Adekunle Emmanuel Alagbe1, John Ayodele Olaniyi1,2 and Oladapo Wale Aworanti1.
1 Department of Haematology, University College Hospital, Ibadan, Nigeria.
2 Department of Haematology, College of Medicine, University of Ibadan, Nigeria.
Corresponding
author: Dr John Ayodele Olaniyi. Department of Haematology, College of
Medicine, University of Ibadan,Queen Elizabeth Road, Mokola. PMB 5116,
Ibadan, Nigeria. Tel: +234 802 345 1509. E-mail:
ayodeleolaniyi8@gmail.com
Published: March 1, 2018
Received: October 15, 2017
Accepted: January 11, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018017 DOI
10.4084/MJHID.2018.017
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objectives:
Inflammatory markers that influence bone pain crisis (BPC) and other
complications of sickle cell anaemia (SCA) are numerous and play
various roles. This study determined the plasma levels of tumour
necrosis factor (TNF) - α, interleukin - 8 (IL-8), and endothelin - 1
(ET-1) in adult SCA patients during BPC and in steady state. In
addition, the plasma levels of these cytokines were correlated with the
severity of BPC of the patients.
Methods and Materials:
Sixty adult SCA patients (30 during BPC and 30 during steady state) and
30 haemoglobin A controls were enrolled for this cross-sectional study.
The severity of BPC was assessed clinically, and questionnaires were
filled. Plasma levels of TNF- α, IL-8 and ET-1 were quantified by
ELISA, and haematological parameters were determined using a 5-part
auto-analyzer. Plasma levels were correlated with the severity of bone
pain crisis. Results were considered statistically significant if
p<0.05.
Results: Plasma
TNF-α, IL-8, and ET-1 were significantly elevated in the BPC group than
in the steady state group and the controls. Plasma TNF-α, IL-8 and ET-1
were markedly higher in the severe BPC groups than the steady state and
control groups, There was a positive correlation between TNF-α and ET-1
in the bone pain crisis group.
Conclusion:
Elevated levels of plasma TNF-α, IL-8, and ET-1 further establish the
chronic inflammatory state in SCA and equally affirm their significant
contribution, not only to pathogenesis but also to the severity of pain
in SCA.
|
Introduction
Vaso-occlusive crisis (VOC) is the commonest acute presentation of sickle cell anaemia (SCA) patients.[1]
Bone pain crisis (BPC) in SCA is the most prevalent form of VOC, and it
is the specific term for VOC affecting bones; thus BPC is used in this
literature.[2] BPC is an acute episodic painful crisis
that results from microcirculatory obstruction by sickle erythrocytes
leading to ischaemic-reperfusion injury of bone and necrosis of bone
marrow. Bone marrow necrosis is accompanied by the release of several
inflammatory mediators.[2] In addition to other
functions, inflammatory mediators have the ability to bind specific
nociceptive receptors on neurons of peripheral nerves. Hence,
inhibition of secretion or neutralization of these peptides would
ameliorate pain.[3] Episodes of BPC in SCA patients
are highly variable, ranging from one to six per year and up to 10
events per year for some homozygous patients depending on certain
environmental and genetic factors.[1] Following
haemolysis, released haem contributes to activation of leucocytes,
platelets and endothelial cells. This activation induces nuclear factor
kappa B (NF-kB), signal transduction and transcription 3 (STAT3), and
other transcription factor pathways to increase production of
pro-inflammatory and anti-inflammatory cytokines. Up-regulation of
these transcription pathways leads to an imbalance between the pro- and
anti-inflammatory cytokines, that is characteristic of the chronic
inflammatory state seen in SCA patients.[4-6]
Recently, studies have shown that targeting a specific inflammatory
pathway may be sufficient to reduce vaso-occlusion and serve as
potential therapeutic options.[7,8]
Pro-inflammatory
mediators have been extensively studied in SCA, but there is a paucity
of information that relates the cytokines to the severity of bone pain
crisis. TNF-α is a pro-inflammatory cytokine that stimulates tumour
necrosis via its receptors, TNFR55 and TNFR75.[9]
TNF-α is produced mainly by monocytes/macrophages and to a less extent
by T-cells, smooth muscle cells, adipocytes, and fibroblasts.[10] TNF-α worsens vaso-occlusion in SCA by enhancing endothelial adhesiveness, activating leucocytes, and coagulation cascade.[10-12] Several studies have shown altered levels of some cytokines in SCA patients.[12-20]
Some found a significantly higher level of TNF-α in SCA patients in VOC
and/or during steady state than in healthy HbA controls.[16-18]
Contrarily, Tavakkoli et al. and Graido-Gonzalez et al. demonstrated
insignificantly elevated TNF-α level in SCA patients in VOC compared
with patients in the steady-state group and controls.[13,21]
Interleukin-8
(IL-8) is a CXC chemokine that stimulates endothelial cell
proliferation and angiogenesis through its receptors (CXCR1 and CXCR2),
which are expressed mainly by neutrophils.[20,22]
IL-8 is produced by neutrophils, endothelial cells, macrophages,
fibroblasts, and platelets. IL-8 activates re-arrangement of the
cytoskeleton, changes in intracellular Ca++ levels, integrins,
promotion of protein-granule exocytosis, and respiratory burst in
neutrophils.[23,24] IL-8 contributes to the
initiation and propagation of inflammation by activating neutrophils,
which are the first line recruits to the site of vascular injury.
Similar to the role of IL-1 and TNF-α, IL-8 increases the endothelial
adhesiveness of the sickle red cells leading to impairment of
microcirculation and exacerbating painful episodes.[11,15,25]
The most potent vasoconstrictor known, endothelins (ET), are a family
of 21 amino acid peptides and ET-1 is the most prevalent subtype of the
four subtypes characterised.[26] ET-1 constricts
large vessels, resistance arterioles and even post-capillary venules,
the usual site of vaso-occlusion in SCA.[27]
Endothelin is produced by endothelial cells at a steady rate that is
increased following endothelial injury or activation. The release of
ET-1 is modulated by TNF-α and other inflammatory mediators.[28]
Therefore, the ET-1 level is expectedly elevated in SCA patients
because of the ongoing endothelial activation and elevated
pro-inflammatory cytokines. Endothelins act via two specific
G-protein–coupled membrane receptors, ETR-A and ETR-B, which are on
vascular smooth muscle cells and the smooth muscle contraction results
from inositol-triphosphate–mediated increases in intracellular calcium.
Though ET-1 is rapidly internalised and cleared from circulation by the
lungs within minutes, its vasoconstrictive effect lasts as long as 1
hour.[29] In in vitro assays, endothelin stimulated
monocyte production of inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8
etc.), neutrophil production of platelet-activating factor (PAF). ET-1
also enhances monocyte and neutrophil chemotaxis.[30-32]
Endothelins upregulate endothelial cell expression of intercellular
adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1
(VCAM-1), and E-selectin, which are adhesion molecules that participate
in the recruitment of leukocytes to sites of inflammation.[33]
Conversely, neutrophil proteases play a crucial role in cleaving
bioactive ET-1 from its precursor molecule, thereby leading to the
production of active ET and resulting in a vicious cycle with worsening
inflammation.[21,34] We, therefore,
hypothesize that pro-inflammatory mediators would increase with the
severity of bone pain in SCA patients. The aim of our study was to
determine the plasma levels of TNF- α, IL-8 and ET-1 in SCA patients
during bone pain crisis compare with those of SCA patients in steady
state and to correlate these with the severity of pain.
Materials and Methods
Study participants.
This cross-sectional study consisted of 90 adult individuals enrolled
and divided into three groups as follows - Bone pain crisis (BPC) group
made up of 30 SCA patients enrolled consecutively at presentation
during acute bone pain crisis at the Haematology day care unit (HDCU)
of University College Hospital (UCH), Ibadan, South-West Nigeria. BPC
was defined as the occurrence of pain in the extremities, back, and/or
chest (ribs and/or sternum) that led to a hospital presentation, and
could not be explained except by sickle cell anaemia;[1,2]
Steady state (steady) group made up of 30 SCA patients enrolled during
routine follow up visit. Steady state was defined as stable health
state in SCA patients who did not have bone pain or any other crisis
and no blood transfusions in the previous two months,[18]
and Control (HbA) group composed of 30 HbA individuals who were
students and workers in the study hospital. The control participants
were healthy (HbA) age- and sex-matched adults without previous
clinical evidence of haemoglobinopathies. The patients (in both BPC and
steady groups) were diagnosed according to their haemoglobin profile as
having homozygous haemoglobin S (HbSS) by alkaline electrophoresis and
High-Performance Liquid Chromatography (HPLC). The control participants
were confirmed as having haemoglobin A (HbAA) by HPLC. The individuals
with concurrent overt infection, other acute complications than bone
pain crisis, pregnancy, other haemoglobinopathies, and those on
hydroxyurea (HU) were excluded.
The researcher/attending Physician
interviewed all SCA participants, and questionnaires were completed.
The survey contained sections on bio-data, past medical history
obtained from the patients’ notes, and clinical assessment for the
management of the bone pain crisis. University of Ibadan/University
College Hospital ethics review committee approved the study
(UI/EC/14/0089), and all participants gave written informed consent.
Clinical severity assessment.
With the aid of a questionnaire, all SCA patients were assessed for
bone pain after clerking for general and organ-specific signs and
symptoms. Pain assessment included pain site (ribs, sternum, back,
lower or upper limbs), pain duration (up to 4 days; 5-7 days or more
than 7 days), and pain intensity based on single dimensional verbal
pain numerical rating system (that is the patient’s perception of pain
on a scale of 1-10). The grading of pain was essential because its
grade guided the type or class of analgesia administered to abate the
pain. The pain was managed as mild for a verbal, numerical score 1-4;
as moderate for a score of 5-6; and as severe for a score of 7-10.[35,36]
However, for this study, the assessment of patient’s pain included the
doctor’s perception of the patient’s pain. This aspect had questions on
behaviour of patient during painful episode (normal, agitated, very
disturbed, or too quiet) and the analgesic used to abort the pain
(Paracetamol; Non-Steroidal Anti-inflammatory Drugs [NSAIDs]; Opioids;
patient-controlled analgesia [PCA]; or intensive care unit [ICU] care)
adapted from WHO analgesic ladder.[25,37]
For
ease of comparability of variables in this study, patient’s and
doctor’s perceptions were analyzed and summarized as total summary pain
score (TSPS). TSPS was adapted for this study and calculated as follows:
TSPS = [patient’s pain score x duration of pain] + [patient’s behaviour] + [analgaesia used]
Each
characteristic of patient’s pain was scored as follows: pain intensity
was accorded 1, 2 or 3 respectively for verbal, numerical score 1-4,
5-6, 7-10 respectively. Duration of pain prior to patient’s
presentation accorded 1, 2 or 3 for a pain that was respectively less
than 4 days, or lasted 5-6 days; or lasted more than 7 days. The same
was done for the immediate analgesic intervention to abate the pain:
accorded 1, 2, or 3 respectively for Paracetamol/NSAIDs; Opioids; or
for the pain that necessitated patient-controlled analgesia/intensive
care. Patient’s behaviour attracted a maximum of four (4) for a patient
who was too quiet, 3 for a very disturbed patient, 2 for an agitated
patient and 1 for a patient who appeared normal.
Venous blood was
collected from all the participants at the time of presentation to the
hospital and dispensed into two EDTA vacutainers. One for analysis of
the haematological parameters, the second tube was centrifuged, and
plasma was stored in aliquots at -20°C until cytokines were assayed.
Haematological parameters.
Complete blood count (CBC) was performed using Sysmex XS -1000i (Sysmex
Corporation, Kobe, Japan), a fully automated 5-part counter.
Plasma Cytokine Assays.
Plasma TNF-α, IL-8 and ET-1 were quantified using high-sensitivity
commercial enzyme-linked immunosorbent assay (ELISA) kits (Span® Biotec
Limited, Shenzhen, China) in accordance with the manufacturer’s
instructions.
Statistical analysis.
Data were analyzed using SPSS version 22.0 (Statistical Package for
Social Sciences, Inc., Chicago, Ill.). The descriptive data were
presented as means ± standard deviation except otherwise stated.
Frequencies were shown in tables and graphs. Kruskal-Wallis test was
used to compare means of the independent variables. Significant results
were subjected to post hoc analyses for pairwise comparisons. Spearman
rho analysis was performed for correlation of the haematological
parameters and/or cytokines with the severity of bone pain crisis.
Results were considered statistically significant if p<0.05. Results
Demographic and haematological parameters of the participants. Demographic characteristics of the study participants, as well as the haematological parameters, are summarized in Table 1.
Of the 90 adults evaluated, there were 30 SCA patients (15 males and 15
females) in bone pain crisis, 30 (12 males and 18 females) SCA patients
in steady state and 30 HbA controls (12 males and 18 females). While
the total leucocyte and platelet counts were significantly elevated in
the BPC compared to those of HbA controls (p=0.000 in each case), the
haematocrit was significantly lower in both SCA groups than the HbA
(p=0.000).
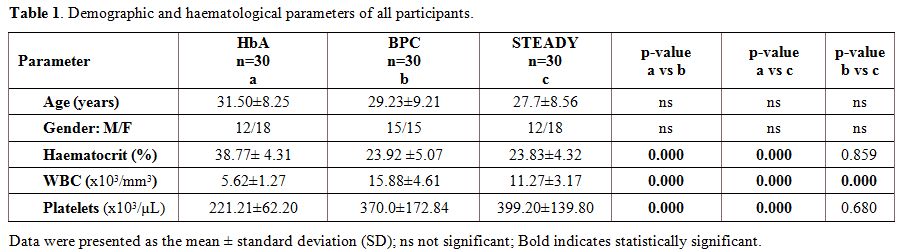 |
Table 1. Demographic and haematological parameters of all participants.. |
Plasma levels of TNF-α, IL-8 and ET-1 in sickle cell anaemia patients and haemoglobin A controls. The plasma levels of TNF-α, IL-8 and ET-1 of the different groups were compared in table 2.
The mean plasma TNF-α was significantly higher in the BPC group
(373.78±354.95ng/L) than in the steady state group (99.77±92.86 ng/L,
p=0.000), and in the HbA controls (82.20±29.32 ng/L, p=0.000).
Similarly, the mean plasma IL-8 level was significantly higher in the
BPC group (464.11±475.99 ng/L, p=0.005) than in the steady state group
(233.57±294.35ng/L, p=0.005) and in the HbA group (183.92±198.58 ng/L,
p=0.002). The mean plasma ET-1 was significantly higher in the BPC
group (164.90±214.76 ng/L) than in the steady state (56.16±48.34 ng/L,
p=0.038) and in the HbA groups (51.18±28.70 ng/L, p=0.042).
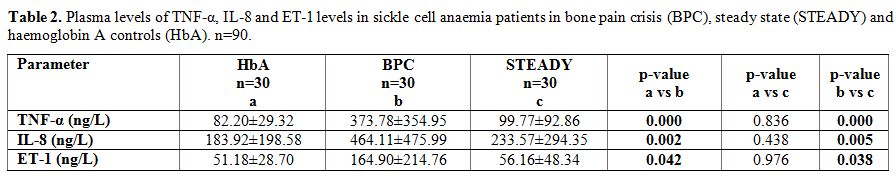 |
Table 2. Plasma levels of TNF-α, IL-8 and
ET-1 levels in sickle cell anaemia patients in bone pain crisis (BPC),
steady state (STEADY) and haemoglobin A controls (HbA). n=90. |
Severity of bone pain crisis in the bone pain crisis group. Table 3
summarizes the clinical severity of bone pain crisis in the SCA
patients at presentation. Of the 30 patients in the BPC group, a
majority 15 (50%) reported a verbal, numerical pain score of 7-10 while
two (6.7%) reported a mild pain score of 1-4 and the remaining 13
reported a moderate score of 5-6. Half (15) of the BPC patients
reported that they had experienced bone pain for a few hours to 4 days
and nine (30%) had experienced pain for 5 to 7 days. Six (20%) of the
patients had pain for more than 7 days prior to presentation. From the
physician’s perception, five patients (16%) were normal in their
behaviour, 15 (50%) were agitated, eight (26.7%) were very disturbed,
and only two (6.7%) were too quiet during clinical examination. Most of
the patients were relieved of pain following administration of opioids,
22 (73.3%); or paracetamol/NSAIDs, 8 (23.3%). None of the patients
required patient-controlled analgesia or intensive care unit to relieve
pain.
The perception of the patient and physician were summed up for ease of comparability as the total summary pain score (TSPS), Table 4. Most patients 15 (50%) had a moderate TSPS and 12 (40%) had a mild TSPS while three (10%) had severe TSPS.
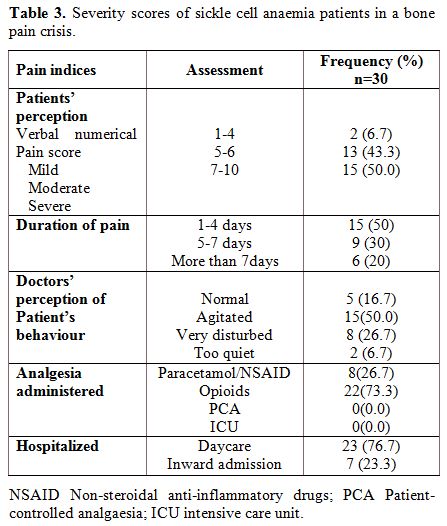 |
Table 3.
Severity scores of sickle cell anaemia patients in a bone pain crisis. |
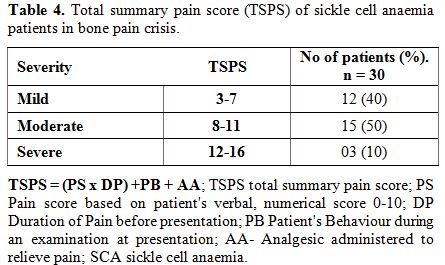 |
Table 4. Total summary pain score (TSPS) of sickle cell anaemia patients in bone pain crisis. |
Site of bone pain crisis in the BPC group.
Concerning the site of bone pain, multiple sites were involved. SCA
patients in bone pain crisis presented with pain in the various parts
of the body: The upper limbs were the most frequently involved parts
with right being 13/76 (17%) and Left 17/76 (22%). The other sites
involved were right lower limb 9/76 (12%); left lower limb 21/76 (16%);
spine 12/76 (16%); and chest (ribs and/or sternum) 09/76 (12%).
Comparing the plasma levels of TNF-α, IL-8 and ET-1 in the bone pain severity groups.
Plasma levels of the pro-inflammatory markers (TNF-α, IL-8 and ET-1)
were compared among the BPC severity groups and shown in Figures 1A-C.
Plasma levels of the pro-inflammatory mediators were highly variable.
The severe BPC group had the most elevated mean plasma TNF-α (610.49 ±
352.82 ng/L), IL-8 (691.61 ± 966.20 ng/L), and ET-1 (336.40 ±24.38
ng/L). Mean plasma TNF-α level was significantly higher in the mild,
moderate and severe than steady state group (p=0.000, p=0.004 and
p=0.001 respectively), figure 1A. The IL-8 level was significantly higher in the mild and moderate groups than the steady state group (p=0.037 and p=0.019), figure 1B. Plasma ET-1 level was significantly higher in the mild and severe groups than the steady state group (p=0.013 and p=0.006), figure 1C.
Post hoc (i.e. within the group) comparison of plasma level of TNF-α,
IL-8 and ET-1 between mild and moderate; moderate and severe; and mild
and severe bone pain severity groups were not statistically significant.
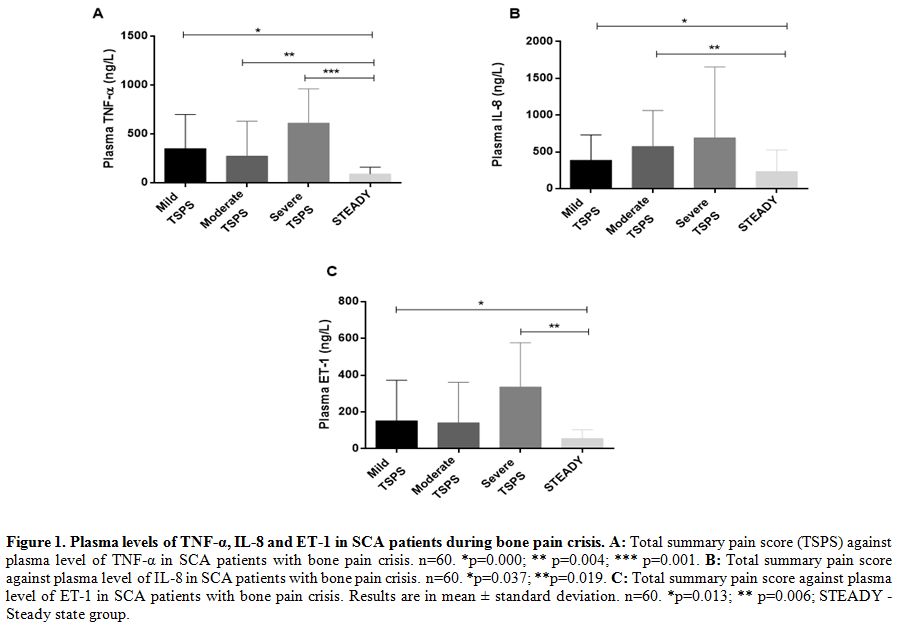 |
Figure 1. Plasma levels of TNF-α, IL-8 and ET-1 in SCA patients during bone pain crisis. A:
Total summary pain score (TSPS) against plasma level of TNF-α in SCA
patients with bone pain crisis. n=60. *p=0.000; ** p=0.004; ***
p=0.001. B: Total summary pain score against plasma level of IL-8 in SCA patients with bone pain crisis. n=60. *p=0.037; **p=0.019. C:
Total summary pain score against plasma level of ET-1 in SCA patients
with bone pain crisis. Results are in mean ± standard deviation. n=60.
*p=0.013; ** p=0.006; STEADY - Steady state group. |
Correlation of TNF- α, IL-8 and ET-1 in sickle cell anaemia patients during bone pain crisis. The correlation was performed for TNF- α and ET-1 in SCA patients during bone pain crisis (n=30), Figure 2.
Spearman rho correlation analysis among the SCA patients in the bone
pain crisis group revealed a significant positive correlation (r=0.854,
p=0.000) for TNF-α and ET-1. IL-8 and ET-1 were not significantly
correlated (r=0.017, p=0.927) in the BPC group. Similarly, IL-8 and
TNF- α (r=0.004, p=0.985) were not significantly correlated.
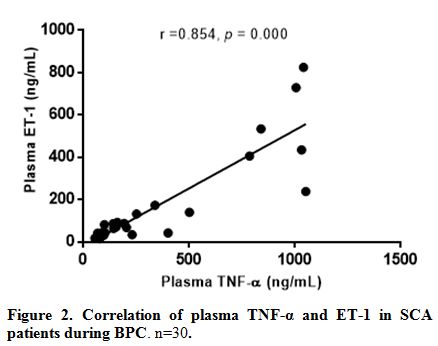 |
Figure 2. Correlation of plasma TNF-α and ET-1 in SCA patients during BPC. n=30. |
Discussion
The
findings in the present study are similar to those of other studies,
who found that levels of pro-inflammatory mediators were higher in SCA
patients than in controls. Contrary to the study by Graido-Gonzalez et
al. who found only ET-1 to be significantly higher in SCD patients
before VOC than after crisis, the index study found that the three
peptides studied were elevated in the BPC group.[21]
The smaller sample size and short post-crisis period of 1-3 weeks may
have accounted for the lack of significant difference in the previously
referenced study. Elevated levels of cytokines observed may be due to
increased secretion by the leucocytes and platelets that were also
significantly elevated in the SCA patients especially during BPC
compared to controls.[28] IL-8 is known to be a
chemotactic factor for neutrophils. Activation of neutrophils seen in
SCA patients during VOC is believed to be mediated by IL-8 and
augmented by other pro-inflammatory mediators.[18,38]
Elevated levels of the IL-8 in patients with SCA agrees with those of
other researchers who found higher plasma IL-8 level during acute chest
syndrome and in patients with vaso-occlusion.[15,21]
IL-8 propagate inflammation by increasing the adherence of sickle red
cells to endothelium via the α4β1 integrin receptors on sickle
reticulocytes.[25,39] The active
process of endothelial adhesion contributes to the passive mechanical
obstruction that leads to vaso-occlusion in SCA.[6,40]
Significantly
elevated TNF-α observed in the SCA groups is similar to findings of
Lanaro et al. and Goncalves et al. Both groups of researchers found
that SCA patients have increased circulating TNF-α and IL-8 levels at
steady state and during the crisis.[15,16] Apart from
enhancing endothelial adhesiveness, TNF-α activates leucocytes and the
coagulation cascade, leading to the elevation of plasma levels of
acute-phase plasma proteins such as fibrinogen that aid erythrocyte
adhesion to endothelium.[41,42] These contribute to
the development of vascular occlusion in SCA patients. Endothelin-1 is
a potent vasoconstrictor of arterioles and post-capillary venules that
contribute to vaso-occlusion, bone pain crisis, acute chest syndrome
and nephropathy. ET-1 upregulates synthesis of adhesion molecules like
ICAM -1, VCAM-1, and E-selectin in endothelial cells thereby
participating in leucocyte adhesion at sites of inflammation.[21,27,28,43]
An elevated level of ET-1 results from low oxygen tension in SCA
patients because hypoxia is a potent stimulus for the production and
release of ET-1 by vascular endothelial cells.[21,39]
Comparing
the verbal pain numerical scoring system with the total severity score
system (adopted in this study for ease of comparability of variables),
only three (10%) of the bone pain crisis SCA group were in severe pain
at presentation. It would then not be surprising that most of the
patients 23(76.7%) were managed as outpatients and none had pain that
was severe enough to warrant patient-controlled analgesia (PCA) or
intensive care unit (ICU) admission. This finding is similar to the
outcome of the study by Etienne-Julan et al., in which an episodic pain
index was adopted for children with SCD.[25] From
those above, it could be inferred that elevated levels of
pro-inflammatory mediators contributed to the severity of BPC in the
patients. Pain and the immune system influence each other in such a
manner that makes it difficult to determine whether blocking
nociception or reducing the production of these pro-inflammatory
cytokines would results in less severe pain.[44]
Pro-inflammatory cytokines stimulate pain via the
cyclooxygenase-1/prostaglandin E2 induction at the tissue injury site
and the spinal cord thus, increasing neuronal sensitivity to pain
stimuli. This mechanism corroborates the observation relating to the
location of pain in the BPC group. In this study (data not shown) BPC
involving the spinal vertebrae was more frequent among the moderate and
the severe pain groups probably because of the proximity of the
infarctive injury to the spinal cord.[44,45] IL-8
like other chemokines are responsible primarily for migration of
leucocytes to the sites of tissue injury or inflammation as seen in
VOC. It also participates in synaptic transmission and formation of
secondary messenger systems in neurons and glial cells, hence favouring
nociception.[45] Tumour necrosis factor-α and ET-1
were mostly positively correlated with the bone-pain crisis severity
groups indicating the role of both peptides in the pathogenesis of the
BPC.
Conclusions
The
persistently elevated levels of these pro-inflammatory cytokines have
further confirmed that SCA is a chronic inflammatory state and
contribute significantly to the pathogenesis and the severity of pain
in SCA. Therapies that target these inflammatory peptides could help to
ameliorate or forestall bone pain crisis in SCA.
Limitation of the study
The
fact that many patients would have used some analgesia to relieve pain
prior to presentation could have affected the assessment of severity
score. This possible error was catered for by the bi-directional
assessment of pain using the adapted total severity pain scoring system.
.
References
- Platt OS, Thorington BD, Brambilla DJ, Milner PF,
Rosse WF, Vichinsky E, Kinney TR. Pain in Sickle Cell Disease. New
England Journal of Medicine. 1991; 325 (1):11-6. https://doi.org/10.1056/NEJM199107043250103 PMid:1710777

- Adewoyin
AS. Management of Sickle Cell Disease: A Review for Physician Education
in Nigeria (Sub-Saharan Africa). Anemia. 2015;2015:21. https://doi.org/10.1155/2015/791498 PMid:25667774 PMCid:PMC4312619

- Delicou S, Maragkos K. Pain Management in Patients with Sickle Cell Disease-A Review. Hematology. 2013.

- Steinberg
MH, Rodgers GP, editors. Pathophysiology of sickle cell disease: role
of cellular and genetic modifiers. Seminars in hematology; 2001:
Elsevier.
- Rother RP, Bell L,
Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis
and extracellular plasma hemoglobin: a novel mechanism of human
disease. Jama. 2005;293(13):1653-62. https://doi.org/10.1001/jama.293.13.1653 PMid:15811985

- Setty
BNY, Betal SG, Zhang J, Stuart MJ. Heme induces endothelial tissue
factor expression: potential role in hemostatic activation in patients
with hemolytic anemia. Journal of Thrombosis and Haemostasis.
2008;6(12):2202-9. https://doi.org/10.1111/j.1538-7836.2008.03177.x PMid:18983524

- Embury
SH, Matsui NM, Ramanujam S, Mayadas TN, Noguchi CT, Diwan BA, Mohandas
N, Cheung AT. The contribution of endothelial cell P-selectin to the
microvascular flow of mouse sickle erythrocytes in vivo. Blood.
2004;104(10):3378-85. https://doi.org/10.1182/blood-2004-02-0713 PMid:15271798

- Belcher
JD, Mahaseth H, Welch TE, Vilback AE, Sonbol KM, Kalambur VS, Bowlin
PR, Bischof JC, Hebbel RP, Vercellotti GM. Critical role of endothelial
cell activation in hypoxia-induced vasoocclusion in transgenic sickle
mice. American Journal of Physiology-Heart and Circulatory Physiology.
2005;288(6):H2715-H25.

- Ikram N, Hassan K, Tufail S. Classes of Cytokines (Table. International Journal of Pathology. 2004;2(1):47-58
 .
.
- Popa
C, Netea MG, Van Riel PL, van der Meer JW, Stalenhoef AF. The role of
TNF-a in chronic inflammatory conditions, intermediary metabolism, and
cardiovascular risk. Journal of lipid research. 2007;48(4):751-62. https://doi.org/10.1194/jlr.R600021-JLR200 PMid:17202130

- Pitanga
TN, Vilas-Boas W, Cerqueira BAnV, Seixas MO, Barbosa CG, Adorno EV,
Goncalves MS. Cytokine profiles in sickle cell anemia: Pathways to be
unraveled. Advances in Bioscience and Biotechnology. 2013;Vol.04 No.07:7
 .
.
- Pathare
A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D.
Cytokine profile of sickle cell disease in Oman. American Journal of
Hematology. 2004;77(4):323-8. https://doi.org/10.1002/ajh.20196 PMid:15551290

- Tavakkoli
F, Nahavandi M, Wyche MQ, Perlin E. Plasma Levels of TNF-alpha in
Sickle Cell Patients Receiving Hydroxyurea. Hematology. 2004;9(1):61-4.
https://doi.org/10.1080/1024533032000158869 PMid:14965870

- Cajado
C, Cerqueira BAV, Couto FD, Moura-Neto JP, Vilas-Boas W, Dorea MJ, et
al. TNF-alpha and IL-8: Serum levels and gene polymorphisms
(-308G>A and -251A>T) are associated with classical
biomarkers and medical history in children with sickle cell anemia.
Cytokine. 2011;56(2):312-7. https://doi.org/10.1016/j.cyto.2011.07.002 PMid:21802960

- Goncalves
MS, Queiroz IL, Cardoso SA, Zanetti A, Strapazoni AC, Adorno E, et al.
Interleukin 8 as a vaso-occlusive marker in Brazilian patients with
sickle cell disease. Brazilian journal of medical and biological
research = Revista brasileira de pesquisas medicas e
biologicas/Sociedade Brasileira de Biofisica [et al].
2001;34(10):1309-13. https://doi.org/10.1590/S0100-879X2001001000011

- Lanaro
C, Franco-Penteado CF, Albuqueque DM, Saad STO, Conran N, Costa FF.
Altered levels of cytokines and inflammatory mediators in plasma and
leukocytes of sickle cell anemia patients and effects of hydroxyurea
therapy. Journal of Leukocyte Biology. 2009;85(2):235-42. https://doi.org/10.1189/jlb.0708445 PMid:19004988

- Qari
MH, Dier U, Mousa SA. Biomarkers of Inflammation, Growth Factor, and
Coagulation Activation in Patients With Sickle Cell Disease. Clinical
and Applied Thrombosis/Hemostasis. 2012;18(2):195-200. https://doi.org/10.1177/1076029611420992 PMid:21949038

- Keikhaei
B, Mohseni AR, Norouzirad R, Alinejadi M, Ghanbari S, Shiravi F, et al.
Altered levels of pro-inflammatory cytokines in sickle cell disease
patients during vaso-occlusive crises and the steady-state condition.
European cytokine network. 2013;24(1):45-52.
PMid:23608554

- Musa
BO, Onyemelukwe GC, Hambolu JO, Mamman AI, Isa AH. Pattern of serum
cytokine expression and T-cell subsets in sickle cell disease patients
in vaso-occlusive crisis. Clinical and vaccine immunology: CVI.
2010;17(4):602-8. https://doi.org/10.1128/CVI.00145-09 PMid:20130127 PMCid:PMC2849336

- Alagbe
AE, Justo Junior AS, Ruas LP, Tonasse WV, Santana RM, Batista THC, et
al. Interleukin-27 and interleukin-37 are elevated in sickle cell
anemia patients and inhibit in vitro secretion of interleukin-8 in
neutrophils and monocytes. Cytokine. 2017. https://doi.org/10.1016/j.cyto.2017.12.001 PMid:29221667

- Graido-Gonzalez
E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma
endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell
disease and acute vaso-occlusive sickle crisis. Blood.
1998;92(7):2551-5. PMid:9746797

- van
der Land V, Peters M, Biemond BJ, Heijboer H, Harteveld CL,
Fijnvandraat K. Markers of endothelial dysfunction differ between
subphenotypes in children with sickle cell disease. Thrombosis
Research.132(6):712-7. https://doi.org/10.1016/j.thromres.2013.10.006 PMid:24182550

- Groves
D, Jiang Y. Chemokines, a family of chemotactic cytokines. Critical
Reviews in Oral Biology & Medicine. 1995;6(2):109-18. https://doi.org/10.1177/10454411950060020101

- Su
S-B, Mukaida N, Matsushima K. Rapid secretion of intracellularly
pre-stored interleukin-8 from rabbit platelets upon activation. Journal
of leukocyte biology. 1996;59(3):420-6. https://doi.org/10.1002/jlb.59.3.420

- Etienne-Julan
M, Belloy M-S, Decastel M, Dougaparsad S, Ravion S, Hardy-Dessources
M-D. Childhood sickle cell crises: clinical severity, inflammatory
markers and the role of interleukin-8. Haematologica. 2004;89(7):863-4.
PMid:15257940

- Yanagisawa
M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A
novel potent vasoconstrictor peptide produced by vascular endothelial
cells. Nature. 1988;332(6163):411-5. https://doi.org/10.1038/332411a0 PMid:2451132

- Boric
MP, Donoso V, Fournier A, Pierre SS, Huidobro-Toro JP. Endothelin
reduces microvascular blood flow by acting on and venules of the
hamster cheek pouch. European Journal of pharmacology.
1990;190(1):123-33. https://doi.org/10.1016/0014-2999(90)94119-I

- Makis AC, Hatzimichael EC, Bourantas KL. The role of cytokines in sickle cell disease. Ann Hematol. 2000;79(8):407-13. https://doi.org/10.1007/s002770000173 PMid:10985359

- De
Nucci G, Thomas R, D'Orleans-Juste P, Antunes E, Walder C, Warner TD,
Vane, JR. Pressor effects of circulating endothelin are limited by its
removal in the pulmonary circulation and by the release of prostacyclin
and endothelium-derived relaxing factor. Proceedings of the National
Academy of Sciences. 1988;85(24):9797-800. https://doi.org/10.1073/pnas.85.24.9797

- McMillen
MA, Huribal M, Cunningham ME, Kumar R, Sumpio BE. Endothelin-1
increases intracellular calcium in human monocytes and causes
production of interleukin-6. Critical care medicine. 1995;23(1):34-40. https://doi.org/10.1097/00003246-199501000-00009 PMid:8001384

- Cunningham
ME, Huribal M, Bala R, McMillen MA. Endothelin-1 and endothelin-4
stimulate monocyte production of cytokines. Critical care medicine.
1997;25(6):958-64. https://doi.org/10.1097/00003246-199706000-00011 PMid:9201047

- Wright
CD, Cody WL, Dunbar JB, Doherty AM, Hingorani GP, Rapundalo ST.
Characterization of endothelins as chemoattractants for human
neutrophils. Life sciences. 1994;55(21):1633-41. https://doi.org/10.1016/0024-3205(94)00330-0

- McCarron
RM, Wang L, Stanimirovic DB, Spatz M. Endothelin induction of adhesion
molecule expression on human brain microvascular endothelial cells.
Neuroscience letters. 1993;156(1):31-4. https://doi.org/10.1016/0304-3940(93)90432-K

- Sessa
WC, Kaw S, Hecker M, Vane JR. The biosynthesis of endothelin-1 by human
polymorphonuclear leukocytes. Biochemical and biophysical research
communications. 1991;174(2):613-8. https://doi.org/10.1016/0006-291X(91)91461-K

- Johnson
C. Measuring pain. Visual analog scale versus numeric pain scale: what
is the difference? Journal of chiropractic medicine. 2006;4(1):43-4. https://doi.org/10.1016/S0899-3467(07)60112-8

- McMaffery M, Pasero C. Pain: Clinical Manual. St. Louis, MO: Mosby. Inc; 1999.
- Vargas-Schaffer
G. Is the WHO analgesic ladder still valid? Twenty-four years of
experience. Canadian Family Physician. 2010;56(6):514-7. PMid:20547511
PMCid:PMC2902929

- Zhang J-M, An J. Cytokines, Inflammation and Pain. International anesthesiology clinics. 2007;45(2):27-37. https://doi.org/10.1097/AIA.0b013e318034194e PMid:17426506 PMCid:PMC2785020

- Kasschau
MR, Barabino GA, Bridges KR, Golan DE. Adhesion of sickle neutrophils
and erythrocytes to fibronectin. Blood. 1996;87(2):771-80. PMid:8555502

- Okpala
I. The intriguing contribution of white blood cells to sickle cell
disease - a red cell disorder. Blood Rev. 2004;18(1):65-73. https://doi.org/10.1016/S0268-960X(03)00037-7

- Francis
RB, Jr. Elevated Fibrin D-Dimer Fragment in Sickle Cell Anemia:
Evidence for Activation of Coagulation during the Steady State as well
as in Painful Crisis. Pathophysiology of Haemostasis and Thrombosis.
1989;19(2):105-11. https://doi.org/10.1159/000215901

- Mohandas
N, Evans E. Adherence of sickle erythrocytes to vascular endothelial
cells: requirement for both cell membrane changes and plasma factors.
Blood. 1984;64(1):282-7. PMid:6733278

- Brett
Heimlich J, Speed JS, O'Connor PM, Pollock JS, Townes TM, Meiler SE, et
al. Endothelin-1 contributes to the progression of renal injury in
sickle cell disease via reactive oxygen species. British Journal of
Pharmacology. 2016;173(2):386-95. https://doi.org/10.1111/bph.13380 PMid:26561980 PMCid:PMC4940621

- Shavit
Y, Fridel K, Beilin B. Postoperative pain management and
proinflammatory cytokines: animal and human studies. Journal of
Neuroimmune Pharmacology. 2006;1(4):443-51. https://doi.org/10.1007/s11481-006-9043-1 PMid:18040817

- Abbadie
C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White
FA. Chemokines and pain mechanisms. Brain research reviews.
2009;60(1):125-34. https://doi.org/10.1016/j.brainresrev.2008.12.002 PMid:19146875 PMCid:PMC2691997

[TOP]














 .
. 
 .
. 































