Sophia Delicou1, Konstantinos Maragkos1, Maria Tambaki2, Dimitrios Kountouras2 and John Koskinas2.
1 Thalassemia and Sickle Cell Department, Hippocratio General Hospital Athens.
2 Second
Academic Department of Medicine, School of Medicine, National and
Kapodistrian University of Athens, Hippokratio General Hospital Athens.
Correspondence to:
Sophia Delicou – MD, Hematologist, Thalassemia and Sickle Cell
Department, Hippocratio General Hospital Athens. Address:114 Leof.
Vasilissis Sofias, Athens 115 27. E-mail:
sophiadelicou@hippocratio.gr
Published: September 1, 2018
Received: April 24, 2018
Accepted: August 6, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018049 DOI
10.4084/MJHID.2018.049
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Sickle
cell disease patients often need regular blood transfusions to improve
both the quality of life and survival from the veno-occlusive
complications of the disease. Deferasirox, a convenient long acting
oral agent, has recently been introduced in clinical practice with
promising efficacy.
This study aims to evaluate the association
of liver stiffness and possible fibrosis with iron deposition and
confirm the use of elastography as a validated test of responding to
chelation with low cost and easy access.
15 patients with sickle
cell disease and systemic or occasional transfusions were evaluated
with MRI, transient elastography and biochemistry, for liver iron(LIC)
and liver stiffness(LSM) before onset and one year after taking
Deferasirox. All patients completed the study.
Our results
showed improvement in hepatic iron and hepatic stiffness after
chelation therapy; Furthermore ALT, AST, LDH and ferritin levels have
improved after 12 months of therapy with deferasirox. During the study
no serious adverse events were encountered indicating the safety of the
drug.
Transient liver elastography findings correlate with serum
ferritin and LIC in patients with sickle cell disease and it is a
useful tool for assessing the response of liver iron chelation therapy.
|
Introduction
Chronic
transfusion therapy is being used more frequently to prevent and treat
the complications of sickle cell disease. Previous studies have shown
that the iron overload that results from such therapy in other patient
populations is associated with significant morbidity and mortality.[1]
Deferoxamine has been the standard drug for iron chelation therapy over
the past four decades. However, its major disadvantage is
non-compliance of patients, because it needs an 8- to 12-hr parenteral
administration since it has a short half-life and a very poor oral
bioavailability. Deferiprone was the first extensively studied oral
chelating agent in the early 2000s for patients who were unable to use
deferoxamine effectively or safely. Although deferiprone-treated
patients had good compliance in thalassemic patients, some serious side
effects such as neutropenia and agranulocytosis were reported that
limited its use in sickle cell disease patients especially in
combination with hydroxyurea.[2,3]
A more
convenient oral iron chelator, deferasirox, has recently become
available showing promising efficacy. Many studies have shown that
deferasirox has an acceptable profile of safety and tolerability in
thalassemic patients.[2,4,5]
Liver
iron concentration has been regarded as the reference standard for
estimating body iron load in thalassemic patients and has been shown to
predict total body iron stores accurately. In sickle cell anemia, the
liver is one of the target organs of the disease itself, except the
transfusional iron overload. The term "sickle cell hepatopathy" has
sometimes been used to reflect the overlapping acute and chronic causes
of liver dysfunction in these patients. Studies in patients that have
been hospitalized due to an acute vasoocclusive crisis have estimated
the frequency of liver involvement ranging from 10% to 39% and an
autopsy study of sickle cell patients has revealed the presence of
hepatic infarction in 34% of patients.[6,7]
Prior
studies have based on data from hereditary hemochromatosis and
thalassemia major showing that elevated hepatic iron content determined
by liver biopsy and imaging techniques over 7 mg/g liver dry weight is
a risk factor for hepatic fibrosis. Therefore this value has been used
as a guide to start chelation therapy.[4,5,7,8]
Transient
elastography and has been extensively validated in chronic liver
diseases and is currently used for detection and staging of liver
fibrosis.
In the last few years, liver stiffness measurement (LSM)
by transient elastography (TE) has been shown to be closely related to
the degree of hepatic fibrosis assessed by biopsy in thalassemic
patients.[9,10]
However, hepatic involvement has
been shown to affect liver stiffness in patients with sickle cell
disease during acute vaso-occlusive crisis measured with transient
elastography.[11]
The study aimed to evaluate
the role of elastography (Liver Stiffness Measurement, LSM, kPascals,
FibroScan, Echosens, Paris, France) in patients with SCD and explore
possible correlations with clinical and laboratory characteristics,
mainly those associated with iron overload.
Materials and Methods
Study Design and Patient Population.
Patients maintained on transfusion therapy either currently, or
previously, were screened for eligibility between April 2014 and
April 2015.
Fifteen patients with SCD who are followed-up in the
Thalassemia and Sickle Cell Unit of Hippokrateion General Hospital in
Athens, Greece were enrolled in the study. All patients completed the
study.
Five patients had HbS/HbS, and thirteen had
HbS/beta-thal; their median age was 45,8 years (range: 19–75
years). Seven patients were males and eight females.
Patients
received regular blood transfusions or had sporadically transfused with
at least 20 units of packed red blood cells during the last five
years. Exchange or simple transfusions were allowed. Transfused
red cells were negative for hemoglobin S, phenotypically matched and
depleted of leukocytes and were delivered in a volume of approximately
10 to 15 ml per kilogram of packed cells per transfusion. The goal of
the transfusion protocol for all patients was to maintain their
hemoglobin S (HbS) percentage at or below 50% and the pre-hemoglobin
and post-hemoglobin greater than 9 g/dL and less than 12 g/dL,
respectively.
The initial dose of DFX was calculated based on
the patient's body weight (10-40 mg/kg/day). The 20-mg/kg dose was
considered appropriate for patients requiring reduction of a moderate
iron burden, and a higher dose of 30-40 mg/kg was felt to be
appropriate for patients with high iron burdens requiring major
reduction of excess iron. Lower doses of 10-mg/kg were selected
for maintenance use in patients with lower LIC values. DFX was taken
daily every morning 30 minutes before breakfast, dispersed in a glass
of water. Prior chelation therapy was permitted but was not
mandatory. The serum ferritin level for entry into the study was ≥500
μg/l.
Patients eligible for entry into the study had performed MRI
using a multi-gradient recalled echo (MGRE) sequence which allowed the
determination of liver T2*, a relaxation time constant sensitive to the
presence of liver iron, inversely proportional to liver LIC (Liver Iron
Concentration).
They also had performed Liver stiffness
measurement (LSM) using transient elastography (Fibroscan). A
pulse-echo ultrasound acquisition is used to follow the propagation of
the shear wave and to measure its velocity, which is directly related
to tissue stiffness and the severity of liver fibrosis.
The
patients were evaluated at the enrollment and at the end of the study.
Laboratory assessments were performed at least monthly and included
complete blood counts with differential counts; Alanine Transaminase
[ALT], Aspartate Transaminase [AST] Lactate Dehydrogenase [LDH]
and ferritin. The concentrations of high sensitive C-reactive protein
were also evaluated.
Urinary testing performed on random
collections included determination of creatinine, total protein, and
albumin. Physical examinations, electrocardiograms (ECGs), audiometry
and ophthalmological tests were performed at baseline. The study
duration was 52 weeks (12 months).
Patients were excluded if
they had a serum creatinine above the upper limit of normal if they had
significant proteinuria or if they had active or chronic hepatitis B or
C. Other exclusion criteria were second and third
atrioventricular block, QT interval prolongation, or therapy with
digoxin or similar medications. Treatment with β-blockers or
angiotensin-converting enzyme inhibitors was permitted. Patients with
chelation therapy-associated ocular toxicity were excluded. No one
patient had clinical or imaging findings suggesting the presence of
liver cirrhosis at baseline or at the end of the study.
Statistical analysis.
Data are reported as mean ± SD. Comparisons among groups were made
using one-way analysis of variation (ANOVA) analyses, where P < 0·05
was considered statistically significant.
Post hoc tests
were Student’s t-test for paired variables and Wilcoxon
nonparametric test. All P values are two-sided and considered
significant with P ≤ 0.05. The Pearson's correlation coefficient r with
p-value is used to measure the strength of a linear association between
Ferritin, LIC biochemistry and Fibroscan and hs-CRP variables.
Statistical analyses were performed using MedCalc for Windows, version
15.0 (MedCalc Software, Ostend, Belgium).
Results
The
study completed 15 patients. Summary of the parameters and evaluated
values at baseline and at the end of the study are given in table 1.
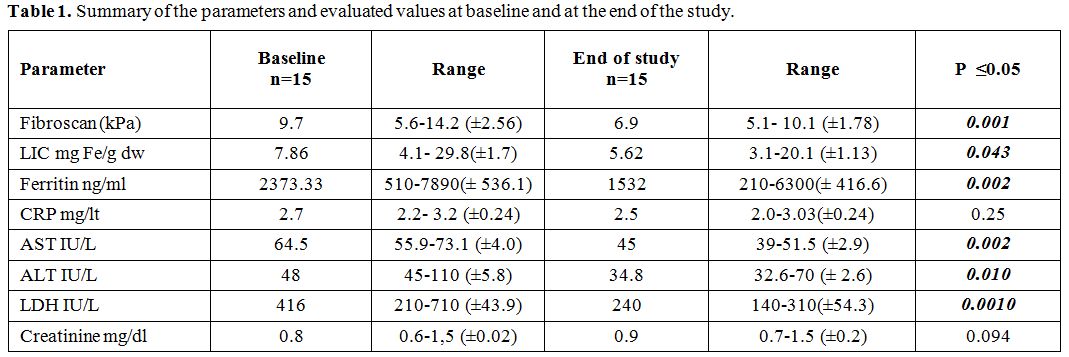 |
Table 1. Summary of the parameters and evaluated values at baseline and at the end of the study. |
After
12 months (52 weeks) of deferasirox therapy, a significant improvement
in LIC from 7.86 to 5.62 mg range:3.1-20.1 mg Fe/g dry
weight p=0.043 was found (Figure A1), followed by significant improvement of serum ferritin mean from 2373.33 to 1532 ng/ml range: 210-6300, p=0.002 (Figure A2).
The above findings were followed by an improvement in liver stiffness from 9.7 kPa to 6.7 kPa range:5.1-10.1, p=0.001 (Figure A3).
A significant improvement in AST, ALT, and LDH at 52 weeks was also
noted. There was no significant difference in hs-CRP and serum
creatinine from baseline to end of the study.
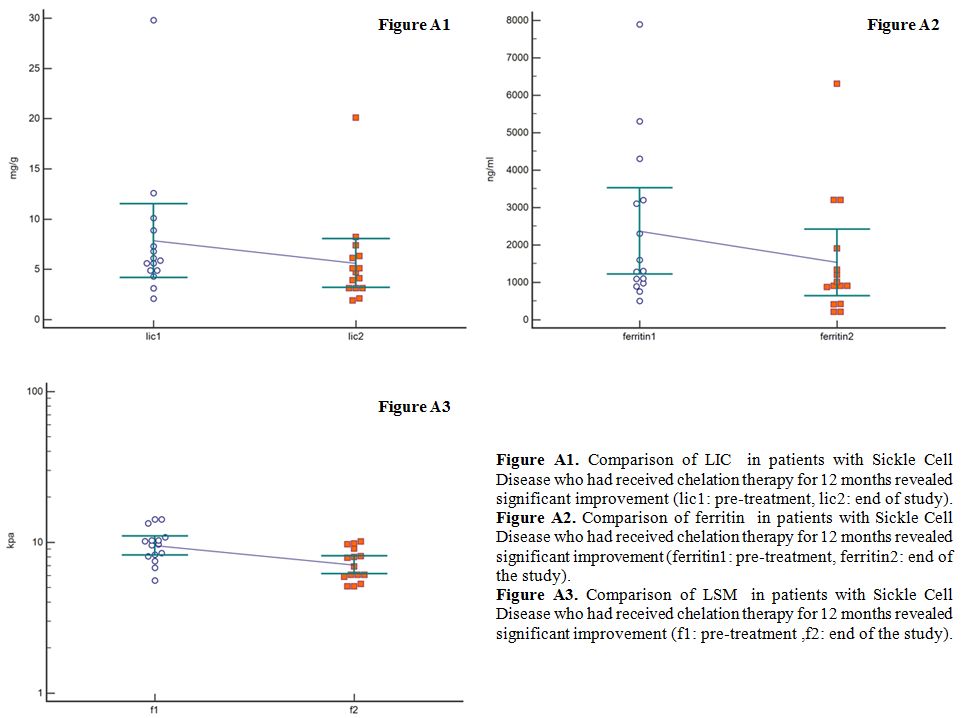 |
Figure A1. Comparison of
LIC in patients with Sickle Cell Disease who had received
chelation therapy for 12 months revealed significant improvement (lic1:
pre-treatment, lic2: end of study).
Figure A2. Comparison of
ferritin in patients with Sickle Cell Disease who had received
chelation therapy for 12 months revealed significant improvement
(ferritin1: pre-treatment, ferritin2: end of the study).
Figure
A3. Comparison of LSM in patients with Sickle Cell Disease who
had received chelation therapy for 12 months revealed significant
improvement (f1: pre-treatment , f2: end of the study). |
A
significant correlation (r1: pre-treatment, r2: after treatment)
between ferritin levels and LIC (r1=0.862 and r2=0.9298) and
between ferritin and LSM (r1=0.6905 r2=0.7936) was found respectively.
Furthermore, the correlation between LIC and LSM was
statistically significant at baseline(r1=0.6344) and at the end of the
study (r2=0.6075). No correlations were found between the other
parameters (Table 2).
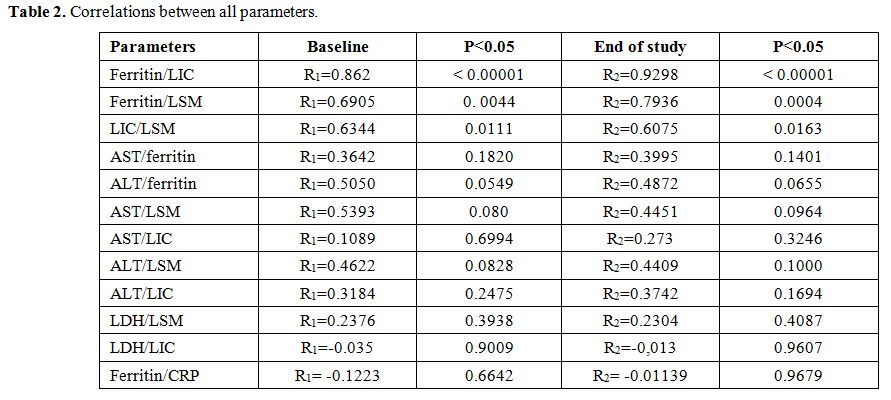 |
Table
2. Correlations between all parameters. |
Safety and tolerability.
During the study 39 Adverse Events were reported. The most common AEs
reported were abdominal pain 41% (7/39), diarrhea 38,5% (15/39), nausea
10,3% (4/39), and nasopharyngitis 7,7% (3/39). Nausea and abdominal
pain were reported on the same day in those patients. Sickle cell
painful crisis 2,6% (1/39) was managed at home. No serious AEs were
experienced during the study.
Serum creatinine levels were mostly
stable during the study. Calculated creatinine clearance with Cockcroft
and Gault formula remained stable during deferasirox treatment. Eight
patients who received concomitant hydroxycarbamide during the study
remained relatively stable in the liver and renal function.
Discussion
Transfusion
therapy is a key intervention in decreasing morbidity and mortality in
patients with sickle cell disease. Transfusions and/or exchange
transfusions first demonstrated their effectiveness in reducing
recurrent strokes in SCD. Transfusions have also proved to be effective
prophylaxis in high risk patients for the first stroke and in other
complications such as acute chest syndrome.[12,13]
The
severity and mechanism of body iron overload in SCD is completely
different compared to the iron overload that occurs in thalassemia
major. Transfusion-acquired iron overload in the heart is rare in
sickle cell disease, probably because iron released by transfusion and
haemolysis is efficiently handled by the effective erythropoiesis of
sickle cell disease, but not as well by the ineffective erythropoiesis
in thalassaemia.[2,14,15]
Sickle
cell disease is also associated with a chronic inflammatory state and
increased haemolytic status related to vasocclusive crisis.
Consequently,
guidelines such as those in the UK currently recommend initiating iron
chelation therapy in patients with SCD once LIC increases to ‡7 mg Fe/g
dry weight if serum ferritin steady-state levels are >1000
μg/l, or at least 20 top-up transfusions.[16]
The
gold standard for assessing liver iron stores is the hepatic iron
content determined by liver biopsy, but this technique is limited
because it is invasive and carries a risk of complications. Noninvasive
methods including lood tests and imaging techniques have been
evaluated and considered in greater detail.[17,18,19]
Studies
of liver biopsies in patients with SCD have linked transfusional iron
load with LIC, fibrosis, and cirrhosis. If transfusion is given without
chelation, portal fibrosis can develop as early as two years after
transfusion. With sequential biopsies, increased fibrosis was found in
1/3 of patients with LIC values > 9 mg/g dry weight and in direct
proportion to the LIC.[20,16]
TE
is an ultrasound-based tool for measuring liver stiffness as a
surrogate of fibrosis that is widely used due to its high accuracy for
the diagnosis of fibrosis stage.[21,22]
Liver
stiffness also correlates with cirrhosis complications including
variceal hemorrhage, ascites, and hepatocellular carcinoma (HCC). The
iron effect in the pathogenesis of fibrosis due to increased oxidative
stress and other pathological modes of action of HCV, ethanol, and
steatosis, lead to mitochondrial dysfunction and hepatocyte apoptosis.[23]
Hepatocellular carcinoma following liver cirrhosis as a complication of
chronic hepatitis C and iron overload has been reported in thalassemia
patients.[24,25] Cirrhosis, is the strongest and the
most common known risk factor for HCC, is frequently found in
thalassaemia patients as it has been described from the Italian
Registry.[24,25,26,27]
In Drasar’s research
paper (2016) have shown that transfusion and markers of iron overload
were weakly but significantly correlated with T.E and enhanced liver
fibrosis score (ELF) using standard markers of liver function.[21]
In
our study LIC, LSM and serum ferritin level were significantly reduced
after 12 months of deferasirox treatment indicating that over 12
months, deferasirox significantly reduced liver iron burden in these
iron-overloaded patients with SCD.
Also, ALT, AST, and LDH
levels significantly decreased after 12 months of therapy suggestive an
improvement of liver inflammation. Furthermore, ferritin and LIC
significantly correlate with hepatic stiffness before and after
deferasirox administration suggesting that reduction of iron in the
liver leads to stiffness improvement. Our results are in agreement with
Deugnier (2011) and Adams (2011) that deferasirox can lead
to regression of fibrosis, improving liver stiffness and correlating
with iron removed.[28,29]
Adequate chelation therapy is mandatory to prevent liver disease progression in sickle cell disease patients.
Additionally
both AST and ALT showed significant improvement over the course of the
study but showed no correlation with liver stiffness. Although
transaminases are markers of inflammatory liver reaction and is well
established that high levels influence the elastographic findings, the
absence of correlation in our study is due to mildly increased levels
of transaminases at the onset of the study.
Hs-CRP remained stable from the beginning to the end of the study, and show no correlation with ferritin or other parameters.
In
addition, studies of deferasirox in patients with thalassemia provided
evidence of significant reduction in hepatic fibrosis irrespective of
hepatic iron concentration or HCV prevalence. Our results clearly
demonstrate that the observed improved of hepatic stiffness is mainly
associated with the reduction of hepatic iron and not by the
improvement of inflammatory status.[30,31]
Patients
with the sickle cell disease are at risk for significant hepatic
complications, and better definitions and markers could be utilized to
understand the pathophysiology of hepatic involvement. Taken together,
these results suggest that TE is a useful and less expensive than the
MRI tool to identify the stage of stiffness/fibrosis in patients
with SCD at steady state and to monitor the efficacy of chelation
therapy.
Conclusions
In this study, LSM
shows a strong relationship with LIC. Both baseline LSM and LIC changed
and correlated with ferritin at the end of the study. The efficacy of
deferasirox in hepatic iron removal in patients with sickle cell
disease also improves the inflammatory response. Transient Elastography
(TE) is a useful tool for assessing the response of liver iron
chelation, it is much more widely accessible, and it is also useful for
more intensive surveillance of liver stiffness. TE is a relatively low
cost easy to perform the test, with high accuracy evaluating liver
stiffness in this patients reflecting both fibrosis and hemosiderosis
on the top of surrogates markers of iron overload.
References
- Chaturvedi Shruti, and Michael R. DeBaun. Evolution
of sickle cell disease from a life‐threatening disease of children to a
chronic disease of adults: The last 40 years. American Journal of
Hematology 91.1 (2016): 5-14. https://doi.org/10.1002/ajh.24235 PMid:26547630

- Porter
John and Maciej Garbowski. Consequences and management of iron
overload in sickle cell disease. ASH Education Program Book 2013.1
(2013): 447-456. https://doi.org/10.1182/asheducation-2013.1.447

- Lucania
Gaetano et al. "Chelation treatment in sickle‐cell‐anaemia: much ado
about nothing?." British Journal of Haematology 154.5 (2011): 545-555. https://doi.org/10.1111/j.1365-2141.2011.08769.x PMid:21707578

- Cappellini
Maria Domenica et al. Tailoring iron chelation by iron intake and serum
ferritin: the prospective EPIC study of deferasirox in 1744 patients
with transfusion-dependent anemias. Haematologica 95.4 (2010): 557-566.

- Vichinsky
Elliott et al. Long‐term safety and efficacy of deferasirox (Exjade®)
for up to 5 years in transfusional iron‐overloaded patients with sickle
cell disease. British Journal of Haematology 154.3 (2011): 387-397. https://doi.org/10.1111/j.1365-2141.2011.08720.x PMid:21592110 PMCid:PMC3170481

- Ebert
Ellen C., Michael Nagar and Klaus D. Hagspiel. Gastrointestinal and
hepatic complications of sickle cell disease. Clinical Gastroenterology
and Hepatology 8.6 (2010): 483-489. https://doi.org/10.1016/j.cgh.2010.02.016 PMid:20215064

- Karam
Lina B. et al. Liver biopsy results in patients with sickle cell
disease on chronic transfusions: poor correlation with ferritin levels.
Pediatric Blood & Cancer 50.1 (2008): 62-65. https://doi.org/10.1002/pbc.21215 PMid:17457853

- Brittenham
Gary M. Iron-chelating therapy for transfusional iron overload. New
England Journal of Medicine 364.2 (2011): 146-156. https://doi.org/10.1056/NEJMct1004810 PMid:21226580 PMCid:PMC3078566

- Stebbing
Justin et al. A meta-analysis of transient elastography for the
detection of hepatic fibrosis. Journal of Clinical Gastroenterology
44.3 (2010): 214-219. https://doi.org/10.1097/MCG.0b013e3181b4af1f PMid:19745758

- Fraquelli
Mirella et al. Transient elastography in the assessment of liver
fibrosis in adult thalassemia patients. American Journal of Hematology
85.8 (2010): 564-568. https://doi.org/10.1002/ajh.21752 PMid:20658587

- Koh
Christopher et al. Liver stiffness increases acutely during sickle cell
vaso‐occlusive crisis. American Journal of Hematology 88.11 (2013):
E250-E254. https://doi.org/10.1002/ajh.23532 PMid:23828202 PMCid:PMC3808506

- Smith‐Whitley
Kim and Alexis A. Thompson. Indications and complications of
transfusions in sickle cell disease. Pediatric Blood & Cancer 59.2
(2012): 358-364. https://doi.org/10.1002/pbc.24179 PMid:22566388

- Chou Stella T. Transfusion therapy for sickle cell disease: a balancing act. ASH Education Program Book 2013.1 (2013): 439-446. https://doi.org/10.1182/asheducation-2013.1.439

- Walter
Patrick B., Paul Harmatz, and Elliott Vichinsky. Iron metabolism
and iron chelation in sickle cell disease. Acta Haematologica 122.2-3
(2009): 174-183.

- Marsella
Maria and Caterina Borgna-Pignatti. Transfusional iron overload and
iron chelation therapy in thalassemia major and sickle cell disease.
Hematology/Oncology Clinics 28.4 (2014): 703-727.

- Sickle
Cell Society. Standards for the clinical care of adults with sickle
cell disease in the UK. Sickle Cell Society, 2008.

- Castera Laurent. Noninvasive assessment of liver fibrosis. Digestive Diseases 33.4 (2015): 498-503. https://doi.org/10.1159/000374097 PMid:26159265

- Patel
Keyur, Pierre Bedossa and Laurent Castera. Diagnosis of liver fibrosis:
present and future. Seminars in Liver Disease. Vol. 35. No. 02. Thieme
Medical Publishers, 2015.

- Ou
George et al. Utility of Transient Elastography in Estimating Hepatic
Iron Concentration in Comparison to Magnetic Resonance Imaging in
Patients Who are Transfusion-Dependent: A Canadian Center Experience.
Hemoglobin 41.1 (2017): 21-25. https://doi.org/10.1080/03630269.2017.1307763 PMid:28532285

- Hoffbrand, A. Victor, Ali Taher and Maria Domenica Cappellini. How I treat transfusional iron overload. Blood (2012). https://doi.org/10.1182/blood-2012-05-370098

- Drasar
Emma et al. Interim assessment of liver damage in patients with sickle
cell disease using new non‐invasive techniques. British Journal of
Haematology 176.4 (2017): 643-650. https://doi.org/10.1111/bjh.14462 PMid:27984631 PMCid:PMC5303160

- Voskaridou
Ersi et al. Liver transient elastography (FibroScan) correlates with
liver iron concentration and reflects liver fibrosis in patients with
sickle cell disease. Blood 2010 116:1646.

- Philippe
Marie A., Richard G. Ruddell, and Grant A. Ramm. Role of iron in
hepatic fibrosis: one piece in the puzzle. World Journal of
Gastroenterology: WJG 13.35 (2007): 4746.

- Kountouras
Dimitrios et al. Liver disease in adult transfusion‐dependent
beta‐thalassaemic patients: investigating the role of iron overload and
chronic HCV infection. Liver International 33.3 (2013): 420-427. https://doi.org/10.1111/liv.12095 PMid:23402611

- Maakaron
Joseph E. et al. Hepatocellular carcinoma in hepatitis-negative
patients with thalassemia intermedia: a closer look at the role of
siderosis. Annals of Hepatology 12.1 (2013): 142-146. PMid:23293206

- Restivo
Pantalone Gaetano et al. Hepatocellular carcinoma in patients with
thalassaemia syndromes: clinical characteristics and outcome in a long
term single centre experience. British Journal of Haematology 150.2
(2010): 245-247.

- Borgna‐Pignatti
Caterina, et al. Hepatocellular carcinoma in thalassaemia: an update of
the Italian Registry. British Journal of Haematology 167.1 (2014):
121-126. https://doi.org/10.1111/bjh.13009 PMid:24992281

- Deugnier
Yves et al. Improvement in liver pathology of patients with
β-thalassemia treated with deferasirox for at least 3 years.
Gastroenterology 141.4 (2011): 1202-1211. https://doi.org/10.1053/j.gastro.2011.06.065 PMid:21741344

- Adams
Paul C. Chelation therapy for secondary iron overload: is the primary
effect less iron or less liver fibrosis?. Gastroenterology 141.4
(2011): 1142-1143. https://doi.org/10.1053/j.gastro.2011.08.022 PMid:21871454

- Angelucci
E. et al. Iron Chelation Therapy with Deferasirox (Exjade®, ICL670) or
Deferoxamine Is Effective in Reducing Iron Overload in Patients with
Advanced Fibrosis and Cirrhosis. Blood 2005 106:2696.

- Deugnier
Y. et al. 189 Effect of Iron Chelation Therapy with Deferasirox
(EXJADE®, ICL670) or Deferoxamine on Hepatocellular Inflammation and
Liver Function in Patients with Transfusiondependent Anemia. Journal of
Hepatology 48 (2008): S79-S80. https://doi.org/10.1016/S0168-8278(08)60191-9

[TOP]


































