Shuvra Neel Baul1, Rajib De1, Prakas Kumar Mandal1, Swagnik Roy2, Tuphan Kanti Dolai1 and Prantar Chakrabarti1.
1 Faculty; Department of Haematology, NRS Medical College, Kolkata.
2 Department of Microbiology, KPC Medical College and Hospital; Jadavpur, Kolkata
Correspondence to: Rakas Kumar Mandal, Department of Hematology, Nrs Medical College; Kolkata. E-mail:
prakas70@gmail.com
Published: September 1, 2018
Received: March 9, 2018
Accepted: July 20, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018051 DOI
10.4084/MJHID.2018.051
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background. Burkholderia cepacia,
an aerobic gram-negative bacillus, is a frequent colonizer of fluids
used in the hospital ward. It poses little risk of infection to healthy
people; however it is a known important opportunistic pathogen causing
morbidity and mortality due to its intrinsic resistance to most of the
antibiotics in hospitalized patients. Small hospital outbreaks are
frequent. B. cepacia may occur as an opportunistic infection in hemato-oncology patients. Here we present an outbreak of Burkholderia cepacia infection in hematology ward of our institute.
Methods.
Febrile episodes as defined by IDSA guideline, 2010 were followed, and
blood for culture and sensitivity was sent in all the events. The
culture was done by an automated method using Bactalert 3d Biomeriux
& sensitivity pattern by Microscan Siemens method and subsequently
detected by PCR based method.
Results.
During September 2016 to February 2017 (six months), a total of 498
blood cultures were sent during febrile episodes. Out of which 60 (12%)
came out to be positive for different microorganisms. Out of all
positive cultures, Burkholderia cepacia
was detected in 29 (48%) patients, which reduced drastically following
the change in antibiotic administration practice. All isolates showed
sensitivity to pipercillin+tazobactum, cefoperazone+sulbactum,
fluoroquinolones, cotrimoxazole and carbapenems and resistance to
polymyxin B and colistin. With timely intervention by appropriate
intravenous antibiotics as per culture sensitivity result and change in
antibiotic preparation practice, overall mortality was low 1 (4%) out
of 29 culture positive episodes.
Conclusion. Change of antibiotic preparation practice was the key to control this outbreak, and overall mortality was low.
|
Introduction
Burkholderia cepacia (B. cepacia) is an aerobic gram-negative bacillus which is catalase negative, non-lactose fermenting gram-negative bacterium[1] B. cepacia
is frequent colonizer of fluids used in the hospital ward (e.g.,
irrigation solutions, intravenous fluids, antiseptic solutions).[2] B. cepacia
poses little risk of infection to healthy people; however it is a known
important opportunistic pathogen causing morbidity and mortality due to
its intrinsic resistance to most of the antibiotics in hospitalized
patients.[3] Small hospital outbreaks are frequent and
are usually due to single contaminated source such as disinfectant,
intravenous solutions, nebulizer solutions, mouthwash, and medical
devices, including respiratory therapy equipment.[2,4]
Infection with B. cepacia is uncommon in the hematological setting. However, B. cepacia may occur as an opportunistic infection in hemato-oncology patients.[5] Here we present an outbreak of B. cepacia infection in hematology ward of our institute.
Materials and Methods
Setting and patients:
Department of Haematology, Nil Ratan Sircar Medical College, and
Hospital, a state-run medical college under Government of West Bengal,
is a dedicated Haematology & Haemato-oncology set up where admitted
patients (either neutropenic or non-neutropenic) having febrile
episodes as a consequence of chemotherapy or other intensive
treatments. Febrile neutropenia was defined as per Infectious Disease
Society America (IDSA) 2010 guidelines.[6] Considering
the immune-compromised status in these group of patients, we also
routinely do perform blood culture test in any febrile episodes even in
non- neutropenic state. We collaborated with the Microbiology
Department of KPC Medical College and Hospital, Jadavpur within the
same city of Kolkata for microbiological assessment of the specimens.
Bacterial cultures and identification.
This is a retrospective analysis of total 498 febrile neutropenic
episodes during the period from September 2016 to February 2017 (six
months). Blood cultures were sent in all febrile episodes in Bactalert
FA plus culture bottles (bioMerieux, France). A standard protocol was
followed for proper storage of culture bottles as mentioned in
manufacturer instructions. Blood was collected with the utmost
sterility, and 10 ml blood was cultured in each BacT/ALERT bottles.
Further identification of microorganisms and sensitivity pattern to
antibiotics were carried out by Microscan NBC42panel (Siemens
Healthcare Diagnostics, West Sacramento, CA, USA).
Environmental sampling and culture.
As part of the outbreak investigation, the following items were
cultured: oxygen masks, stock materials of fluids for intravenous (IV)
administration, venous catheters, intravenous sets, antiseptic
solutions, a swab from hospital floor. These environmental samples were
primarily cultured in 10 mL Brain Heart Infusion broth (HIMEDIA LAB,
INDIA) and B. cepacia Agar Base and B. cepacia Selective Supplement. CODE: SR0189 and CODE: CM0995; manufactured by Oxoid Scientific (Thermo), UK.
B. cepacia complex
(Bcc) species identification and assessment of clonal relatedness: We
used conventional polymerase chain reaction (PCR) method to identify
the organism; the details of primers used are given table 1, where we followed the PCR method for Burkholderia as per Lynch report.[7]
He reported two sets of primer pairs detecting Burkholderia species and
the second one specific for Bcc. 20 µL mixture was prepared using 200
µM of dNTP, 1.5 Mm MgCl2, 50 pmol of
each primer, 1.25 units of Taq polymerase and 1X PCR buffer. The PCR
was performed on a gradient PCR (Verity, Applied Biosystems) by the
following programme: 96°C for 4 minutes, followed by 35 cycles at 96°C,
59°C and 72°C each for 1 minute and lastly 59°C for 2 minutes.
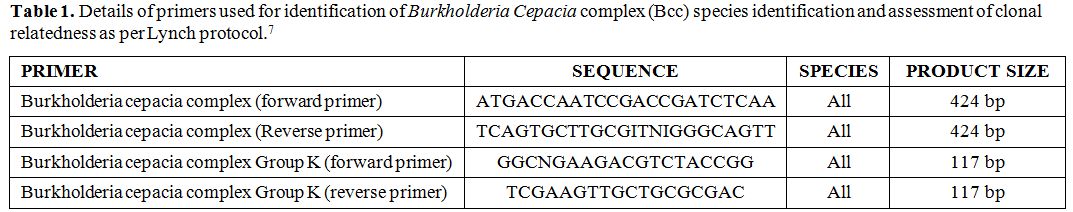 |
Table 1. Details of primers used for identification of Burkholderia Cepacia complex (Bcc) species identification and assessment of clonal relatedness as per Lynch protocol |
Genotyping.
DNA polymorphisms of all isolates were evaluated by PFGE (pulsed-field
gel electrophoresis) with specimen I, 20 DNA patterns were compared by
standard DNA marker interpreted by visual inspection. PCR products were
separated on 0.8% (wt/vol) agarose gels in 1X Tris-acetate-EDTA
(pH 8.0).
For every case, the MIC50 and MIC90 values against each antibiotic were recorded meticulously as per the CLSI guidelines,[8] and the sensitivity/resistance pattern was determined.
Results
This
outbreak occurred in our hematology ward from September 2016 to
February, 2017. All patients included in this study were having various
hematological malignancies (as shown in figure 1A) including acute leukemia (ALL, AML, MPAL) and high-grade lymphomas (DLBCL). As shown in figure 1B,
the patients were on different intensive chemotherapy protocols
including BFM-90, UKALL, R-CODOX-M. All patients had a central venous
catheter (CVC’s), the majority a peripherally introduced central
catheter (PICC). The dressings at the insertion site were changed
regularly by using povidone-iodine solution for antisepsis. The
demographic profile of the patients during febrile episodes are shown
in table 2. Mean age, mean
total leukocyte count (TLC) and absolute neutrophil count (ANC) of the
patients were 21 years (range: 6-48), 1440/μl (range: 640-5000) and
620/μl (300-2800).
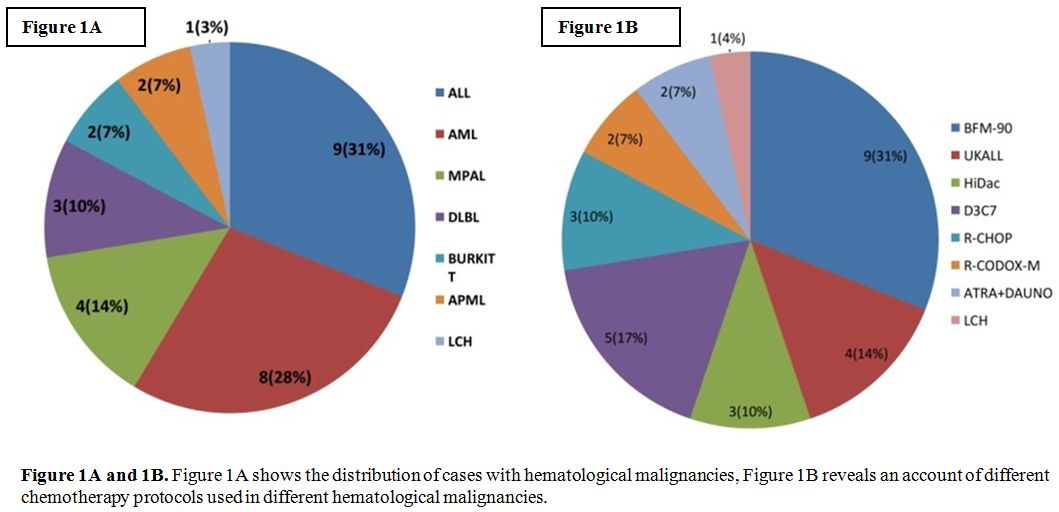 |
Figures 1A and 1B.
Figure 1A shows the distribution of cases with hematological
malignancies, Figure 1B reveals an account of different chemotherapy
protocols used in different hematological malignancies. |
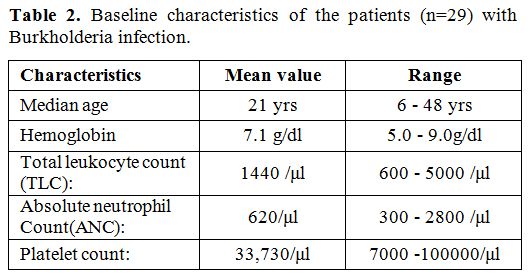 |
Table 2. Baseline characteristics of the patients (n=29) with Burkholderia infection. |
Total
of 498 febrile episodes occurred during this period. Blood culture was
positive in 60 episodes representing only 12%. There was the growth of B. cepacia in 29 episodes of febrile neutropenia. So, out of 60 positive culture episodes, B. cepacia represented 48% of all culture-positive organisms. On further analysis, B. cepacia alone was the major pathogen isolated. On genotyping with the forward and reverse primer used for clonal related of B. cepacia
complex, we first got a gene product of 424 bp size that was further
characterized by using the specific primer for the species
identification as mentioned in table 1
and got a gene product of 117 bp size. And, in all cases, we could
confirm the clonal relatedness to Burkholderia and the species
identified as Bcc.
For every case, the MIC50 and MIC90 value
against each antibiotic were noted as per the CLSI guidelines. Amongst
the most resistant group of antibiotics (cephalosporins), specially
ceftazidime showed MIC50 and MIC90 values of 2 and 32 μg/ml,
respectively (sensitive ≤8 μg/ml and resistant ≥32 μg/ml according to
CLSI guideline). Likewise, the MIC50 and MIC90 values against each
antibiotic were noted meticulously.
Depending upon the
sensitivity pattern of microorganisms, as an institutional policy,
within one hour of onset of febrile episodes, we use to start initial
antibiotic therapy with either piperacillin-tazobactam or
cefoperazone-sulbactam intravenously. After the culture reports were
available, either we continued with the antibiotic started initially or
changed according to the sensitivity pattern. The sensitivity pattern
of B. cepacia
to various antibiotics was analyzed in detail and analysis showed that
the organism was sensitive to piperacillin-tazobactam, carbapenems,
fluoroquinolones, cotrimoxazole and chloramphenicol (Figure 2). On further analysis of the resistance pattern of B. cepacia,
we found that it was resistant to aminoglycosides, cephalosporins,
third and fourth generation cephalosporins, colistin and polymyxin B
and few had shown intermediate sensitivity pattern (Figure 2).
For those, initially started with cefepime/amikacin, subsequently
changed to the appropriate antibiotic as soon as the culture report was
made available.
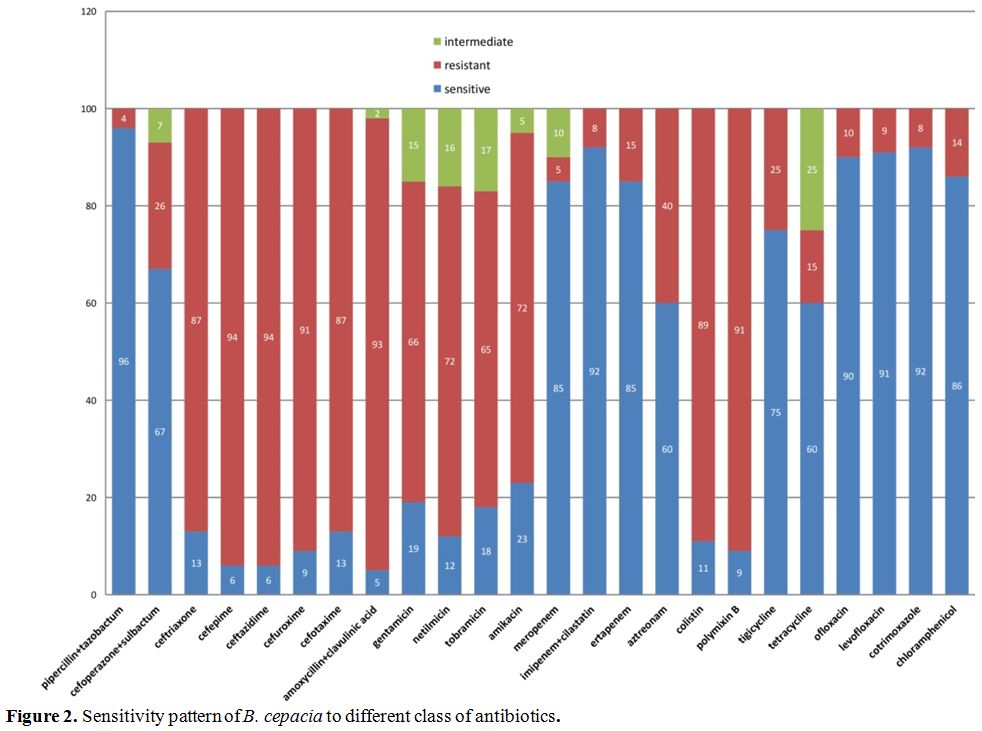 |
Figure 2. Risk Factors for infections in MDS High-risk. += risk factor; + =no
risk factor. |
One patient of acute myeloid leukemia on day10 of induction chemotherapy with B. cepacia
infection died during the study period. This patient had also CVC
induced thrombosis and possible fungal infection in lungs as suggested
by ground glass pattern of infiltration in high-resolution computed
tomography (HRCT). In all other 28 episodes, patients responded to
appropriate antibiotics as per culture and sensitivity pattern. To
identify the possible sources of infection culture samples were sent
from the hospital floor, walls, and antiseptic solutions. No growth
of B. cepacia was identified from any site.
Our
tertiary care teaching hospital with hematology-oncology care facility
catering to people of low socio-economic status mostly, have many
resources constraints such as- provision/facility of no separate room
for the patients undergoing intensive chemotherapy sharing multiple
beds in a single room, common bathroom, very low nurse-patient ratio.
On further departmental inquiry for the possible cause of this
outbreak, it was revealed that the health care providers used to
prepare the intravenous antibiotics at late night supposed to be given
in the next morning and store in the refrigerator. Since Bcc is an
organism with a capacity to grow in antiseptic solutions, it might have
grown in prepared antibiotic solutions. So we considered these practice
could have precipitated the B. cepacia
outbreak. This practice was stopped with our intervention following
which Burkholderia outbreak came down sharply from 48% to 9% in the
next three months (Figure 3).
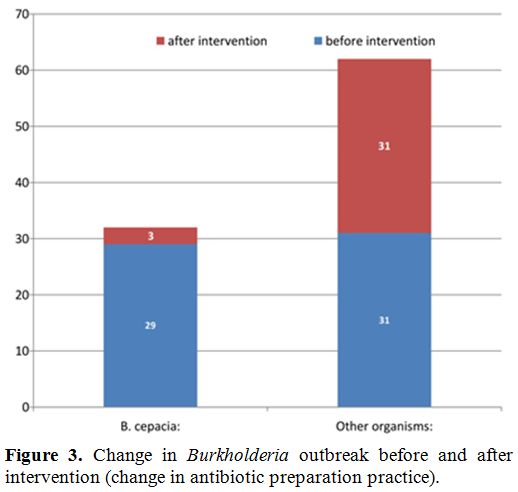 |
Figure 3. Change in Burkholderia outbreak before and after intervention (change in antibiotic preparation practice). |
Discussion
In our study, we found that B. cepacia
infection is an opportunistic gram-negative infection in patients with
hematological malignancy receiving chemotherapy. Such outbreaks are not
uncommon.[9] Fever with chills and rigor was the
universal manifestation in all episodes as initial presentation. In a
recent study in febrile neutropenia with hematological malignancy done
by Mandal PK et al[10] from eastern part of India
showed that, eight commonest isolates are Pseudomonas aeruginosa (14.10
%), methicillin-resistant Staphylococcus aureus (MRSA-12.82 %),
Acinetobacter species (11.53 %), coagulase-negative Staphylococcus
(10.25 %), Klebsiella pneumoniae (8.97 %), Escherichia coli (8.97 %),
ESBL E. coli (6.41 %), methicillin-sensitive S. aureus (MSSA-6.41 %).
B. cepacia is a gram-negative organism and is a common infection in patients with cystic fibrosis[11]
with lung involvement or chronic granulomatous disease, usually due to
contamination of medical devices or products. Its intrinsic resistance
to many disinfectants, antiseptic solutions, and antibiotics makes
infection control particularly problematic.[12]
Though many reports of Bcc outbreaks are there in intensive care units,
hemodialysis clinic, and also oncology departments, but reports
regarding hematology patients are few.[8,12,13]
The very pertinent issues regarding these organisms is that the
virulence of sepsis is no different from other gram-negative organism
and considering the prolonged hospital stay of our patients the
organism may become multi-antibiotic resistant.[14]
Moreover Burkholderia outbreak can occur due to the growth of the
organism in saline, antiseptic solutions as established by others.[2,4]
Another very interesting fact about B. cepacia
infection is that cross-transmission is the very common mode of spread
although all the studies are in patients with cystic fibrosis.[15] While investigating an outbreak of B. cepacia bacteremia in a tertiary care center by Abdelfattah R et al.[16], B. cepacia
was isolated from the blood cultures of 14 patients resulting from
contamination of the gel applied to the ultrasound probe used to guide
the insertion of a central venous catheter. Gill JS et al[17]
from a tertiary care hospital in India reported 17 cases of cardiac
pacemaker pocket infection including multidrug-resistant B. cepacia
(n=3) infection and concluded that every hospital should formulate
their antibiotic policy based on the pattern of the hospital flora and
their drug sensitivity.
All possible sources from our ward were
cultured, and no other causative source was identified and thus,
strengthened our proposal of overnight refrigeration of prepared
intravenous antibiotics was a possible cause for the outbreak of B. cepacia.
We
very meticulously noted the MIC50 and MIC90 values against every
antibiotic as per CLSI guidelines. Though other studies showed
sensitivity to ceftazidime,[11,18] but our study showed resistance to this antibiotic including many other cephalosporins as shown in figure 3. As shown in the study by Vardi et al[12],
the organisms had shown resistance to polymyxin B, Imipenem, and
aminoglycosides; our study also showed a similar type of resistance to
the antibiotics except for imipenem (including all other carbapenems)
which had shown excellent sensitivity. All the patients received
empirical antibiotics with Cefoperazone-Sulbactum or
Piperacillin-Tazobactum and became afebrile within three days of
intravenous antibiotic therapy. We didn’t have to escalate to
carbapenems in any of our patients. Antibiotics were continued for
10-14 days depending on the clinical scenario, and the repeat blood
culture report was negative. As shown in table 2,
the patients in the study had a mean ANC of 600/μl (range: 300-2800).
Thus, many patients had no neutropenia at the onset of fever. And this
signifies that patients may get infected with opportunistic organisms
like B. cepacia
due to the immune-compromised status related to primary hematological
malignancy and/or chemotherapy, even if the patient may not be in
neutropenic phase.
When we analyzed our outcome regarding mortality related to this outbreak, it was not disappointing. Only one patient with B. cepacia
sepsis suffering from acute myeloid leukemia died of sepsis on day 10
of her chemotherapy with DA 3+7 standard protocol. The death cannot
surely be attributed to B. cepacia sepsis as she also had developed possible fungal pneumonia with multi-organ failure.
We
work in a tertiary care university hospital with hematology-oncology
care facility catering to people of low socio-economic status, mostly.
Due to obvious reasons, there are many resource constraints such as-
provision/facility of no separate room for the patients undergoing
intensive chemotherapy, multiple beds in a single room with very little
inter-bed distance, common toilet facility, very low nurse-patient
ratio. On trying to search for the possible cause of this outbreak, it
was suddenly discovered that the health care providers used to
reconstitute the intravenous antibiotics and store them in the syringes
late at night in anticipation of administration of the drug on the next
morning. The reconstituted antibiotics used to be stored in the
refrigerator. Since Bcc is an organism with a capacity to grow in
antiseptic solutions, it might have grown in prepared antibiotic
solutions. So we hypothesized that this practice could have
precipitated the Bcc outbreak. In our urgency to save the lives of the
patients, the practice was immediately discontinued, and the
intervention resulted in the incidence coming down sharply from 48% to
9% in the next three months (Figure 3).
On hindsight, we realized that in our enthusiasm to act fast, we had
not sent the suspected fluid for culture and sensitivity. And, we
successfully managed to control the propagation of this outbreak
further by changing our antibiotic preparation practice.
Conclusion
B. cepacia,
an opportunistic infection initially reported in patients of cystic
fibrosis with lung involvement or chronic granulomatous disease,
usually is due to contamination of medical devices or products. Its
intrinsic resistance to many disinfectants, antiseptic solutions, and
antibiotics makes infection control particularly problematic. Many
reports of Bcc outbreaks are from intensive care units, hemodialysis
clinic, and also oncology departments, but reports regarding hematology
patients are few. Burkholderia sepsis in any clinical ward is a matter
of concern, as it may be considered as a surrogate indicator of
deficient barrier nursing care and breach of principles of asepsis. By
continued surveillance and active supervision of this unusual outbreak
could be checked and thus protected the at-risk immune-compromised
patients in the hemato-oncology ward.
References
- Mahenthiralingam E, Urban TA, and Goldberg JB. The
multifarious, multi replicon Burkholderia cepacia complex. Nature
Reviews Microbiology 2005; 3: 144–56. https://doi.org/10.1038/nrmicro1085 PMid:15643431

- Heo
ST, Kim SJ, Jeong YG, et al. Hospital outbreak of Burkholderia stabilis
bacteraemia related to contaminated chlorhexidine in haematological
malignancy patients with indwelling catheters; J Hosp Infect 2008; 70:
241-45. https://doi.org/10.1016/j.jhin.2008.07.019 PMid:18799235

- Baylan
O. An opportunistic pathogen frequently isolated from immunocompromised
patients: Burkholderia cepacia complex. Mikrobiyol Bul 2012; 46:
304-18. PMid:22639321

- De
Smet B, Veng C, Kruy L, et al. Outbreak of Burkholderia cepacia
bloodstream infections traced to the use of Ringer lactate solution as
multiple-dose vial for catheter flushing, Phnom Penh, Cambodia. Clin
Microbiol Infect 2013; 19: 832-37. https://doi.org/10.1111/1469-0691.12047 PMid:23173820

- T.
Mann, D. Ben-David, A. Zlotkin, et al. An outbreak of Burkholderia
cenocepacia bacteremia in immunocompromised oncology patients.
Infection 2010; 38: 187–94. https://doi.org/10.1007/s15010-010-0017-0 PMid:20358245

- Freifeld
AG, Bow EJ, Sepkowitz KA, et al. Clinical Practice Guideline for the
Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010
Update by the Infectious Diseases Society of America; Clinical
Infectious Diseases 2011; 52: e56–e93. https://doi.org/10.1093/cid/cir073 PMid:21258094

- Lynch
KH, Dennis JJ. Development of a Species-Specific fur Gene-Based Method
for Identification of the Burkholderia Cepacia Complex. J Clin
Microbiol 2008; 46: 447-55. https://doi.org/10.1128/JCM.01460-07 PMid:18057135 PMCid:PMC2238088

- Performance
standards for antimicrobial susceptibility testing; Twentieth
informational supplement. Clinical and Laboratory Standards Institute
(CLSI): Wayne, PA; 2010
- Abe K, D'Angelo
MT, Sunenshine R, et al. Outbreak of Burkholderia cepacia bloodstream
infection at an outpatient hematology and oncology practice. Infect
Control Hosp Epidemiol 2007; 28: 1311–313. https://doi.org/10.1086/522679 PMid:17926285

- Mandal
PK, Maji SK, Dolai TK, et al. Micro-organisms Associated with Febrile
Neutropenia in Patients with Haematological Malignancies in a Tertiary
Care Hospital in Eastern India. Indian J Hematol Blood Transfus 2015;
31: 46–50. https://doi.org/10.1007/s12288-014-0393-1 PMid:25548444 PMCid:PMC4275510

- Jones
AM, Dodd ME, Govan JRW, et al. Burkholderia cenocepacia and
Burkholderia multivorans: influence on survival in cystic fibrosis.
Thorax 2004; 59: 948–51, https://doi.org/10.1136/thx.2003.017210 PMid:15516469 PMCid:PMC1746874

- Vardi
A, Sirigou A, Lalayanni C, et al. An outbreak of Burkholderia cepacia
bacteremia in hospitalized hematology patients selectively affecting
those with acute myeloid leukemia. Am J Infect Control 2013; 41: 312-6.
https://doi.org/10.1016/j.ajic.2012.04.325 PMid:23040605

- Metan
G, Demiraslan H, Kaynar LG, et al. Factors influencing the early
mortality in haematological malignancy patients with nosocomial Gram
negative bacilli bacteraemia: a retrospective analysis of 154 cases.
Braz J Infect Dis 2013; 17: 143-9. https://doi.org/10.1016/j.bjid.2012.09.010 PMid:23485438

- Burns
JL, Wadsworth CD, Barry JJ, et al. Nucleotide sequence analysis of a
gene from Burkholderia (Pseudomonas) cepacia encoding an outer membrane
lipoprotein involved in multiple antibiotic resistance. Antimicrob
Agents Chemother 1996; 40: 307-313. PMid:8834871 PMCid:PMC163107

- Ledson
MJ, Gallagher MJ, Corkill JE, Hart CA, Walshaw MJ; Cross infection
between cystic fibrosis patients colonised with Burkholderia cepacia.
Thorax 1998; 53: 432-36. https://doi.org/10.1136/thx.53.5.432 PMid:9708241 PMCid:PMC1745231

- Abdelfattah
R, Al-Jumaah S, Al-Qahtani A, et al. Outbreak of Burkholderia cepacia
bacteraemia in a tertiary care centre due to contaminated ultrasound
probe gel. J Hosp Infect 2018; 98: 289-294. https://doi.org/10.1016/j.jhin.2017.09.010 PMid:28923373

- Gill
JS, Singh N, Khanna SP. Risk of cardiac pacemaker pocket infection in a
tertiary care hospital. Indian J Pathol Microbiol 2017; 60: 185-188. https://doi.org/10.4103/IJPM.IJPM_190_16 PMid:28631632

- Roy
P, Ahmed NH, Biswal I, et al. Antimicrobial Susceptibility Pattern of
Burkholderia cepacia isolates from Patients with Malignancy. J Glob
Infect Dis 2014; 6: 90 - 91. https://doi.org/10.4103/0974-777X.132064 PMid:24926173 PMCid:PMC4049049

[TOP]






















