Ibrahim Kanbour1, Prem Chandra2, Ashraf Soliman3, Vincenzo De Sanctis4, Abdulqadir Nashwan5, Sandra Abusamaan6, Abbas Moustafa6 and Mohamed A Yassin7.
1 Medical Registrar, Dijon University Hospital, Dijon, France
2 Senior Biostatistician, Medical Research Center, Hamad Medical Corporation (HMC), Doha, Qatar
3 Department of Paediatrics, University of Alexandria, Alexandria, Egypt
4 Paediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy
5 Nurse Research Scientist, Cancer Clinical Trials Unit, NCCCR, Hamad Medical Corporation (HMC), Doha, Qatar
6 Department of Radiology, Hamad Medical Corporation (HMC), Doha, Qatar
7 Hematology Section, National Center for Cancer Care and Research, Hamad Medical Corporation, (HMC), Doha, Qatar
Correspondence to: Mohamed A Yassin, MD. Consultant Haematologist,
Hematology Section, National Center for Cancer Care and Research, Hamad
Medical Corporation, Doha (Qatar). E-mail:
Yassinmoha@gmail.com
Published: November 1, 2018
Received: August 20, 2018
Accepted: September 22, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018062 DOI
10.4084/MJHID.2018.062
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction:
Chronic blood transfusion is the mainstay of care for individuals with
β-thalassemia major (BTM). However, it causes iron-overload that
requires monitoring and management by long-term iron chelation therapy
to prevent endocrinopathies and cardiomyopathies, which can be fatal.
Hepatic R2 MRI method (FerriScan®) has been validated as the gold
standard for evaluation and monitoring liver iron concentration (LIC)
that reflects the total body iron-overload. Although adequate oral iron
chelation therapy (OIC) is promising for the treatment of transfusional
iron-overload, some patients are less compliant with it, and others
suffer from long-term effects of iron overload.
Objective:
The aim of our study was to evaluate the prevalence of endocrinopathies
and liver dysfunction, in relation to LIC and serum ferritin level, in
a selected group of adolescents and young adult BTM patients with
severe hepatic iron overload (LIC from 15 to 43 mg Fe/g dry weight).
Patients
and Methods: Twenty-four selected BTM patients with severe LIC, due to
transfusion-related iron-overload, followed at the Haematology Section,
National Centre for Cancer Care and Research, Hamad Medical Corporation
of Doha (Qatar), from April 2015 to July 2017, were retrospectively
evaluated. The prevalence of short stature, hypogonadism,
hypothyroidism, hypoparathyroidism, impaired fasting glucose (IFG),
diabetes, and adrenal insufficiency was defined and assessed according
to the International Network of Clinicians for Endocrinopathies in
Thalassemia (ICET) and American Diabetes Association criteria.
Results:
Patients’ most common transfusion frequency was every three weeks
(70.8%). At the time of LIC measurements, their median age was 21.5
years with a mean age of 21.7± 8.0 years. Mean LIC was 32.05 ± 10.53 mg
Fe/g dry weight (range: 15 to 43 mg Fe/g dry weight), and mean serum
ferritin level was 4,488.6 ± 2,779 µg/L. LIC was correlated
significantly with serum ferritin levels (r = 0.512; p = 0.011). The
overall prevalence of short stature was 26.1% (6/23), IFG was 16.7%
(4/24), sub-clinical hypothyroidism was 14.3% (3/21), hypogonadotropic
hypogonadism was 14.3% (2/14), diabetes mellitus was 12.5% (3/24), and
biochemical adrenal insufficiency was 6.7% (1/15). The prevalence of
hepatitis C positivity was 20.8% (5/24). No case of clinical
hypothyroidism, adrenal insufficiency or hypoparathyroidism was
detected in this cohort of patients. The prevalence of IFG impaired
fasting glucose was significantly higher in BTM patients with very high
LIC (>30 mg Fe/g dry liver) versus those with lower LIC (p = 0.044).
The prevalence of endocrinopathies was not significantly different
between the two groups of patients with LIC above and below 15 mg Fe/g
dry weight.
Conclusions: A
significant number of BTM patients, with high LIC and endocrine
disorders, still exist despite the recent developments of new oral iron
chelating agents. Therefore, physicians’ strategies shall optimize
early identification of those patients to optimise their chelation
therapy and to avoid iron-induced organ damage. We believe that further
studies are needed to evaluate if serial measurements of quantitative
LIC may predict the risk for endocrine complications. Until these data
are available, we recommend a close monitoring of endocrine and other
complications, according to the international guidelines.
|
Introduction
Chronic
blood transfusion (CBT) is the mainstay of care for individuals with
β-thalassemia major (BTM) for preventing growth retardation, skeletal
changes that result from the expansion of the bone marrow, and
development of masses from extramedullary hematopoiesis.[1]
In spite of that, CBT causes iron-overload that requires monitoring and
management through long-term iron chelation therapy. With inadequate
chelation therapy, cardiac arrhythmias, cardiomyopathy, and heart
failure are the predominant causes of death, while endocrine
abnormalities and chronic liver disease contribute significantly to
morbidity and mortality.[1]
In general, iron chelation therapy
should be started as soon as the patient becomes significantly iron
loaded. Significant iron load correlates with serum ferritin of
approximately 1,000 ng/mL. Liver iron concentration (LIC) is considered
the best measure of total iron loading. LIC should be at least 3 mg
Fe/g dry weight before starting chelation.[1]
Overall, iron
chelation therapy results in better overall survival, especially if it
is instituted early and compliance is maintained. In particular, the
second-generation of oral agents appears to be associated with improved
overall and event-free survival in transfusion-dependent patients with
β-thalassemia.[2]
Very little is known about the relationship
between mild/moderate iron overload assessed by LIC and
endocrinopathies in patients with BTM,[3] and nothing has been reported
in the literature, to the best of our knowledge, about the prevalence
of disease-specific endocrinopathies in patients with severe iron
overload.
The present study aims to report the prevalence of
endocrinopathies and liver dysfunction, in relation LIC and serum
ferritin level, in a selected group of adolescents and young adults
with BTM and severe iron overload, assessed by hepatic R2 MRI method
(FerriScan®). Patients and Methods
This
retrospective cohort study was performed on 24 adolescents and young
adults with BTM and severe LIC (from 15 to 43 mg Fe/g dry weight),
secondary to transfusion-related iron-overload, followed at the
National Centre for Cancer Care and Research (NCCCR), Doha (Qatar) over
27 months (from April 2015 to July 2017).
The prevalence of
endocrinopathies in 28 TM patients followed at the NCCCR with a LIC
strictly under 15 mg Fe/g dry weight was also registered to assess the
differences between the two groups of patients.
The patients'
medical history (including blood transfusion regime and chelation
therapy), the growth percentiles and pubertal stages (standing height,
weight, body mass index - BMI - and Tanner's stages) were also
reviewed.
Laboratory investigations included measurement of
their serum ferritin (SF), alkaline phosphatase (ALP), lactate
dehydrogenase (LDH), total bilirubin, alanine transferase (ALT),
aspartate transferase (AST), fasting blood glucose (FBG), hemoglobin
A1C (HbA1c), morning cortisol level (Cort-AM), insulin-like growth
factor (IGF-1), parathyroid hormone (PTH), corrected calcium (Ca Corr),
phosphate (PO4), luteinizing hormone (LH), follicle-stimulating hormone
(FSH), testosterone (in males), estradiol (in females), thyroid
stimulating hormone (TSH), and free thyroxine (FT4).
SF and
hormonal parameters were measured by immune-enzymatic and
electrochemiluminescence immunoassays. The manufacturer’s normal
reference range values for SF were 30–350 ngm/L, in males, and 15–150
ng/mL in females.
Iron status was classified as mild (serum
ferritin <1,000 ng/mL), moderate (serum ferritin >1,000 ng/ml and
<2,000 ng/mL) or severe (serum ferritin >2,000 ng/mL).
Liver
iron content (LIC) was measured by FerriScan ® and values were
expressed in mg Fe/g weight.[4] Four classes of LIC have been reported
in thalassemic patients: Class 1=normal LIC < 3 mg Fe/g dry liver,
Class 2=mild overload LIC 3–7 mg Fe/g dry liver, Class 3=moderate LIC
overload 7–15 mg Fe/g dry liver, and Class 4=severe LIC overload ≥15 mg
Fe/g dry liver.[5]
Short stature and endocrine complications were
assessed and defined according to the International Network of
Clinicians for Endocrinopathies in Thalassemia (ICET) and American
Diabetes Association criteria.[6,7]
Ethical approval for the study
was obtained by the IRB of Medical Research Center at Hamad Medical
Corporation [http://www.wma.net]. All procedures were carried out with
the adequate understanding and consent of patients.
The
Kolmogorov-Smirnov (K-S) test and PP plot were used to test for
normality of the data. Associations between two or more qualitative
variables were assessed using chi-square (χ2),
Fisher Exact or Yates corrected Chi-square statistical tests as
appropriate. Quantitative data between the two independent groups were
analysed using unpaired ‘t’ test or Mann Whitney U test as appropriate.
The relationship between two quantitative variables was examined using
Pearson’s correlation coefficients. Pictorial presentations of the key
were made using appropriate statistical graphs. All p values presented
were two-tailed, and p values <0.05 was considered as statistically
significant. All Statistical analyses were done using statistical
packages SPSS 22.0 (SPSS Inc. Chicago, IL) and Epi-info (Centres for
Disease Control and Prevention, Atlanta, GA) software.
Results
Out
of the twenty-four transfusion-dependent BTM, 63% (15/24) were males
and 37% (9/24) were females. There were eight different nationalities,
but Pakistanis accounted for almost one-third (29.2%) and Qataris for
one-fifth (20.8%), whereas Egyptians, Sudanese, Bangladeshis, Iranians,
Palestinians and Bahrainis accounted for 12.5%, 12.5%, 8.3%, 8.3%, 4.2%
and 4.2%, respectively.
BTM patients had been regularly transfused
over the past 19.7±8.0 years (range: 7-33 years), starting from the age
of 2 years. Transfusion frequencies were as follows: every three weeks
accounted for 70.8% (17/24 patients), every four weeks 16.7% (4/24) and
every two weeks 12.5% (3/24).
21 out of 24 BTM patients referred a
"regular" chelation therapy; 18/24 were using deferasirox, 1/24 was
using deferoxamine, and 2/24 were using combined deferasirox and
deferoxamine therapy. Moreover, 3 out of 24 patients referred a poor
chelation therapy (Table 1).
Before LIC assessment by FerriScan®, patients using deferasirox, deferoxamine or both (Table 1)
had been on iron chelation therapy, starting from the age of 4 years,
for the past 15.3±5.4 years. Their mean age at the time of LIC
measurements was 21.7±8.0 years (range: 9-35 years).
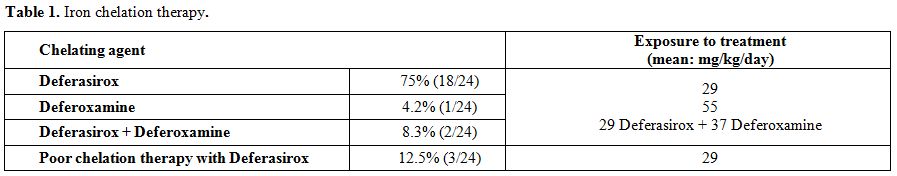 |
Table 1. Iron chelation therapy. |
Patients’ mean standing height was 158.2±11.4 cm, mean weight was 54.1±13.9 kg, and mean BMI was 21.5±5 Kg/m2.
The mean LIC at the time of the study was 32.05±10.53 mg Fe/g dry
weight (range:15- 43 mg Fe/g dry weight; 43 mg Fe/g dry weight being
the upper limit for FerriScan® values). Mean SF at LIC measurements was
4,488±2,779 ng/mL (range: 453-11,117 ng/mL). Biochemical (AST, ALT,
ALP, LDH and total bilirubin) and hormonal data are shown in table 2.
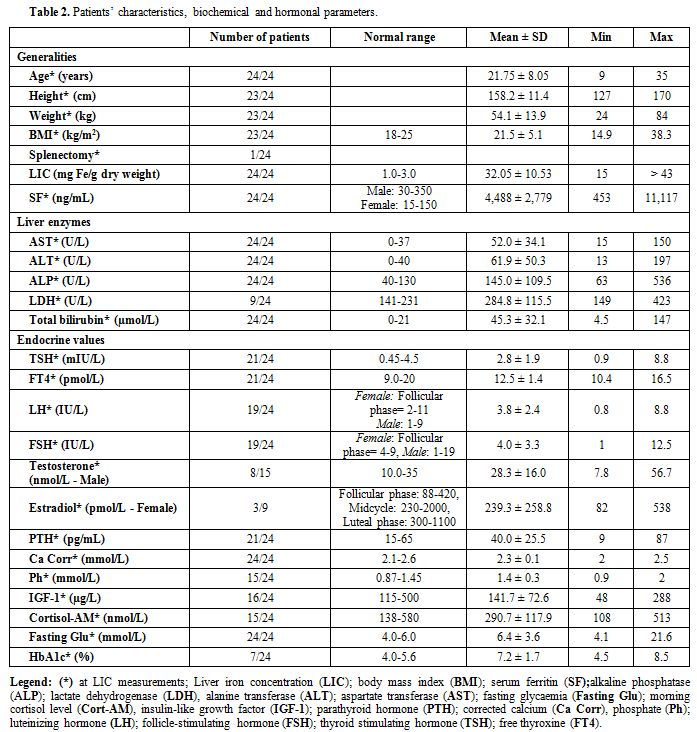 |
Table
2. Patients’ characteristics, biochemical and hormonal parameters. |
LIC
was correlated significantly with morning cortisol levels (r = 0.539, p
= 0.038), but not with any of the hormonal levels. There was also a
significant correlation between LIC and SF in BTM patients (r = 0.512,
p = 0.011).
SF was correlated significantly with TSH (r = 0.603, p = 0.004) and IGF-1 (r = -0.611, p = 0.012) concentrations (Table 3 and Figure 1).
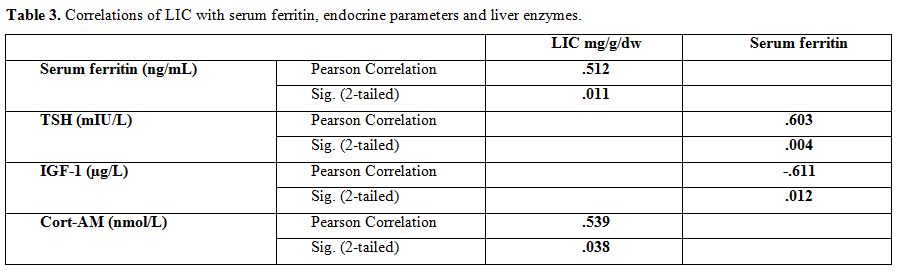 |
Table 3.
Correlations of LIC with serum ferritin, endocrine parameters and liver enzymes. |
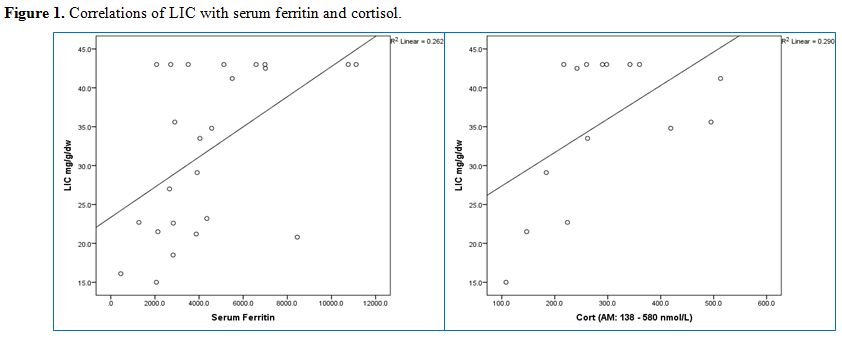 |
Figure 1. Correlations of LIC with serum ferritin and cortisol. |
The
overall prevalence of endocrinopathies was as follows = short stature:
26.1% (6/23), IFG: 16.7% (4/24), sub-clinical hypothyroidism: 14.3%
(3/21), hypogonadotropic hypogonadism: 14.3% (2/14), diabetes insulin
dependent: 12.5% (3/24), and biochemical hypocortisolism: 6.7% (1/15).
A single endocrinopathy was present in 7 patients out of 24 (29.2%) and
2 or more endocrinopathies in five patients (20.8%). Five out of 24
patients (20.8%) of patients were hepatitis C antibody positive.
The
prevalence of IFG was significantly higher in BTM patients with LIC
> 30 vs those with < 30 mg Fe/g dry weight [30.8% (4/13) vs 0 %
(0/11), p = 0.044]. On the contrary, the prevalence of short stature
and sub-clinical hypothyroidism were non-significantly higher in the
group of BTM patients with LIC > 30 vs those with < 30 mg Fe/g
dry weight [33.3% (4/12) vs 18.2% (2/11), p = 0.408 and 16.7% (2/12) vs
11.1% (1/9), p = 0.719, respectively] as well as the prevalence of
hypogonadism, diabetes and hepatitis C among the two groups of patients.
We
also compared these data with those of 28 BTM patients followed at
NCCCR with a LIC <15 mg Fe/g dry weight (median age: 19 years, mean
age: 21.7 years, range: 8 – 35 years). No statistical differences were
observed between the two groups of patients (Table 4).
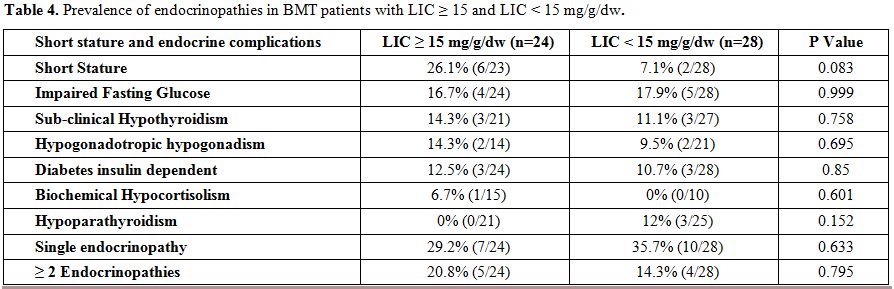 |
Table 4. Prevalence of endocrinopathies in BMT patients with LIC ≥ 15 and LIC < 15 mg/g/dw. |
Discussion
BTM
is an important cause of morbidity and mortality worldwide.
Complications related to chronic transfusion therapy are numerous,
affect almost every organ in the body, and vary considerably among
patients over the time.[1,2,6] Endocrine organ dysfunction and failures
are among the important complications of excessive iron overload.
Sixty-eight
patients with BTM and 14 with thalassemia intermedia are regularly
followed at the Haematology Section, National Centre for Cancer Care
and Research, Hamad Medical Corporation of Doha (Qatar). 24 out of 68
(35.2%) patients with BTM and elevated levels of iron storage, assessed
by hepatic R2 MRI method (FerriScan®), were selected for our study
because very few data comparing the prevalence of endocrinopathies in
relation to the degree of iron overload in adolescents and young adults
with BTM are available in the literature.[8,9]
The liver is the
main iron storage organ in the body, containing approximately 70% of
the total content of the body. Liver iron can be assessed by needle
biopsy or, more recently, by non-invasive magnetic resonance imaging
(MRI). As liver iron correlates with total body iron, an alternative to
evaluating body iron overload is the measurement of LIC.
Measurements
of LIC above 1.6 mg Fe/g of dry weight are considered high; there is a
small risk of complications when under 7 mg Fe/g dry weight, between 7
and 15 mg Fe/g dry weight are intermediate values and patients with
above 15 mg Fe/g dry weight have a risk of serious injury, including
fibrosis and cirrhosis of the liver, and cardiac failure.[8]
The
lowest LIC level in our patients was 15 mg Fe/g dry weight and the
highest 43 mg Fe/g dry weight (the upper limit for FerriScan®
assessment), with a mean of 32±10.5 mg Fe/g dry weight.
Despite
the relatively young patients' age (mean: 21.7 years; range 9-35 years)
and duration of oral iron chelation (OIC; mean:15.3 years), our
subjects had a high prevalence of short stature, IFG, hypogonadism,
diabetes and subclinical hypothyroidism.
A cross-sectional
analytic study, carried out in 2016 on 43 older BTM patients, aged
45-60 years, showed that 88.4% of them suffered from at least one
endocrine complication. The majority of patients developed endocrine
complications in the second decade of life when serum ferritin level
was very high (above 5,000 ng/mL). The very high peak level of serum
ferritin registered in these patients was probably due to inadequate
doses of deferoxamine in the first years of life, combined with poor
compliance to treatment during the peri-pubertal or pubertal age, and
to the increased amount of transfused blood not balanced by an
increased iron chelating treatment.[9]
Another study done in 2006
from 31 centres in the USA, Canada and the UK found that 56% of BTM
patients (age: 25.8±8.1 years) had more than one endocrinopathy with
LIC = 19.4±11.4 mg Fe/g dry weight. Endocrinopathies in their patients
included diabetes (13%), hypogonadism (40%) and hypothyroidism
(10%).[10]
Comparing these data with our study, a lower
incidence of hypogonadism was documented, despite the higher LIC
levels. There are several potential reasons why BTM patients may not
demonstrate the same degree of endocrine organ injury. It is possible
that a critical level with prolonged exposure may be necessary to
achieve target organ injury.[11] Alternatively, the duration of OIC in
our younger patients with BTM (mean age: 21.7±8.0 years), could have
partially protected their hypothalamic-pituitary-gonadal axis from the
deleterious effects of chronic iron overload.
Furthermore, when
compared the prevalence of endocrinopathies with a cohort of 28 BTM
patients with a LIC <15 mg Fe/g dry weight (with a relatively same
age), the difference between the two groups was not statistically
significant. These findings furtherly support the hypothesis that a
prolonged iron overload exposure is required in order to induce a
detrimental target organ failure.
A low rate of new endocrine
disorders and a stabilization of those pre-exisisting were observed in
a clinical practice setting by Casale et al. in 86 patients with BTM
(mean age: 23.0±12.6 years, range: 5–49).[12]
However, due to
the multifactorial causes of endocrine disorders in BTM patients, other
factors such as: duration and adherence to prescribed chelation
regimens, transfusional iron burden and biological responsiveness to a
given chelation regimen could play an additional role in determining
the degree of iron overload and development of organs damage.[13-16]
In
the Casale et al. study, the median duration of exposure to deferasirox
was 6.5 years (range: 3–10) and the mean dose was 25.2±5.1 mg/kg/day
(range: 16–36). Improvements were noted in all iron overload indices.
LIC values decreased from 5.3 mg Fe/g dry weight (1.0–28.0) to 4.5 mg
Fe/g dry weight (p: 0.020). A reduction in serum ferritin level was
also noted, from a median of 1,261.0 ng/mL (range 202–7,661) to 1,017
ng/mL (137–7,234), although the change was not statistically
significant (p: 0.165). Overall, 19 (22%) patients interrupted therapy
temporarily and 3 (3%) definitively due to alopecia, hemorrhagic
gastritis, and skin rash. Reasons for temporary interruptions to
treatment included pregnancy in six patients and poor compliance for a
12-month period of the study in one patient.
In our BTM patients,
LIC was correlated significantly with morning cortisol levels (r =
0.539, p = 0.038), but not with any of the hormonal levels. SF was
correlated significantly with TSH (r = 0.603, p = 0.004) and IGF-1 (r =
-0.611, p = 0.012) concentrations.
Is it possible that a single
quantitative measurement of LIC is not predictive of endocrinopathy, as
suggested by Vichinsky et al.,[17] and multiple serum ferritin
measurements over time could be more valuable in predicting
complications of iron overload than a single liver biopsy as reported
by Telfer et al.[8] Supporting the predictive value of serum ferritin,
Gamberini et al.[18] reported that levels of approximately 2,000 ng/mL
were found to correlate with hypogonadism, and 3,000 ng/mL for
hypothyroidism, hypoparathyroidism and diabetes mellitus, and Shalitin
et al.[19] found that an average serum ferritin above 2,500 ng/mL,
during puberty, was predictive of hypogonadism and above 3,000 ng/mL,
during the first decade of life, was predictive of final short stature.
Therefore, though serum ferritin can be much less accurate in
patients with hepatitis C and is weakly correlated with degree of
cardiac siderosis,[20] its monthly measurement could still have some
value in predicting endocrine complications.
Potential limitations
of the study include (a) a single centre with (b) a relatively small
sample population, and (c) a detailed knowledge of patients' compliance
to chelation therapy. On the other hand, a potential benefit of this
single-centre study is the uniformity of the clinical approach and the
personal knowledge of patients by doctors and nurses. This is an
advantage in particular compared to larger national or international
multicentre datasets in which the approaches to patient care across
centers and countries maybe variable over the time.
Conclusions
In
our study of BTM patients with severe iron overload, short stature was
the most common complication followed by IFG, sub-clinical
hypothyroidism, hypogonadism, and diabetes mellitus. The prevalence of
IFG was significantly higher in BTM patients with LIC >30 mg Fe/g
dry weight versus those with lower LIC. Whereas the prevalence of
endocrinopathies was not significantly different between the two groups
of patients with LIC above and below 15 mg Fe/g dry weight. SF was
correlated significantly with TSH and IGF-1 levels, and LIC correlated
significantly with SF levels. Guidelines
for the management of TM patients recommend, based mostly on expert
opinion, that LIC assessment should be done at 1–2 year intervals (or
more frequently depending on iron overload status).[21-23]
We believe that further studies are needed to evaluate if serial
measurements of quantitative LIC may predict the risk for endocrine
glands damage. Until these data are available, we recommend a close
monitoring of endocrine and other complications, according to the
international guidelines, although some aspects of BTM management
remain controversial with clear absence of strong evidence-based
recommendations. References
- Galanello R, Origa R. Beta-thalassemia. Orphanet J Rare Dis. 2010 May 21;5:11. doi: 10.1186/1750-1172-5-11. https://doi.org/10.1186/1750-1172-5-11
- Ballas
SK, Zeidan AM, Duong VH, DeVeaux M, Heeney MM. The effect of iron
chelation therapy on overall survival in sickle cell disease and
β-thalassemia: A systematic review. Am J Hematol. 2018;93:943-952. https://doi.org/10.1002/ajh.25103 PMid:29635754
- Yassin
MA, Soliman AT, De Sanctis V, Abdula MA, Riaz LM, Ghori FF, Yousaf A,
Nashwan AJ, Abusamaan S, Moustafa A, Kohla S, Soliman DS. Statural
Growth and Prevalence of Endocrinopathies in Relation to Liver Iron
Content (LIC) in Adult Patients with Beta Thalassemia Major (BTM) and
Sickle Cell Disease (SCD). Acta Biomed. 2018;89(2-S):33-40.
- St
Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK,
Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging
of liver iron concentrations using proton magnetic resonance. Blood.
2005;105:855-861. https://doi.org/10.1182/blood-2004-01-0177 PMid:15256427
- Verlhac
S, Morel M, Bernaudin F, Béchet S, Jung C, Vasile M. Liver iron
overload assessment by MRI R2* relaxometry in highly transfused
pediatric patients: an agreement and reproducibility study. Diagn
Interv Imaging. 2015;96:259-264. https://doi.org/10.1016/j.diii.2014.11.021 PMid:25533496
- De
Sanctis V, Soliman AT, Elsedfy H, Skordis N, Kattamis C, Angastiniotis
M, Karimi M, Yassin MA, El Awwa A, Stoeva I, Raiola G, Galati MC,
Bedair EM, Fiscina B, El Kholy M. Growth and endocrine disorders in
thalassemia: The international network on endocrine complications in
thalassemia (I-CET) position statement and guidelines. Indian J
Endocrinol Metab. 2013;17:8-18. https://doi.org/10.4103/2230-8210.107808 PMid:23776848 PMCid:PMC3659911
- International
Diabetes Federation. Definition and diagnosis of diabetes mellitus and
intermediate hyperglycaemia: report of a WHO/IDF consultation. Geneva:
World Health Organization; 2006.
- Telfer
PT, Prestcott E, Holden S, Walker M, Hoffbrand AV, Wonke B. Hepatic
iron concentration combined with long-term monitoring of serum ferritin
to predict complications of iron overload in thalassaemia major. Br J
Haematol. 2000;110:971-977. https://doi.org/10.1046/j.1365-2141.2000.02298.x PMid:11054091
- De
Sanctis V, Elsedfy H, Soliman AT, Elhakim IZ, Soliman NA, Elalaily R,
Kattamis C. Endocrine profile of β-thalassemia major patients followed
from childhood to advanced adulthood in a tertiary care center. Indian
J Endocrinol Metab. 2016 ;20:451-459. https://doi.org/10.4103/2230-8210.183456 PMid:27366710 PMCid:PMC4911833
- Fung
EB, Harmatz PR, Lee PD, Milet M, Bellevue R, Jeng MR, Kalinyak KA,
Hudes M, Bhatia S, Vichinsky EP; Multi-Centre Study of Iron Overload
Research Group. Increased prevalence of iron-overload associated
endocrinopathy in thalassaemia versus sickle-cell disease. Br J
Haematol. 2006;135:574-582. https://doi.org/10.1111/j.1365-2141.2006.06332.x PMid:17054676
- Vichinsky
E, Butensky E, Fung E, Hudes M, Theil E, Ferrell L, Williams R, Louie
L, Lee PD, Harmatz P. Comparison of organ dysfunction in transfused
patients with SCD or beta thalassemia. Am J Hematol. 2005;80:70-74. https://doi.org/10.1002/ajh.20402 PMid:16138345
- Casale
M, Citarella S, Filosa A, De Michele E, Palmieri F, Ragozzino A,
Amendola G, Pugliese U, Tartaglione I, Della Rocca F, Cinque P, Nobili
B, Perrotta S. Endocrine function and bone disease during long-term
chelation therapy with deferasirox in patients with β-thalassemia
major. Am J Hematol. 2014;89:1102-1106. https://doi.org/10.1002/ajh.23844 PMid:25197009
- Al-Akhras
A, Badr M, El-Safy U, Kohne E, Hassan T, Abdelrahman H, Mourad M,
Brintrup J, Zakaria M. Impact of genotype on endocrinal complications
in β-thalassemia patients. Biomed Rep. 2016;4:728-736. https://doi.org/10.3892/br.2016.646 PMid:27284414 PMCid:PMC4887852
- Skordis
N, Michaelidou M, Savva SC, Ioannou Y, Rousounides A, Kleanthous M,
Skordos G, Christou S. The impact of genotype on endocrine
complications in thalassaemia major. Eur J Haematol. 2006;77:150-156. https://doi.org/10.1111/j.1600-0609.2006.00681.x PMid:16800840
- Cunningham
MJ, Macklin EA, Neufeld EJ, Cohen AR; Thalassemia Clinical Research
Network. Complications of beta-thalassemia major in North America.
Blood. 2004;104:34-39. https://doi.org/10.1182/blood-2003-09-3167 PMid:14988152
- Chirnomas
D, Smith AL, Braunstein J, et al. Deferasirox pharmacokinetics in
patients with adequate versus inadequate response. Blood. 2009;
114:4009–4013. https://doi.org/10.1182/blood-2009-05-222729 PMid:19724055 PMCid:PMC2774541
- Vichinsky
E, Butensky E, Fung E, Hudes M, Theil E, Ferrell L, Williams R, Louie
L, Lee PD, Harmatz P. Comparison of organ dysfunction in transfused
patients with SCD or beta thalassemia. Am J Hematol. 2005;80:70-74. https://doi.org/10.1002/ajh.20402 PMid:16138345
- Gamberini
MR, De Sanctis V, Gilli G. Hypogonadism, diabetes mellitus,
hypothyroidism, hypoparathyroidism: incidence and prevalence related to
iron overload and chelation therapy in patients with thalassaemia major
followed from 1980 to 2007 in the Ferrara Centre. Pediatr Endocrinol
Rev. 2008M 6 (Suppl 1):158-169. PMid:19337172
- Shalitin
S, Carmi D, Weintrob N, Phillip M, Miskin H, Kornreich L, Zilber R,
Yaniv I, Tamary H. Serum ferritin level as a predictor of impaired
growth and puberty in thalassemia major patients. Eur J Haematol.
2005;74:93-100. https://doi.org/10.1111/j.1600-0609.2004.00371.x PMid:15654898
- Wood
JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading
in transfusion-dependent thalassemia and sickle cell disease. Blood.
2004; 103:1934–1936. https://doi.org/10.1182/blood-2003-06-1919 PMid:14630822
- Ho
PJ, Tay L, Lindeman R, Catley L, Bowden DK. Australian guidelines for
the assessment of iron overload and iron chelation in
transfusion-dependent thalassaemia major, sickle cell disease and other
congenital anaemias. Intern Med J. 2011;41:516-524. https://doi.org/10.1111/j.1445-5994.2011.02527.x PMid:21615659
- Sayani
F, Warner M, Wu J, Wong-Rieger D, Humphreys K, Odame I. Guidelines for
the Clinical Care of Patients with Thalassemia in Canada. Anemia
Institute for Research & Education, Thalassemia Foundation of
Canada, ON, Canada.2009.
- Cappellini
MD, Cohen A, Porter J, Taher A, Viprakasit V, editors. Guidelines for
the Management of Transfusion Dependent Thalassaemia (TDT) [Internet].
3rd edition. Nicosia (CY): Thalassaemia International Federation; 2014.
https://www.ncbi.nlm.nih.gov/pubmed/25610943
[TOP]




