by the Takeda
Science Foundation and Astra Zeneca (JK).
.
References
- Cancer Stat Facts: Myeloma. National Cancer
Institute, Surveillance, Epidemiology, and End Results Program.
Accessed 25 December 2017 https://seer.cancer.gov/statfacts/html/mulmy.html
- Ferlay
J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW,
Comber H, Forman D, Bray F (2013) Cancer incidence and mortality
patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer
49:1374-403. https://doi.org/10.1016/j.ejca.2012.12.027

- Center for Cancer Control and Information Service, National Cancer Center, Japan. 25 December 2017 https://ganjoho.jp/reg_stat/statistics/stat/summary.html
- Kumar
SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK,
Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz
MA. Improved survival in multiple myeloma and the impact of novel
therapies. Blood. 2008;111:2516-20 https://doi.org/10.1182/blood-2007-10-116129 PMid:17975015 PMCid:PMC2254544

- Kumar
SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P,
Dingli D, Hayman SR, Leung N, Lust J, McCurdy A, Russell SJ, Zeldenrust
SR, Kyle RA, Rajkumar SV. Continued improvement in survival in multiple
myeloma: changes in early mortality and outcomes in older patients.
Leukemia. 2014;28:1122-28 https://doi.org/10.1038/leu.2013.313 PMid:24157580 PMCid:PMC4000285

- Benboubker
L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, Pinto A,
Weisel K, Ludwig H, Bahlis N, Banos A, Tiab M, Delforge M, Cavenagh J,
Geraldes C, Lee JJ, Chen C, Oriol A, de la Rubia J, Qiu L, White DJ,
Binder D, Anderson K, Fermand JP, Moreau P, Attal M, Knight R, Chen G,
Van Oostendorp J, Jacques C, Ervin-Haynes A, Avet-Loiseau H, Hulin C,
Facon T; FIRST Trial Team. Lenalidomide and dexamethasone in
transplant-ineligible patients with myeloma. N Engl J Med.
2014;371:906-17 https://doi.org/10.1056/NEJMoa1402551 PMid:25184863

- Palumbo
A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I, Nagler A,
Petrucci MT, Hajek R, Pezzatti S, Delforge M, Patriarca F, Donato F,
Cerrato C, Nozzoli C, Yu Z, Boccadifuoco L, Caravita T, Benevolo G,
Guglielmelli T, Vincelli D, Jacques C, Dimopoulos MA, Ciccone G, Musto
P, Corradini P, Cavo M, Boccadoro M. Continuous therapy versus fixed
duration of therapy in patients with newly diagnosed multiple myeloma.
J Clin Oncol. 2015;33:3459-66.

- Lonial
S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I,
Walter-Croneck A, Moreau P, Mateos MV, Magen H, Belch A, Reece D,
Beksac M, Spencer A, Oakervee H, Orlowski RZ, Taniwaki M, Röllig C,
Einsele H, Wu KL, Singhal A, San-Miguel J, Matsumoto M, Katz J,
Bleickardt E, Poulart V, Anderson KC, Richardson P; ELOQUENT-2
Investigators. Elotuzumab Therapy for relapsed or refractory multiple
myeloma. N Engl J Med. 2015;373:621-31 https://doi.org/10.1056/NEJMoa1505654 PMid:26035255

- Palumbo
A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I,
Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin
X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P; CASTOR Investigators.
Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl
J Med. 2016;375:754-66 https://doi.org/10.1056/NEJMoa1606038 PMid:27557302

- Dimopoulos
MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N,
Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoon SS, Ben Yehuda D,
Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O'Rourke L,
Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P; POLLUX Investigators.
Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N
Engl J Med. 2016;375:1319-31 https://doi.org/10.1056/NEJMoa1607751 PMid:27705267

- Chari
A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ,
Weiss BM, Krishnan A, Lentzsch S, Comenzo R, Wang J, Nottage K, Chiu C,
Khokhar NZ, Ahmadi T, Lonial S. Daratumumab plus pomalidomide and
dexamethasone in relapsed and/or refractory multiple myeloma. Blood.
2017;130:974-81 https://doi.org/10.1182/blood-2017-05-785246 PMid:28637662 PMCid:PMC5570682

- Badros
A, Hyjek E, Ma N, Lesokhin A, Dogan A, Rapoport AP, Kocoglu M, Lederer
E, Philip S, Milliron T, Dell C, Goloubeva O, Singh Z. Pembrolizumab,
pomalidomide, and low-dose dexamethasone for relapsed/refractory
multiple myeloma. Blood. 2017;130:1189-97 https://doi.org/10.1182/blood-2017-03-775122 PMid:28461396

- Hsi
ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M,
Powers D, Nanisetti A, Zhang Y, Rice AG, van Abbema A, Wong M, Liu G,
Zhan F, Dillon M, Chen S, Rhodes S, Fuh F, Tsurushita N, Kumar S,
Vexler V, Shaughnessy JD Jr, Barlogie B, van Rhee F, Hussein M, Afar
DE, Williams MB. CS1, a potential new therapeutic antibody target for
the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775-84 https://doi.org/10.1158/1078-0432.CCR-07-4246 PMid:18451245 PMCid:PMC4433038

- Tai
Y-T, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K,
Hideshima T, Rice AG, van Abbema A, Jesaitis L, Caras I, Law D, Weller
E, Xie W, Richardson P, Munshi NC, Mathiot C, Avet-Loiseau H, Afar DE,
Anderson KC. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits
myeloma cell adhesion and induces antibody-dependent cellular
cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329-37 https://doi.org/10.1182/blood-2007-08-107292 PMid:17906076 PMCid:PMC2515112

- Iida
S, Tobinai K, Taniwaki M, Shumiya Y, Nakamura T, Chou T. Phase I dose
escalation study of high dose carfilzomib monotherapy for Japanese
patients with relapsed or refractory multiple myeloma. Int J Hematol.
2016;104:596-604 https://doi.org/10.1007/s12185-016-2070-7 PMid:27460677

- Suzuki
K, Sunami K, Ohashi K, Iida S, Mori T, Handa H, Matsue K, Miyoshi M,
Bleickardt E, Matsumoto M, Taniwaki M. Randomized phase 3 study of
elotuzumab for relapsed or refractory multiple myeloma: ELOQUENT-2
Japanese patient subanalysis. Blood Cancer J. 2017;7(3):e540. https://doi.org/10.1038/bcj.2017.18 PMid:28282035 PMCid:PMC5380903

- Dimopoulos
MA, Lonial S, White D, Moreau P, Palumbo A, San-Miguel J, Shpilberg O,
Anderson K, Grosicki S, Spicka I, Walter-Croneck A, Magen H, Mateos MV,
Belch A, Reece D, Beksac M, Bleickardt E, Poulart V, Sheng J, Sy O,
Katz J, Singhal A, Richardson P. Elotuzumab plus
lenalidomide/dexamethasone for relapsed or refractory multiple myeloma:
ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival
and tumour growth. Br J Haematol. 2017;178:896-905 https://doi.org/10.1111/bjh.14787 PMid:28677826

- Dimopoulos
MA, Lonial S, White D, Moreau P, Mateos MV, San-Miguel J, Anderson KC,
Shpilberg O, Grosicki S, Spicka I, Walter-Croneck A, Magen H, Belch A,
Reece DE, Beksac M, Mekan S, Sy O, Anil K. Singhal AK, Richardson PG ,
Weisel K (Abstract release date: May 18, 2017). Phase 3 ELOQUENT-2
Study: Extended 4-year follow-up of elotuzumab plus
lenalidomide/dexamethasone vs lenalidomide/dexamethasone in
relapsed/refractory multiple myeloma. EHA Learning Center. Dimopoulos
M. Jun 24, 2017; 181743 https://learningcenter.ehaweb.org/eha/2017/22nd/181743/meletios.a.dimopoulos.phase.3.eloquent-2.study.extended.4-year.follow-up.of.html
- Zonder
JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, Fry J,
Afar DE, Singhal AK. A phase 1, multicenter, open-label, dose
escalation study of elotuzumab in patients with advanced multiple
myeloma. Blood. 2012;120:552-9 https://doi.org/10.1182/blood-2011-06-360552 PMid:22184404 PMCid:PMC4467882

- Jakubowiak
AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A,
Richardson PG, Afar DE, Singhal AK, Anderson KC. Phase I trial of
anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib
in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol.
2012;30:1960-5 https://doi.org/10.1200/JCO.2011.37.7069 PMid:22291084 PMCid:PMC4874204

- Lonial
S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, Kaufman JL,
Leleu X, Tsao LC, Westland C, Singhal AK, Jagannath S. Elotuzumab in
combination with lenalidomide and low-dose dexamethasone in relapsed or
refractory multiple myeloma. J Clin Oncol. 2012;30:1953-9 https://doi.org/10.1200/JCO.2011.37.2649 PMid:22547589

- Richardson
PG, Jagannath S, Moreau P, Jakubowiak AJ, Raab MS, Facon T, Vij R,
White D, Reece DE, Benboubker L, Zonder J, Tsao LC, Anderson KC,
Bleickardt E, Singhal AK, Lonial S; 1703 study investigators.
Elotuzumab in combination with lenalidomide and dexamethasone in
patients with relapsed multiple myeloma: final phase 2 results from the
randomised, open-label, phase 1b-2 dose-escalation study. Lancet
Haematol. 2015;2(12):e516–e27 https://doi.org/10.1016/S2352-3026(15)00197-0

- Berenson
J, Manges R, Badarinath S, Cartmell A, McIntyre K, Lyons R, Harb W,
Mohamed H, Nourbakhsh A, Rifkin R. A phase 2 safety study of
accelerated elotuzumab infusion, over less than 1 h, in combination
with lenalidomide and dexamethasone, in patients with multiple myeloma.
Am J Hematol. 2017;92:460-6 https://doi.org/10.1002/ajh.24687 PMid:28213943

- Jakubowiak
A, Offidani M, Pégourie B, De La Rubia J, Garderet L, Laribi K, Bosi A,
Marasca R, Laubach J, Mohrbacher A, Carella AM, Singhal AK, Tsao LC,
Lynch M, Bleickardt E, Jou YM, Robbins M, Palumbo A. Randomized phase 2
study: elotuzumab plus bortezomib/dexamethasone vs
bortezomib/dexamethasone for relapsed/refractory MM. Blood.
2016;127:2833-40 https://doi.org/10.1182/blood-2016-01-694604 PMid:27091875 PMCid:PMC4900953

- Mateos
MV, Granell M, Oriol A, Martinez-Lopez J, Blade J, Hernandez MT, Martín
J, Gironella M, Lynch M, Bleickardt E, Paliwal P, Singhal A, San-Miguel
J. Elotuzumab in combination with thalidomide and low-dose
dexamethasone: a phase 2 single-arm safety study in patients with
relapsed/refractory multiple myeloma. Br J Haematol. 2016;175:448-56 https://doi.org/10.1111/bjh.14263 PMid:27434748

- Boles
KS, Mathew PA. Molecular cloning of CS1, a novel human natural killer
cell receptor belonging to the CD2 subset of the immunoglobulin
superfamily. Immunogenetics. 2001;52:302-7 https://doi.org/10.1007/s002510000274

- Lee
JK, Mathew SO, Vaidya SV, Kumaresan PR, Mathew PA. CS1 (CRACC, CD319)
induces proliferation and autocrine cytokine expression on human B
lymphocytes. J Immunol. 2007;179:4672-8 https://doi.org/10.4049/jimmunol.179.7.4672 PMid:17878365

- Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665-705 https://doi.org/10.1146/annurev-immunol-030409-101302 PMid:21219180

- Boles
KS, Stepp SE, Bennett M, Kumar V, Mathew PA. 2B4 (CD244) and CS1: novel
members of the CD2 subset of the immunoglobulin superfamily molecules
expressed on natural killer cells and other leukocytes. Immunol Rev.
2001;181:234-49 https://doi.org/10.1034/j.1600-065X.2001.1810120.x PMid:11513145

- Schwartzberg
PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence
lymphocyte interactions, development and function. Nat Rev Immunol.
2009;9:39-46 https://doi.org/10.1038/nri2456 PMid:19079134

- Veillette
A, Guo H. CS1, a SLAM family receptor involved in immune regulation, is
a therapeutic target in multiple myeloma. Crit Rev Oncol Hematol.
2013;88:168-7 https://doi.org/10.1016/j.critrevonc.2013.04.003 PMid:23731618

- Veillette
A. SLAM-family receptors: immune regulators with or without SAP-family
adaptors. Cold Spring Harb Perspect Biol. 2010;2(3):a002469. https://doi.org/10.1101/cshperspect.a002469

- Martin
M, Romero X, de la Fuente MA, Tovar V, Zapater N, Esplugues E, Pizcueta
P, Bosch J, Engel P. CD84 functions as a homophilic adhesion molecule
and enhances IFN-gamma secretion: adhesion is mediated by Ig-like
domain 1. J Immunol. 2001;167:3668-76 https://doi.org/10.4049/jimmunol.167.7.3668 PMid:11564780

- Bouchon
A, Cella M, Grierson HL, Cohen JI, Colonna M. Activation of NK
cell-mediated cytotoxicity by a SAP-independent receptor of the CD2
family. J Immunol. 2001;167:5517-21 https://doi.org/10.4049/jimmunol.167.10.5517 PMid:11698418

- Detre
C, Keszei M, Romero X, Tsokos GC, Terhorst C. SLAM family receptors and
the SLAM-associated protein (SAP) modulate T cell functions. Semin
Immunopathol. 2010;32:157-71 https://doi.org/10.1007/s00281-009-0193-0 PMid:20146065 PMCid:PMC2868096

- Cruz-Munoz
ME, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM
family receptor coupled to the adaptor EAT-2, on natural killer cell
function. Nat Immunol. 2009;10:297-305 https://doi.org/10.1038/ni.1693 PMid:19151721

- Wilson
TJ, Garner LI, Metcalfe C, King E, Margraf S, Brown MH: Fine
specificity and molecular competition in SLAM family receptor
signaling. PLoS One. 2014;9:e92184. eCollection 2014

- Chen
J, Zhong MC, Guo H, Davidson D, Mishel S, Lu Y, Rhee I, Pérez-Quintero
LA, Zhang S, Cruz-Munoz ME, Wu N, Vinh DC, Sinha M, Calderon V, Lowell
CA, Danska JS, Veillette A. SLAMF7 is critical for phagocytosis of
haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544:493-7 https://doi.org/10.1038/nature22076 PMid:28424516 PMCid:PMC5565268

- Al-Alem
U, Li C, Forey N, Relouzat F, Fondanèche MC, Tavtigian SV, Wang ZQ,
Latour S, Yin L. Impaired Ig class switch in mice deficient for the
X-linked lymphoproliferative disease gene Sap. Blood. 2005;106:2069-75 https://doi.org/10.1182/blood-2004-07-2731 PMid:15941917

- Kis
LL, Nagy N, Klein G, Klein E. Expression of SH2D1A in five classical
Hodgkin's disease-derived cell lines. Int J Cancer. 2003;104:658-61 https://doi.org/10.1002/ijc.10986 PMid:12594824

- Roncador
G, García Verdes-Montenegro JF, Tedoldi S, Paterson JC, Klapper W,
Ballabio E, Maestre L, Pileri S, Hansmann ML, Piris MA, Mason DY,
Marafioti T. Expression of two markers of germinal center T cells (SAP
and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica.
2007;92:1059-66 https://doi.org/10.3324/haematol.10864 PMid:17640856

- Morra
M, Lu J, Poy F, Martin M, Sayos J, Calpe S, Gullo C, Howie D, Rietdijk
S, Thompson A, Coyle AJ, Denny C, Yaffe MB, Engel P, Eck MJ, Terhorst
C. Structural basis for the interaction of the free SH2 domain EAT-2
with SLAM receptors in hematopoietic cells. EMBO J. 2001;20:5840-52 https://doi.org/10.1093/emboj/20.21.5840 PMid:11689425 PMCid:PMC125701

- Calpe
S, Erdos E, Liao G, Wang N, Rietdijk S, Simarro M, Scholtz B, Mooney J,
Lee CH, Shin MS, Rajnavölgyi E, Schatzle J, Morse HC 3rd, Terhorst C,
Lanyi A. Identification and characterization of two related murine
genes, Eat2a and Eat2b, encoding single SH2-domain adapters.
Immunogenetics. 2006;58:15-25 https://doi.org/10.1007/s00251-005-0056-3 PMid:16425036

- Chan
B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D,
Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors.
Nat Cell Biol. 2003;5:155-60 https://doi.org/10.1038/ncb920 PMid:12545174

- Pérez-Quintero
LA, Roncagalli R, Guo H, Latour S, Davidson D, Veillette A. EAT-2, a
SAP-like adaptor, controls NK cell activation through phospholipase C?,
Ca++, and Erk, leading to granule polarization. J Exp Med.
2014;211:727-42 https://doi.org/10.1084/jem.20132038 PMid:24687958 PMCid:PMC3978279

- offey
AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP,
Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E,
Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini
P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR,
Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH,
Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT,
Meindl A, Bentley DR. Host response to EBV infection in X-linked
lymphoproliferative disease results from mutations in an SH2-domain
encoding gene. Nat Genet. 1998;20:129-35 https://doi.org/10.1038/2424 PMid:9771704

- Sayos
J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo
L, Geha R, Roncarolo MG, Oettgen H, De Vries JE, Aversa G, Terhorst C.
The X-linked lymphoproliferative-disease gene product SAP regulates
signals induced through the co-receptor SLAM. Nature. 1998;395:462-9
https://doi.org/10.1038/26683 PMid:9774102

- Wu
C, Sayos J, Wang N, Howie D, Coyle A, Terhorst C. Genomic organization
and characterization of mouse SAP, the gene that is altered in X-linked
lymphoproliferative disease. Immunogenetics. 2000;51:805-15
https://doi.org/10.1007/s002510000215 PMid:10970095

- Calpe
S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, Terhorst C. The SLAM
and SAP gene families control innate and adaptive immune responses. Adv
Immunol. 2008;97:177-250 https://doi.org/10.1016/S0065-2776(08)00004-7

- Nelson
DL, Terhorst C. X–linked lymphoproliferative syndrome. Clin Exp
Immunol. 2000;122:291-5
https://doi.org/10.1046/j.1365-2249.2000.01400.x PMCid:PMC1905809

- Veillette
A. NK cell regulation by SLAM family receptors and SAP-related
adapters. Immunol Rev. 2006;214:22-34
https://doi.org/10.1111/j.1600-065X.2006.00453.x
PMid:17100873

- Mattoo
H, Mahajan VS, Maehara T, Deshpande V, Della-Torre E, Wallace ZS,
Kulikova M, Drijvers JM, Daccache J, Carruthers MN, Castelino FV, Stone
JR, Stone JH, Pillai S. Clonal expansion of CD4(+) cytotoxic T
lymphocytes in patients with IgG4-related disease. J Allergy Clin
Immunol. 2016;138:825-38 https://doi.org/10.1016/j.jaci.2015.12.1330
PMid:26971690 PMCid:PMC5014627

- Xie
Z, Gunaratne J, Cheong LL, Liu SC, Koh TL, Huang G, Blackstock WP, Chng
WJ: Plasma membrane proteomics identifies biomarkers associated with
MMSET overexpression in T(4;14) multiple myeloma. Oncotarget.
2013;4:1008-18 https://doi.org/10.18632/oncotarget.1049 PMid:23900284
PMCid:PMC3759662

- Kim
JR, Mathew SO, Mathew PA. Blimp-1/PRDM1 regulates the transcription of
human CS1 (SLAMF7) gene in NK and B cells. Immunobiology. 2016;221:31-9
https://doi.org/10.1016/j.imbio.2015.08.005 PMid:26310579

- Tellier
J, Shi W, Minnich M, Liao Y, Crawford S, Smyth GK, Kallies A,
Busslinger M, Nutt SL. Blimp-1 controls plasma cell function through
the regulation of immunoglobulin secretion and the unfolded protein
response. Nat Immunol. 2016;17:323-30 https://doi.org/10.1038/ni.3348
PMid:26779600 PMCid:PMC4757736

- Matsumoto
Y, Horiike S, Ohshiro M, Yamamoto M, Sasaki N, Tsutsumi Y, Kobayashi T,
Shimizu D, Uchiyama H, Kuroda J, Nomura K, Shimazaki C, Taniwaki M.
Expression of master regulators of helper T-cell differentiation in
peripheral T-cell lymphoma, not otherwise specified, by
immunohistochemical analysis. Am J Clin Pathol. 2010;133:281-90
https://doi.org/10.1309/AJCP0SBHYVLY5EML PMid:20093238

- Takeuchi
A, Saito T. CD4 CTL, a Cytotoxic Subset of CD4+ T Cells, Their
Differentiation and Function. Front Immunol. 2017;8:194. doi:
10.3389/fimmu.2017.00194. eCollection 2017.
https://doi.org/10.3389/fimmu.2017.00194

- Xie
Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P,
Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells
differentiated in vivo eradicate established melanoma. J Exp Med.
2010;207:651-67 https://doi.org/10.1084/jem.20091921 PMid:20156973
PMCid:PMC2839147

- Quezada
SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R,
Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive
CD4(+) T cells develop cytotoxic activity and eradicate large
established melanoma after transfer into lymphopenic hosts. J Exp Med.
2010;207:637-50 https://doi.org/10.1084/jem.20091918 PMid:20156971
PMCid:PMC2839156

- Woo
J, Vierboom MP, Kwon H, Chao D, Ye S, Li J, Lin K, Tang I, Belmar NA,
Hartman T, Breedveld E, Vexler V, 't Hart BA, Law DA, Starling GC.
PDL241, a novel humanized monoclonal antibody, reveals CD319 as a
therapeutic target for rheumatoid arthritis. Arthritis Res Ther.
2013;15:R207. doi: 10.1186/ar4400. https://doi.org/10.1186/ar4400

- Collins
SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, Starling
GC, Ciarlariello D, Bhaskar S, Briercheck EL, Hughes T, Yu J, Rice A,
Benson DM Jr. Elotuzumab directly enhances NK cell cytotoxicity against
myeloma via CS1 ligation: evidence for augmented NK cell function
complementing ADCC. Cancer Immunol Immunother. 2013;62:1841-9
https://doi.org/10.1007/s00262-013-1493-8 PMid:24162108 PMCid:PMC4134870

- Guo
H, Cruz-Munoz M-E, Wu N, Robbins M, Veillette A. Immune cell inhibition
by SLAMF7 is mediated by a mechanism requiring src kinases, CD45, and
SHIP-1 that is defective in multiple myeloma cells. Mol Cell Biol.
2015;35:41-51 https://doi.org/10.1128/MCB.01107-14 PMid:25312647
PMCid:PMC4295394

- van
Rhee F, Szmania SM, Dillon M, van Abbema AM, Li X, Stone MK, Garg TK,
Shi J, Moreno-Bost AM, Yun R, Balasa B, Ganguly B, Chao D, Rice AG,
Zhan F, Shaughnessy JD Jr, Barlogie B, Yaccoby S, Afar DE.
Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab
(HuLuc63) and bortezomib against multiple myeloma. Molecular Cancer
Therapeutics. 2009;8: 2616-24
https://doi.org/10.1158/1535-7163.MCT-09-0483 PMid:19723891
PMCid:PMC2748787

- Balasa
B, Yun R, Belmar NA, Fox M, Chao DT, Robbins MD, Starling GC, Rice AG.
Elotuzumab enhances natural killer cell activation and myeloma cell
killing through interleukin-2 and TNF-a pathways. Cancer Immunol
Immunother. 2015;64:61-73 https://doi.org/10.1007/s00262-014-1610-3
PMid:25287778 PMCid:PMC4282702

- Dong
Z, Cruz-Munoz ME, Zhong MC, Chen R, Latour S, Veillette A. Essential
function for SAP family adaptors in the surveillance of hematopoietic
cells by natural killer cells. Nat Immunol. 2009;10:973-80
https://doi.org/10.1038/ni.1763 PMid:19648922

- Caraux
A, Kim N, Bell SE, Zompi S, Ranson T, Lesjean-Pottier S, Garcia-Ojeda
ME, Turner M, Colucci F. Phospholipase C-gamma2 is essential for NK
cell cytotoxicity and innate immunity to malignant and virally infected
cells. Blood. 2006;107:994-1002
https://doi.org/10.1182/blood-2005-06-2428 PMid:16204312

- Kageyama
R, Cannons JL, Zhao F, Yusuf I, Lao C, Locci M, Schwartzberg PL, Crotty
S. The receptor Ly108 functions as a SAP adaptor-dependent on-off
switch for T cell help to B cells and NKT cell development. Immunity.
2012;36:986-1002 https://doi.org/10.1016/j.immuni.2012.05.016
PMid:22683125 PMCid:PMC3389310

- Zhao
F, Cannons JL, Dutta M, Griffiths GM, Schwartzberg PL. Positive and
negative signaling through SLAM receptors regulate synapse organization
and thresholds of cytolysis. Immunity. 2012;36:1003-16.
https://doi.org/10.1016/j.immuni.2012.05.017 PMid:22683123
PMCid:PMC3389133

- Lagrue
K, Carisey A, Morgan DJ, Chopra R, Davis DM. Lenalidomide augments
actin remodeling and lowers NK-cell activation thresholds. Blood.
2015;126:50-60 https://doi.org/10.1182/blood-2015-01-625004
PMid:26002964 PMCid:PMC4551357

- Sehgal
K, Das R, Zhang L, Verma R, Deng Y, Kocoglu M, Vasquez J, Koduru S, Ren
Y, Wang M, Couto S, Breider M, Hansel D, Seropian S, Cooper D, Thakurta
A, Yao X, Dhodapkar KM, Dhodapkar MV. Clinical and pharmacodynamic
analysis of pomalidomide dosing strategies in myeloma: impact of immune
activation and cereblon targets. Blood.2015;125:4042-51
https://doi.org/10.1182/blood-2014-11-611426 PMid:25869284
PMCid:PMC4481593

- Niu
C, Jin H, Li M, Zhu S, Zhou L, Jin F, Zhou Y, Xu D, Xu J, Zhao L, Hao
S, Li W, Cui J. Low-dose bortezomib increases the expression of NKG2D
and DNAM-1 ligands and enhances induced NK and ?d T cell-mediated lysis
in multiple myeloma. Oncotarget. 2017;l8:5954-64

- Yang
G, Gao M, Zhang Y, Kong Y, Gao L, Tao Y, Han Y, Wu H, Meng X, Xu H,
Zhan F, Wu X, Shi J. Carfilzomib enhances natural killer cell-mediated
lysis of myeloma linked with decreasing expression of HLA class I.
Oncotarget. 2015;6:26982-94. https://doi.org/10.18632/oncotarget.4831

- García-Sanz
R, González M, Orfão A, Moro MJ, Hernández JM, Borrego D, Carnero M,
Casanova F, Bárez A, Jiménez R, Portero JA, San Miguel JF. Analysis of
natural killer-associated antigens in peripheral blood and bone marrow
of multiple myeloma patients and prognostic implications. Br J
Haematol. 1996;93:81-8
https://doi.org/10.1046/j.1365-2141.1996.4651006.x PMid:8611480

- Omedé
P, Boccadoro M, Gallone G, Frieri R, Battaglio S, Redoglia V, Pileri A.
Multiple myeloma: increased circulating lymphocytes carrying plasma
cell-associated antigens as an indicator of poor survival. Blood.
1990;76:1375-9 PMid:2119828

- Lopez-Verges
S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ,
Nixon DF, Lanier LL. CD57 defines a functionally distinct population of
mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood.
2010;116:3865-74 https://doi.org/10.1182/blood-2010-04-282301
PMid:20733159 PMCid:PMC2981540

- Tienhaara
A, Pelliniemi TT. Peripheral blood lymphocyte subsets in multiple
myeloma and monoclonal gammopathy of undetermined significance. Clin
Lab Haematol. 1994;16:213-23
https://doi.org/10.1111/j.1365-2257.1994.tb00414.x PMid:7828409

- Pessoa
de Magalhães RJ, Vidriales MB, Paiva B, Fernandez-Gimenez C,
García-Sanz R, Mateos MV, Gutierrez NC, Lecrevisse Q, Blanco JF,
Hernández J, de las Heras N, Martinez-Lopez J, Roig M, Costa ES, Ocio
EM, Perez-Andres M, Maiolino A, Nucci M, De La Rubia J, Lahuerta JJ,
San-Miguel JF, Orfao A; Spanish Myeloma Group (GEM); Grupo
Castellano-Leones de Gammapatias Monoclonales, cooperative study
groups. Analysis of the immune system of multiple myeloma patients
achieving long-term disease control by multidimensional flow cytometry.
Haematologica. 2013;98:79-86
https://doi.org/10.3324/haematol.2012.067272 PMid:22773604
PMCid:PMC3533663

- Pérez-Andres
M, Almeida J, Martin-Ayuso M, Moro MJ, Martin-Nu-ez G, Galende J,
Hernandez J, Mateo G, San Miguel JF, Orfao A; Spanish Network on
Multiple Myeloma; Spanish Network of Cancer Research Centers.
Characterization of bone marrow T cells in monoclonal gammopathy of
undetermined significance, multiple myeloma, and plasma cell leukemia
demonstrates increased infiltration by cytotoxic/Th1 T cells
demonstrating a squed TCR-Vbeta repertoire. Cancer. 2006;106: 1296-305
https://doi.org/10.1002/cncr.21746 PMid:16475149

- Jurisic
V, Srdic T, Konjevic G, Markovic O, Colovic M. Clinical stage-depending
decrease of NK cell activity in multiple myeloma patients. Med Oncol.
2007;24:312-7 https://doi.org/10.1007/s12032-007-0007-y PMid:17873307

- Dosani
T, Carlsten M, Maric I, Landgren O. The cellular immune system in
myelomagenesis: NK cells and T cells in the development of MM and their
uses in immunotherapies. Blood Cancer J. 2015;5:e321. doi:
10.1038/bcj.2015.49. https://doi.org/10.1038/bcj.2015.49

- Famularo
G, D'Ambrosio A, Quintieri F, Di Giovanni S, Parzanese I, Pizzuto F,
Giacomelli R, Pugliese O, Tonietti G. Natural killer cell frequency and
function in patients with monoclonal gammopathies. J Clin Lab Immunol.
1992;37: 99–109 PMid:1285130

- De
Rossi G, De Sanctis G, Bottari V, Tribalto M, Lopez M, Petrucci MT
Fontana L. Surface markers and cytotoxic activities of lymphocytes in
monoclonal gammopathy of undetermined significance and untreated
multiple myeloma. Increased phytohemagglutinin-induced cellular
cytotoxicity and inverted helper/suppressor cell ratio are features
common to both diseases. Cancer Immunol Immunother. 1987;25:133-6
https://doi.org/10.1007/BF00199953 PMid:3664530

- Fauriat
C, Mallet F, Olive D, Costello RT. Impaired activating receptor
expression pattern in natural killer cells from patients with multiple
myeloma. Leukemia. 2006;20:732-733
https://doi.org/10.1038/sj.leu.2404096 PMid:16437151

- Costello
RT, Boehrer A, Sanchez C, Mercier D, Baier C, Le Treut T, Sébahoun G.
Differential expression of natural killer cell activating receptors in
blood versus bone marrow in patients with monoclonal gammopathy.
Immunology. 2013;139: 338-41 https://doi.org/10.1111/imm.12082
PMid:23360454 PMCid:PMC3701180

- El-Sherbiny
YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G,
Feyler S, Richards SJ, Davies FE, Morgan GJ, Cook GP. The requirement
for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated
killing of myeloma cells. Cancer Res. 2007;67:8444-9
https://doi.org/10.1158/0008-5472.CAN-06-4230 PMid:17875681

- Benson
DM Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B,
Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine
SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. The PD-1/PD-L1
axis modulates the natural killer cell versus multiple myeloma effect:
a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody.
Blood. 2010;116:2286-94 https://doi.org/10.1182/blood-2010-02-271874 PMid:20460501 PMCid:PMC3490105

- Ray
A, Das DS, Song Y, Richardson P, Munshi NC, Chauhan D, Anderson KC.
Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell
interactions with T cells, natural killer cells and multiple myeloma
cells. Leukemia. 2015;29:1441-4 https://doi.org/10.1038/leu.2015.11 PMid:25634684 PMCid:PMC5703039

- Bezman
NA, Jhatakia A, Kearney AY, Brender T, Maurer M, Henning K, Jenkins MR,
Rogers AJ, Neeson PJ, Korman AJ, Robbins MD, Graziano RF. PD-1 blockade
enhances elotuzumab efficacy in mouse tumor models. Blood Advances
2017;1:753-65 http://www.bloodadvances.org/content/bloodoa/1/12/753.full.pdf

- Koene
HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc
gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural
killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H
phenotype. Blood. 1997;90:1109-14 PMid:9242542

- Wu
J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE,
Kimberly RP. A novel polymorphism of Fc?RIIIa (CD16) alters receptor
function and predisposes to autoimmune disease. J Clin Invest.
1997;100: 1059-70 https://doi.org/10.1172/JCI119616 PMid:9276722 PMCid:PMC508280

- Weng
WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms
independently predict response to rituximab in patients with follicular
lymphoma. J Clin Oncol. 2003;21:3940-7 https://doi.org/10.1200/JCO.2003.05.013 PMid:12975461

- Cartron
G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier
H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and
polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754-8
https://doi.org/10.1182/blood.V99.3.754 PMid:11806974

- Poulart
V, Jou Y-M, Delmonte T, Robbins M (Abstract release date: May 19,
2016). Fc-gamma receptor polymorphisms and progression-free survival:
Analysis of three clinical trials of elotuzumab in multiple myeloma.
EHA Learning Center. Poulart V. Jun 9, 2016;132830
 https://learningcenter.ehaweb.org/eha/2016/21st/132830/valerie.poulart.fc-gamma.receptor.polymorphisms.and.progression-free.survival.html?f=m3e968
https://learningcenter.ehaweb.org/eha/2016/21st/132830/valerie.poulart.fc-gamma.receptor.polymorphisms.and.progression-free.survival.html?f=m3e968
- dbSNP Short Genetic Variations. NCBI https://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=396991
- Leiba
M, Duek A, Amariglio N, Avigdor A, Benyamini N, Hardan I, Zilbershats
I, Ganzel C, Shevetz O, Novikov I, Cohen Y, Ishoev G, Rozic G, Nagler
A, Trakhtenbrot L. Translocation t(11;14) in newly diagnosed patients
with multiple myeloma: Is it always favorable? Genes Chromosomes
Cancer. 2016;55:710-8 https://doi.org/10.1002/gcc.22372
PMid:27152944

- Walker
BA, Wardell CP, Brioli A, Boyle E, Kaiser MF, Begum DB, Dahir NB,
Johnson DC, Ross FM, Davies FE, Morgan GJ. Translocations at 8q24
juxtapose MYC with genes that harbor superenhancers resulting in
overexpression and poor prognosis in myeloma patients. Blood Cancer J.
2014;4:e191. doi: 10.1038/bcj.2014.13 https://doi.org/10.1038/bcj.2014.13

- Glitza
IC, Lu G, Shah R, Bashir Q, Shah N, Champlin RE, Shah J, Orlowski RZ,
Qazilbash MH Chromosome 8q24.1/c-MYC abnormality: a marker for
high-risk myeloma. Leuk Lymphoma. 2015;56:602-7 https://doi.org/10.3109/10428194.2014.924116 PMid:24844357 PMCid:PMC4333105

- Nagoshi
H, Taki T, Hanamura I, Nitta M, Otsuki T, Nishida K, Okuda K, Sakamoto
N, Kobayashi S, Yamamoto-Sugitani M, Tsutsumi Y, Kobayashi T, Matsumoto
Y, Horiike S, Kuroda J, Taniwaki M. Frequent PVT1 rearrangement and
novel chimeric genes PVT1-NBEA and PVT1-WWOX occur in multiple myeloma
with 8q24 abnormality. Cancer Res. 2012;72:4954-62 https://doi.org/10.1158/0008-5472.CAN-12-0213 PMid:22869583

- Richardson
P, Wong E, Stockerl-Goldstein K, Rosenbaum C, Dhodapkar M, Jou Y-M,
Lynch M, Robbins M, Bleickardt E, Jagannath S (Abstract release date:
May 19, 2016). A phase 2 open-label, multicenter study of elotuzumab
monotherapy in patients with high-risk smoldering multiple myeloma. EHA
Learning Center. Jagannath S. Jun 12, 2016; 135309 https://learningcenter.ehaweb.org/eha/2016/21st/135309/sundar.jagannath.a.phase.2.open-label.multicenter.study.of.elotuzumab.html?f=m3
- Mateos
MV, Hernández MT, Giraldo P, de la Rubia J, de Arriba F, Corral LL,
Rosi-ol L, Paiva B, Palomera L, Bargay J, Oriol A, Prosper F, López J,
Argui-ano JM, Quintana N, García JL, Bladé J, Lahuerta JJ, Miguel JF.
Lenalidomide plus dexamethasone versus observation in patients with
high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up
of a randomised, controlled, phase 3 trial. Lancet Oncol.
2016;17:1127-36 https://doi.org/10.1016/S1470-2045(16)30124-3

- Usmani
SZ, Sexton R, Ailawadhi S, Shah JJ, Valent J, Rosenzweig M, Lipe B,
Zonder JA, Fredette S, Durie B, Hoering A, Bartlett B, Orlowski RZ.
Phase I safety data of lenalidomide, bortezomib, dexamethasone, and
elotuzumab as induction therapy for newly diagnosed symptomatic
multiple myeloma: SWOG S1211. Blood Cancer J. 2015;5:e334. https://doi.org/10.1038/bcj.2015.62 PMid:26252787 PMCid:PMC4558587

- Palumbo
A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, Offidani M,
McCarthy P, Evangelista A, Lonial S, Zweegman S, Musto P, Terpos E,
Belch A, Hajek R, Ludwig H, Stewart AK, Moreau P, Anderson K, Einsele
H, Durie BG, Dimopoulos MA, Landgren O, San Miguel JF, Richardson P,
Sonneveld P, Rajkumar SV: Geriatric assessment predicts survival and
toxicities in elderly myeloma patients: an International Myeloma
Working Group report. Blood. 2015;125:2068-74. Correction in: Blood.
2016 Mar 3; 127(9): 1213. Correction in: Blood. 2016 Aug 18; 128(7):
1020 https://doi.org/10.1182/blood-2014-12-615187 PMid:25628469 PMCid:PMC4375104

- Ozaki
S, Handa H, Saitoh T, Murakami H, Itagaki M, Asaoku H, Suzuki K, Isoda
A, Matsumoto M, Sawamura M, Konishi J, Sunami K, Takezako N, Hagiwara
S, Kuroda Y, Chou T, Nagura E, Shimizu K. Trends of survival in
patients with multiple myeloma in Japan: A multicenter retrospective
collaborative study of the Japanese Society of Myeloma. Blood Cancer J.
2015;5:e349.
https://doi.org/10.1038/bcj.2015.79 PMid:26383822 PMCid:PMC4648525 
[TOP]
































































































 https://learningcenter.ehaweb.org/eha/2016/21st/132830/valerie.poulart.fc-gamma.receptor.polymorphisms.and.progression-free.survival.html?f=m3e968
https://learningcenter.ehaweb.org/eha/2016/21st/132830/valerie.poulart.fc-gamma.receptor.polymorphisms.and.progression-free.survival.html?f=m3e968 






