The extracellular environment is crucial for the physiological development of the nascent sprout interaction; cell surface receptors of the integrin family mediate adhesion to and signaling by the extracellular matrix (ECM). Indeed, the Integrin Receptor αvβ3 for Von Willebrand factor (VWF) is expressed on EC and has been shown to play a crucial role in angiogenesis.[4] In this line, malignant PC promote vessel formation through the expression of angiogenic molecules or their induction in the microenviroment.[5] Effectively, a striking feature of MM is the predominant localization of malignant PC in the bone marrow, close to stromal cells, where they secrete several angiogenic activators, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and HGF hepatocyte growth factor (HGF). They also activate matrix- metalloproteinase-degrading enzymes (MMPs) and regulator of the quiescence/angiogenesis
balance such as angiopoietins (Ang1/Ang2), involved in tumour-induced base membrane matrix remodeling, endothelial cell (EC) migration, and vessel formation.[4-8] The progression from in-situ to invasive, and to metastatic tumor has been described as a switch from avascular to vascular phase.[9,10] In MM, this transition which finally results in tumor growth has been associated with the imbalance of pro- and anti-angiogenic factors in the microenviroment. Active MM may represent the vascular phase, whereas smoldering myeloma (SM) and monoclonal gammopathy of undetermined significance (MGUS) may be considered the avascular phase in the spectrum of PC disorders. Actually, a gradual increase in the angiogenesis rate, the modulation of specific cell-cell adhesion molecules and secretion of MMPs play an important role in changing the bone marrow composition from benign conditions, such as MGUS, to SM and active MM.[10,11]
Based on these considerations, we analyzed the “angiogenic potential”, and the gene expression profiles of cell-cell adhesion molecules in the BM and peripheral blood (PB) PC from patients with MGUS, SM and active MM, in comparison with healthy subjects. To this purpose, we selected several pro-angiogenic factors, cell-cell adhesion molecules and Matrix-Metallo Proteinases, including VEGF, Ang-2, bFGF, MMP-2, MMP-9, VE-Cadh (vascular endothelial cadherin, CDH5), MCAM/MUC18 (endothelial antigen CD146) and E-Cadh (epithelial cadherin, CDH1), this last known to be involved in epithelial cell-cell adhesion processes to analyze all PC expressing CD138.
Sixty-one patients diagnosed with plasma cell diseases according to the International Myeloma Working Group guidelines[12] and 14 healthy donors were enrolled in this prospective study (median age 55 years). All patients were treated at the Hematology Department of “Tor Vergata” University of Rome and gave informed consent before study inclusion, according to the declaration of Helsinki. They were diagnosed and classified as follows: 13 MGUS, 25 SM, 23 MM, (three of which were extramedullary MM). Healthy controls included 14 BM donors (4 males and 10 females, mean age 44.6 years) and 30 PB donors (15 males and 15 females, with median age 45 years; range 18-55 years) accessing our Stem Cell Transplantation Unit. Since the expected frequency of circulating CD138+ cells is about 10-4, at least 30 ml PB was collected. CD138+ PC were isolated using the RosetteSep Multiple Myeloma Enrichment Cocktail as described by the manufacturer (Voden-Stem Cell Technology Inc., Milan, Italy), and purity of the selected population was assessed in selected cases (n=10) using immunophenotype. Briefly, after enrichment cells were stained with predefined optimal concentrations of the specific antibodies (anti-CD19, anti-CD45, anti-CD38 anti-CD138, anti-CD20 and anti-CD56), using standard conditions. At least 10000 events were acquired for each sample, gating for CD138+ cells. Gene expression of the above-mentioned pro-angiogenic factors, cell-cell adhesion molecules and Matrix-Metallo- Proteinases was also analyzed in primary cells (fibroblasts, EDS, endothelial Huvec) and tumor cell lines as positive and negative controls (Table 1). Total RNA was reverted to cDNA using oligod(T)16-18 as primers. Qualitative RT-PCR for all genes was performed on RNA isolated from CD138+ cell samples, using the oligonucleotide listed in the supplementary file and according to the manufacturer’s instructions (Applied BioSystems, Roche Molecular Systems, Inc., Branchburh, New Jersey, USA). All PCR experiments were performed in triplicate using the housekeeping gene beta2-microglobulin as an internal control. Due to the small cohort of samples, a 2-step analysis was performed. Differences between the four groups (MM, SM, MGUS and healthy donors) were analyzed using multiple pairwise comparisons, by the Marascuilo procedure that provides the magnitude of variation in the pairs of proportions and allows to simultaneously test the differences of all pairs of proportions, when there are several populations under investigation.
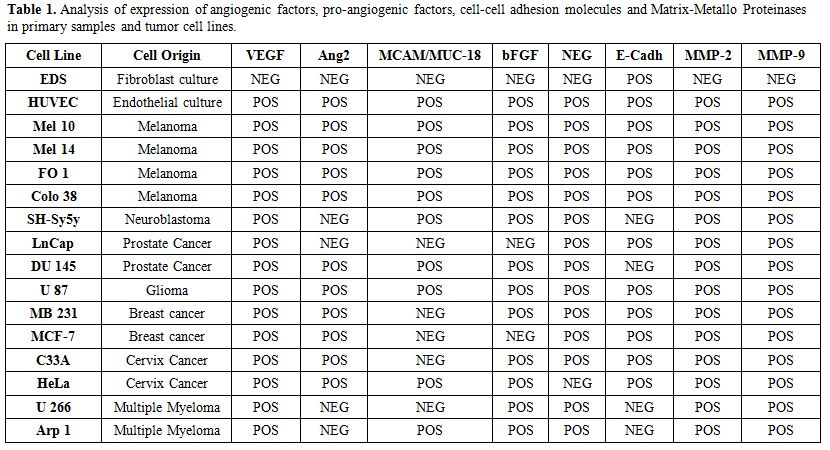 |
Table 1. Analysis of expression of angiogenic factors, pro-angiogenic factors, cell-cell adhesion molecules and Matrix-Metallo Proteinases in primary samples and tumor cell lines. |
In the second step, a binomial logistic regression (corrected for age and sex covariates and with backward stepwise elimination) was performed in order identify the genes whose expression was predictive of the transformation from MGUS to SM, and from SM to active MM.[13] Statistical significance was considered as P<0.01 for Marascuilo procedure, and P<0.05 for logistic regression.
We isolated a median of 2x106 CD138+ PC (range: 1-4 106 cells) from 2 ml BM or PB samples. The purity after enrichment was >96%, as measured by flow cytometry using a PE- conjugated anti-CD138 antibody, (Figure 1). Expression of investigated genes was also performed in primary control cells and tumor cell lines heterogeneous in primary control cells and tumor cell lines as summarized in Table 1. In particular, E-Cadh was expressed in most controls, except the neuroblastoma cell line (SH-Sy5y) and the two MM lines (Arp1 and U266), that tested negative. All four melanoma cell lines (M10, M14, FO1 and Colo38) expressed the endothelial antigen MCAM/MUC18, while the breast cancer cell lines (MB 231, MCF-7) and the androgen- dependent prostate cells (LNCap) were negative. Also, myeloma cell lines, (Arp-1, U266), did not express MCAM/MUC18/CD146, despite the use of a highly sensitive nested-PCR, as reported.[14]
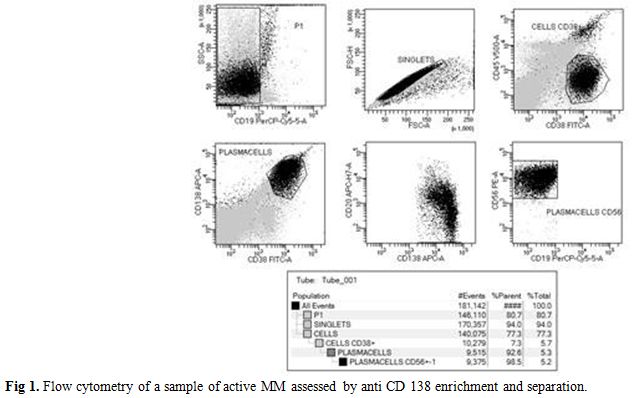 |
Figure 1. Flow cytometry of a sample of active MM assessed by anti CD 138 enrichment and separation. |
Circulating CD 138+PC displayed only weak not statistically significant mRNA expression (bFGF 12 pts, 40%; MMPs 4 pts, 13%; VE-CADH 8 pts, 26.6%; and VEGF 6 pts, 20%). Expression was constantly absent in the BM of healthy donors, except weak VE-Cadh and bFGF positivity. Analysis of BM-PC selected from individual patients showed concomitant expression of the angiogenic factors VEGF, MUC18/MCAM, and Ang-2, that characterized early stages of disease (MGUS and SM). On the other hand, bFGF and MMPs expression was only detectable during disease progression and active MM. E- Cadh was expressed by the PC of active MM (n=20) (100% positivity), excluding the three extra-medullary MM (Table 2 part a). The absence of expression of MMP-9 was predictive of stable MGUS, with no signs of progression to SM (OR=41.5, p=0.016), and the absence of E-Cadh expression defined stable SM, with no signs of evolution to symptomatic MM (OR=15.9. p<0.01). Indeed, MMP-9 expression improved classification of 69% of patients with MGUS and 84.6% of those with SM. Similarly, E-Cadh expression improved the definition of 76.9% of SM and 82.6% of symptomatic MM, including extramedullary multiple myeloma. Therefore, E- Cadh expression resulted not only suggestive but was predictive of the transition from SM to MM (OR=15.9 p<0.01), mostly in association with MMPs and bFGF expression (Table 2 part b).
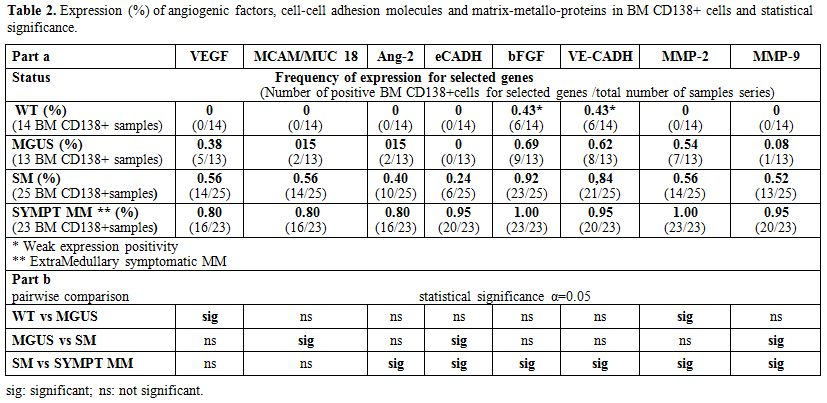 |
Table 2. Expression (%) of angiogenic factors, cell-cell adhesion molecules and matrix-metallo-proteins in BM CD138+ cells and statistical significance |
Angiogenesis and massive secretion of matrix- metalloproteinase-degrading enzymes occur in several solid tumors during invasion and metastasis and play a key role in the pathogenesis and progression of MM.[3-6] The results of our study support these data, reflecting the disease spectrum from MGUS to MM, and indicating an increase of angiogenesis, cell-cell adhesion and secretion of MMPs in the progression towards active MM.
This profile is in agreement with the “angiogenic switch” from the pre-vascular to the vascular phase in solid tumors proposed by Ribatti et al.[8,9]
To our knowledge, we report for the first time that E-Cadh expression, the main epithelial cell- adhesion molecule, was highly predictive of the transition from SM to symptomatic MM.[15] Accordingly, loss of E-Cadh-mediated adhesion characterizes the transition from benign lesions to invasive and metastatic cancer, associated with epithelial-mesenchymal conversion. In this setting, E-Cadh may be considered as a tumor suppressor gene, whose loss allows and enhances the invasion of adjacent normal tissues, increasing the metastatic potential.[15,16] Despite this, some reports documented increased serum concentration of soluble E-Cadh in various forms of epithelial and non-epithelial malignancies.[17] In keeping with the findings reported by Wrobel et al. (2006),[18] that describes high VE-Cadh serum levels in MM patients at diagnosis, we did not confirm a statistical significant mRNA overexpression of this cell-cell adhesion molecule, likely corresponding to this expected serum increase. We also report the constant association of the endothelial antigen MCAM/MUC18/CD146 recently correlated with poor prognosis in malignant melanoma,[14] with the better known VEGF and Ang2 expression which may indicate vascular tumor remodeling in MM-PC.[19,20]
In conclusion, the expression panel described in this report may help to discriminate between stable MGUS and those evolving towards SM and might be proposed as an additional tool to identify patients at risk of progression to MM.
Quantitative RNA and protein expression assays may further validate the prognostic role of E-cadherin and MCAM in the context of a prospective study.