Panagiota Zikidou1, Anastassia Grapsa2, Zoe Bezirgiannidou3, Athanassios Chatzimichael1, and Elpis Mantadakis1*.
1 Department of Pediatrics, Democritus University of Thrace Faculty of Medicine, Alexandroupolis, Thrace, Greece.
2 Department of Medical Microbiology, University General Hospital of Evros, Alexandroupolis, Thrace, Greece.
3 Blood Transfusion Centre, University General Hospital of Evros, Alexandroupolis, Thrace, Greece.
Corresponding
author: Elpis Mantadakis, MD, Ph.D., Associate Professor of Pediatrics
and Pediatric Hematology/Oncology, Democritus University of Thrace
Faculty of Medicine, Department of Pediatrics, University General
Hospital of Evros, 6th Kilometer Alexandroupolis-Makris, 68100
Alexandroupolis, Thrace, Greece. Tel: +30-25513-51411, Fax:
+30-25510-30340, E-mail:
emantada@med.duth.gr
Published: March 1, 2018
Received: November 19, 2017
Accepted: January 24, 2017
Mediterr J Hematol Infect Dis 2018, 10(1): e2018018 DOI
10.4084/MJHID.2018.018
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Human parvovirus B19 (HPV-B19) is the etiologic agent of erythema infectiosum,
of transient aplastic crises in individuals with underlying chronic hemolytic
disorders, and of chronic pure red cell aplasia in immunocompromised
individuals.
Case report: We
describe a 14-year-old girl with long-standing Evans syndrome, who presented
with severe anemia, reticulocytopenia and thrombocytopenia. A bone marrow
aspirate revealed severe erythroid hypoplasia along with the presence of giant
pronormoblasts, while serological studies and real-time PCR of whole blood were
positive for acute parvovirus B19 infection. The patient was initially managed
with corticosteroids, but both cytopenias resolved only after administration of
intravenous gamma globulin 0.8g/kg.
Conclusion: Acute
parvovirus B19 infection should be suspected in patients with immunologic
diseases, who present reticulocytopenic hemolytic anemia and thrombocytopenia.
In this setting, intravenous gamma globulin is effective for both cytopenias.
|
Introduction
Human
parvovirus B19 (HPV-B19) is the etiologic agent of erythema
infectiosum. In individuals with underlying chronic hemolytic
disorders, HPV-B19 causes transient aplastic crises (TAC).[1] In immunocompromised individuals, persistent HPV-B19 viremia presents as chronic pure red cell aplasia (PRCA).[2] Finally, HPV-B19 rarely has been implicated as a cause of autoimmune hemolytic anemia (AIHA) in normal children.[3-7] The cellular receptor of HPV-B19 is the blood group P antigen, which explains the viral tropism for erythroid precursors.[8]
We
present a 14-year-old girl with long-standing Evans syndrome (ES), who
developed severe anemia, reticulocytopenia and thrombocytopenia due to
acute HPV-B19 infection, and briefly review the relevant literature.
Case Report
A
known to us 14-year-old girl with long-standing ES was admitted because
of fatigue that got progressively worse over three days and new-onset
petechiae. The patient was diagnosed with ES at the age of 3 years,
when she presented with symptomatic thrombocytopenia without hemolysis
and was found to have a strongly positive direct antiglobulin test
(DAT) for non-specific warm IgG that persists to nowadays. Over the
years, she had required therapy with corticosteroids and intravenous g
globulin (IVIG) for symptomatic thrombocytopenia only, although she was
treatment-free for almost four years. The patient's last hemogram with
reticulocyte count before this admission was three months ago and was
normal.
On admission to us, she was pale, slightly tachycardic
(heart rate of 108/min), had wet purpura, along with numerous petechiae
and bruises on both lower extremities and a palpable spleen tip.
Laboratory examinations on admission showed leukocytes 4,600/μL (53%
neutrophils, 30% lymphocytes, 12% monocytes, 5% reactive lymphocytes),
hemoglobin 8.4g/dL, hematocrit 24.4%, platelets 2,000/μL and
reticulocytes 0.07%. DAT was 4+ positive for IgG alone. Biochemical
studies revealed serum LDH 330U/L (reference range 120-246 U/L), total
bilirubin 0.8mg/dL, direct bilirubin 0.2 mg/dL, alanine transaminase 14
U/L, aspartate transaminase 15 U/L, g-glutamyl transpeptidase 9 U/L,
ferritin 208 ng/ml, haptoglobin 5.8mg/dL (reference range 30-140),
vitamin B12 460pg/ml, and serum folate 2.87ng/ml (reference range
3-14). An abdominal ultrasonogram showed borderline splenomegaly,
without focal lesions or gallstones. Serological studies were positive
for HPV-B19 IgM (210 U/ml, positive >24U/ml by ELISA kit (RecomWell
Mikrogen GmbH, Neuried, Germany), while real-time PCR of whole blood
using the LightMix® Kit Parvovirus B19 (TIB MOLBIOL GmbH, Berlin,
Germany) for the LightCycler 2.0 instrument (Roche GmbH, Mannheim,
Germany) was positive for DNA of HPV-B19 (5.7x106 copies/ml). Additional real-time PCR of whole blood was negative for CMV, EBV, HSV-1, and HSV-2.
Due
to laboratory evidence of hemolysis (very low serum haptoglobin) in a
child with ES, she was started on intravenous methylprednisolone
80mg/day. The next day, a repeated hemogram showed hemoglobin 6.9g/dL,
hematocrit 20.1%, platelets 14,000/μL and reticulocytes 0.09%, while
indirect bilirubin picked at 1.8mg/dl. Due to worsening anemia with
severe reticulocytopenia, a bone marrow aspirate was performed and
showed a regular maturation of the myeloid precursors and abundant
megakaryocytes. The erythroid series demonstrated severe decrease of
erythroid precursors that were almost exclusively represented by
pronormoblasts, frequently of giant size. On the 3rd
hospital day, due to persistent anemia and thrombocytopenia, a single
dose of IVIG was administered (40g or 830mg/kg). Treatment was
well-tolerated. The next morning, hemoglobin was 8.1g/dL, hematocrit
23.4%, platelets 136,000/μL and reticulocytes 0.23%. Two days later,
the hemoglobin was further increased to 9.6g/dL, hematocrit 28.4%,
platelets 517,000/μL, and reticulocytes 4.05%. The patient was
discharged home to continue a 4-week tapering of oral prednisolone
along with daily oral folic acid 5mg/day. At the end of therapy, she
had a normal full blood count (hemoglobin 12.2g/dL, platelets
277,000/μL) and serum haptoglobin (122 mg/dL). Eight months after the
described events, she remains asymptomatic, off-therapy, with normal
hemogram, but continues to have 4+ positive DAT for IgG.
Discussion
It
is well-known that HPV-B19 is associated with TAC in patients with
shortened erythrocyte life-span and increased erythropoiesis.[1]
Our patient, despite having a strongly positive DAT for years, she had
never developed an episode of hemolysis in the past. We believe that
the data linking HPV-B19 to the described episode of reticulocytopenic
hemolytic anemia are strong since we found laboratory, cytological,
serological and molecular evidence of acute HPV-B19 infection. First,
in typical AIHA cases, with or without ES, the reticulocyte count is
elevated, while our patient had profound reticulocytopenia, a
well-known feature of acute HPV-B19 infection. Second, the observed
bone marrow erythroid hypoplasia along with the presence of giant
pronormoblasts are typical findings of HPV-B19 infection.[9]
Third, HPV-B19 infection was confirmed with appropriate molecular
(real-time PCR) and serological studies (specific IgM). Finally, our
patient had no clinical or laboratory evidence of chronic hemolysis
(normal hemogram and reticulocyte count), when last seen as an
outpatient three months ago. Thus, in all likelihood HPV-B19 triggered
the hemolysis in our patient, who already harbored non-specific warm
IgG anti-erythrocytic autoantibodies confirmed with several screening
tests over the years.
Primary ES is defined by the concurrent or
sequential occurrence of (auto) immune thrombocytopenia (ITP) and DAT
positive AIHA in the absence of an underlying etiology. Active
hemolysis is not always present, but erythrocyte involvement requires a
positive DAT, like in our patient. Primary ES is a diagnosis of
exclusion, and other causes of immune cytopenias such as autoimmune
lymphoproliferative syndrome, systemic lupus erythematosus, IgA
deficiency, common variable immune deficiency, and acquired
immunodeficiency syndrome should be excluded. The natural history of ES
is characterized by a chronic and relapsing course requiring
immunosuppressive therapy. Almost all patients with ES are initially
treated with corticosteroids, especially in cases with clinically
significant autoimmune hemolysis. IVIG is preferred as first-line
therapy in cases of symptomatic thrombocytopenia, but cannot be
recommended as first-line therapy in AIHA, since only about 40% of
patients will respond.[10]
Our patient had
long-lasting primary ES, but without active hemolysis. Over the years,
other causes of immune cytopenias were excluded by appropriate
laboratory studies. The contemporary event of reticulocytopenic
hemolytic anemia and relapse of thrombocytopenia leads us to suspect
acute HPV B19 infection.
HPV-B19-associated aplastic crises in
patients with sickle-cell anemia, thalassemia, spherocytosis, and
glucose-6-phosphate dehydrogenase deficiency are usually managed with
simple erythrocyte transfusions.[2] However, in
patients with ES, blood transfusions are generally not an option due to
the difficulty in finding compatible blood.[11,12]
In
immunocompromised individuals, IVIG therapy for PRCA related HPV-B19
infection appears to be effective. Kurtzman et al. were the first to
report cure of enduring PRCA due to persistent parvovirus B19 infection
with immunoglobulin therapy.[13] Crabol et al.
reviewed the efficacy of IVIG therapy in 133 patients with HPV-B19
PRCA. Hemoglobin was corrected after the first course of IVIG in 93% of
the patients, while disease relapse occurred in 33.9% at a mean of 4.3
months.[14] Our patient received a single dose of
IVIG 0.8g/kg, lower than the typical dose described by Crabol et al.,
and had a quick response, as witnessed by the elevated reticulocyte
count and rapid correction of anemia. Moreover, she demonstrated a
dramatic rise of platelets number, and thrombocytopenia was fully
corrected (platelets>150,000/μL) within two days after
administration of IVIG.
Spontaneous recovery from HPV-B19 occurs
in normal persons and typically correlates with the appearance of
circulating specific antivirus antibodies.[2] Our
patient who suffered from ES despite having a high anti-HPV-B19 IgM
titer had persistent reticulocytopenia that was corrected only after
administration of IVIG. Hence, we believe that her recovery was not
spontaneous, but rather the result of IVIG administration.
HPV-B19
not only causes TAC in patients with reduced red cell survival, but it
also triggers AIHA. Five reported cases of AIHA due to acute HPV-B19
infection in healthy children are summarized in Table 1. As shown, three of the five patients were males, with a median age at presentation of 5 years.[3-7]
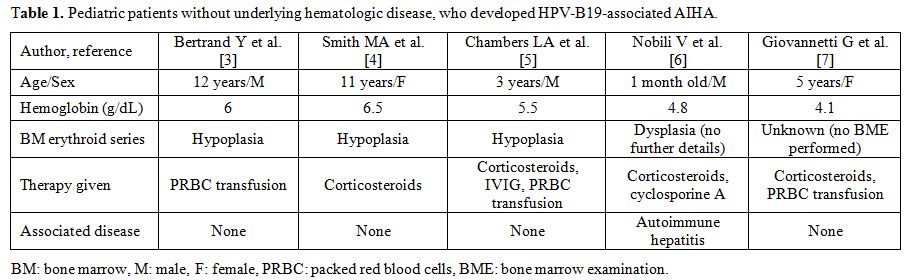 |
Table 1. Pediatric patients without underlying hematologic disease, who developed HPV-B19-associated AIHA. |
Few cases of HPV-B19 induced AIHA associated with the hemophagocytic syndrome have also been published,[15-17]
but our patient’s bone marrow showed no signs of hemophagocytosis or
thrombophagocytosis. The presence of abundant megakaryocytes suggests
that thrombocytopenia in our case was immune-mediated due to peripheral
platelet destruction.
Regarding HPV - B19 - associated
thrombocytopenia, Heegaard et al. studied 47 children with newly
diagnosed ITP and found molecular evidence of recent parvovirus B19
infection in 13% of the patients.[18] Zhang et al.
performed a meta-analysis of eight studies that investigated the
relationship between HPV-B19 infection and childhood ITP. The incidence
of HPV-B19 infection in the ITP group was significantly higher than
that in the control group, confirming an association of HPV-B19 with
ITP.[19]
In conclusion, HPV- B19 infection
should be suspected in patients with immunologic diseases, who present
with anemia, reticulocytopenia, and thrombocytopenia. In this setting,
IVIG therapy is indicated and can achieve rapid and long-standing
correction of both cytopenias. References
- Lefrère JJ, Couroucé AM, Bertrand Y, Girot R,
Soulier JP. Human parvovirus and aplastic crisis in chronic hemolytic
anemias: a study of 24 observations. Am J Hematol. 1986;23:271-275. https://doi.org/10.1002/ajh.2830230311 PMid:3020978

- Brown KE, Young NS. Parvoviruses and bone marrow failure. Stem Cells. 1996;14:151-163. https://doi.org/10.1002/stem.140151 PMid:8991535

- Bertrand
Y, Lefrere JJ, Leverger G, Courouce AM, Feo C, Clark M, Schaison G,
Soulier JP. Autoimmune haemolytic anaemia revealed by human parvovirus
linked erythroblastopenia. Lancet. 1985;2:382-383. https://doi.org/10.1016/S0140-6736(85)92509-7

- Smith
MA, Shah NS, Lobel JS. Parvovirus B19 infection associated with
reticulocytopenia and chronic autoimmune hemolytic anemia. Am J Pediatr
Hematol Oncol. 1989;11:167-169. PMid:2546464

- Chambers
LA, Rauck AM. Acute transient hemolytic anemia with a positive
Donath-Landsteiner test following parvovirus B19 infection. J Pediatr
Hematol Oncol. 1996;18:178-181. https://doi.org/10.1097/00043426-199605000-00017

- Nobili
V, Vento S, Comparcola D, Sartorelli MR, Luciani M, Marcellini M.
Autoimmune hemolytic anemia and autoimmune hepatitis associated with
parvovirus B19 infection. Pediatr Infect Dis J. 2004;23:184-185. https://doi.org/10.1097/01.inf.0000110270.38240.51 PMid:14872194

- Giovannetti
G, Pauselli S, Barrella G, Neri A, Antonetti L, Gentile G, Iacobini M,
Girelli G, Coluzzi S. Severe warm autoimmune haemolytic anaemia due to
anti-Jk(a) autoantibody associated with Parvovirus B19 infection in a
child. Blood Transfus. 2013;11:634-635. PMid:23522895 PMCid:PMC3827410

- Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114-117. https://doi.org/10.1126/science.8211117 PMid:8211117

- Chim CS, Ma SK. Giant pronormoblasts in parvovirus-associated pure red cell aplasia. Am J Hematol. 2000;65:289. https://doi.org/10.1002/1096-8652(200012)65:4<289::AID-AJH5>3.0.CO;2-R

- Flores
G, Cunningham-Rundles C, Newland AC, Bussel JB. Efficacy of intravenous
immunoglobulin in the treatment of autoimmune hemolytic anemia: results
in 73 patients. Am J Hematol. 1993;44:237-42. https://doi.org/10.1002/ajh.2830440404 PMid:8237993

- Mantadakis
E, Farmaki E. Natural history, pathogenesis, and treatment of Evans
syndrome in children. J Pediatr Hematol Oncol. 2017;39:413-419. https://doi.org/10.1097/MPH.0000000000000897 PMid:28654461

- Petz LD. A physician's guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol. 2004;124:712-716. https://doi.org/10.1111/j.1365-2141.2004.04841.x PMid:15009058

- Kurtzman
G, Frickhofen N, Kimball J, Jenkins DW, Nienhuis AW, Young NS. Pure
red-cell aplasia of 10 years' duration due to persistent parvovirus B19
infection and its cure with immunoglobulin therapy. N Engl J Med.
1989;321:519-523. https://doi.org/10.1056/NEJM198908243210807 PMid:2548098

- Crabol
Y, Terrier B, Rozenberg F, Pestre V, Legendre C, Hermine O,
Montagnier-Petrissans C, Guillevin L, Mouthon L; Groupe d'experts de
l'Assistance Publique - Hôpitaux de Paris. Intravenous immunoglobulin
therapy for pure red cell aplasia related to human parvovirus b19
infection: a retrospective study of 10 patients and review of the
literature. Clin Infect Dis. 2013;56:968-977. https://doi.org/10.1093/cid/cis1046 PMid:23243178

- Uike
N, Miyamura T, Obama K, Takahira H, Sato H, Kozuru M. Parvovirus
B19-associated haemophagocytosis in Evans syndrome: aplastic crisis
accompanied by severe thrombocytopenia. Br J Haematol. 1993;84:530-532.
https://doi.org/10.1111/j.1365-2141.1993.tb03113.x PMid:8217805

- Sekiguchi
Y, Shimada A, Imai H, Wakabayashi M, Sugimoto K, Nakamura N, Sawada T,
Komatsu N, Noguchi M. A case of recurrent autoimmune hemolytic anemia
during remission associated with acute pure red cell aplasia and
hemophagocytic syndrome due to human parvovirus B19 infection
successfully treated by steroid pulse therapy with a review of the
literature. Int J Clin Exp Pathol. 2014;7:2624-2635. PMid:24966977
PMCid:PMC4069955

- Puigví
L, Baumann T, Fernández S, Castro P, Pereira A, Merino A. Massive
erythrophagocytosis by peripheral monocytes and neutrophils in
parvovirus-B19 autoimmune hemolytic anemia. Ann Hematol.
2017;96:881-882. https://doi.org/10.1007/s00277-017-2957-2 PMid:28224193

- Heegaard
ED, Rosthøj S, Petersen BL, Nielsen S, Karup Pedersen F, Hornsleth A.
Role of parvovirus B19 infection in childhood idiopathic
thrombocytopenic purpura. Acta Paediatr. 1999;88:614-617. https://doi.org/10.1111/j.1651-2227.1999.tb00009.x PMid:10419244

- Zhang
YD, Hu Q, Liu SY, Liu AG, Wang GL, Xiong H, Sun Y. Association of human
parvovirus B19 infection and childhood idiopathic thrombocytopenic
purpura: a meta-analysis of Chinese literature. Zhongguo Dang Dai Er Ke
Za Zhi. 2009;11:999-1001. PMid:20113609

[TOP]





















