Matteo Chinello¹, Rita
Balter¹, Massimiliano De Bortoli¹, Virginia Vitale¹, Ada Zaccaron¹,
Elisa Bonetti¹, Paola Tonin², Gaetano Vattemi², Valeria Guglielmi² and
Simone Cesaro¹.
1 Pediatric Hematology Oncology, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
2 Department of Neurosciences, Biomedicine and Movement Sciences, Section of Clinical Neurology, University of Verona, Italy.
Correspondence to: Matteo Chinello, M.D. Pediatric Hematology Oncology,
Azienda Ospedaliera Universitaria Integrata, Piazzale A. Stefani 1,
37126, Verona, Italy. Fax: +390458127887, Tel: +390458127816. E-mail:
matteo.chinello@aovr.veneto.it
Published: January 1, 2020
Received: July 24, 2019
Accepted: November 14, 2019
Mediterr J Hematol Infect Dis 2020, 12(1): e2020002 DOI
10.4084/MJHID.2020.002
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Chronic graft versus host disease (cGVHD) occurs in 20-30% of
paediatric patients receiving haemopoietic stem cell transplantation
(HSCT). Neuromuscular disorders such as polymyositis are considered a
rare and distinctive but non-diagnostic manifestation of cGVHD and, in
the absence of other characteristic signs and symptoms, biopsy is
highly recommended to exclude other causes.
Case report:
We report a case of a 17-months-old child affected by hemophagocytic
lymphohistiocytosis who underwent a matched unrelated donor
haematopoietic stem cell transplantation (HSCT). She developed severe
cGVHD-related polymyositis that was successfully treated with high-dose
steroid therapy, rituximab and sirolimus.
Conclusions:
This is the first case of cGVHD-related-polymyositis described in a
pediatric patient which was successfully treated with rituximab.
|
Introduction
Chronic
graft-versus-host-disease (cGVHD) is a late complication of allogeneic
haemopoietic stem cell transplantation (HSCT), occurring more than 100
days after transplantation.[1] It occurs in 20-30% of
patients receiving HSCT with higher frequency in patients with acute
graft-versus-host-disease (aGVHD).[2-4] Early or delayed neurological GVHD-related manifestations occur in 30-60% of allogenic HSCT recipients.[5,6]
These include immune-mediated polyneuropathies and less frequently,
polymyositis, myasthenia gravis, myositis, demyelination,
cerebrovascular complications and immune-mediated encephalitis.[5,6] We report the case of a 17-month-old child with an immune-mediated myopathy as a consequence of cGVHD-related polymyositis.
Case Report
A
one-month-old girl of African origin was admitted to the local
emergency pediatric unit for high fever, trilineage blood cytopenia and
hepatosplenomegaly. Natural killer cells analysis showed a lack of
perforin expression. The diagnosis of hemophagocytic
lymphohistiocytosis (HLH) was confirmed by Next Generation Sequencing
(NGS) analysis on peripheral blood DNA, showing the presence of genomic
variants c.50delT and c.1130G>A in PRF1 gene, both heterozygous.
NM_005041 (PRF1): c.[50delT(;)1130G>A],
p.[Leu17ArgfsTer34(;)Cys377Tyr]. These variants, both homozygous and
compound heterozygous, are described as related to HLH.[7,8]
The patient started treatment with dexamethasone and cyclosporine,
followed by emapalumab, a monoclonal antibody anti-interferon gamma.[9]
She underwent HLA-matched unrelated donor HSCT at the age of 6 months
(HLA-A, DRB1, permissive DPB1 allele mismatches). Conditioning regimen:
busulfan (3x3,2 mg/kg/day), fludarabine (3x50 mg/m2/day), thiotepa (2x5 mg/kg/day) and rabbit antithymocyte globulin (ATG GenzymeTM) (3x4,5 mg/kg/day). The patient received 4.14x108/kg bone marrow total nucleated cells and 7.37x106/kg
of CD34+ cells. GVHD prophylaxis was based on cyclophosphamide (2x50
mg/kg) and cyclosporine and low dose of prednisone (0,4 mg/kg/die). The
pre-engraftment period was complicated by Pseudomonas aeruginosa sepsis
(day + 10) and right lobar pneumonia (day +13). The engraftment
occurred at day +16 with no sign of aGVHD. Therapy with ciclosporin was
interrupted three months after HSCT, and she started therapy with
tacrolimus. The prednisone given as GVHD prophylaxis was interrupted at
month +7 post-HSCT. Full donor chimerism was found at day +50 and
confirmed at month +8 post-HSCT. At 17 months the child was
admitted to the hospital for lack of appetite, elevated liver enzymes
with alanine aminotransferase (ALT) 850 U/L and aspartate
aminotransferase (AST) 499 U/L (normal value 5-45), and polypnea.
Presuming GVHD-related symptoms, she was treated with
methylprednisolone at the dose of 2 mg/kg/die with no clinical
improvement. The child rapidly developed respiratory failure that
required mechanical ventilation. An extensive diagnostic work-up was
performed: blood analysis revealed an increased value of creatine
kinase (CK) of 13830 U/L (normal value 25-190), creatine kinase (CK)-MB
555 ng/L (normal value < 6) and troponin of 2601 ng/L (normal value
< 45); tacrolimus through blood level was in range; immunoglobulin
levels were normal whilst the peripheral blood lymphocyte
subpopulations showed an increase in the content of B-lymphocytes
(2544,34 cells/ul, normal value 123-349); echocardiography showed a
normal biventricular function; electroencephalography (EEG) revealed no
abnormality; serological test and polymerase chain reaction (PCR) assay
revealed no evidence of recent parvovirus B19, Adenovirus, Enterovirus,
Cytomegalovirus, Human herpesvirus 6, Human immunodeficiency virus,
hepatitis B virus, hepatitis C virus or Epstein-Barr virus infection;
cerebrospinal fluid (CSF) findings resulted negative for bacterial and
viral infections; electromyography (EMG) showed a normal pattern of the
motor unit action potential (MUAP) waveform with normal values of F
wave and only sporadic myopathic MUAPs were found; an extensive screen
for autoantibodies related to autoimmune and neuromuscular disease was
negative. In suspicion of a GVHD-related myositis, a biopsy from vastus
lateralis muscle was performed showing necrotic and degenerating muscle
fibres, basophilic regenerating fibres and inflammatory infiltrates
predominantly around vessels (Figure 1).
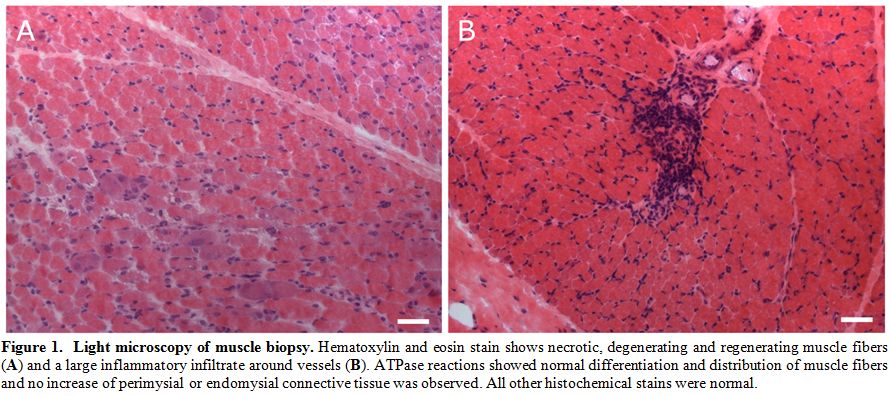 |
Figure
1. Light microscopy of muscle biopsy. Hematoxylin and eosin stain shows necrotic, degenerating and regenerating muscle fibers (A) and a large inflammatory infiltrate around vessels (B).
ATPase reactions showed normal differentiation and distribution of
muscle fibers and no increase of perimysial or endomysial connective
tissue was observed. All other histochemical stains were normal. |
Inflammatory cells
were predominantly composed of CD3+ CD8+ T cells; some CD4+ T cells and
a few CD68+ macrophages, CD20+ B cells and CD57+ natural killer cells
were observed; only rare CD 138+ cells were present (Figure 2).
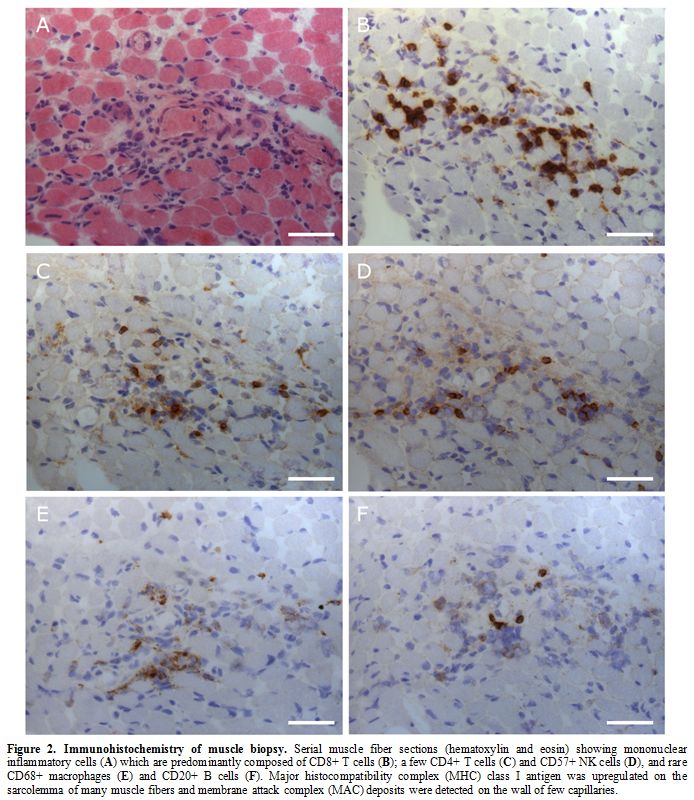 |
Figure 2. Immunohistochemistry of muscle biopsy. Serial muscle fiber sections (hematoxylin and eosin) showing mononuclear inflammatory cells (A) which are predominantly composed of CD8+ T cells (B); a few CD4+ T cells (C) and CD57+ NK cells (D), and rare CD68+ macrophages (E) and CD20+ B cells (F).
Major histocompatibility complex (MHC) class I antigen was upregulated
on the sarcolemma of many muscle fibers and membrane attack complex
(MAC) deposits were detected on the wall of few capillaries. |
These findings
indicated immune-mediated polymyositis; therefore the immunosuppression
treatment was potentiated with methylprednisolone 30 mg/kg for three
days, rituximab (105 mg/m2
once weekly for four doses); tacrolimus was replaced with sirolimus
because of its potential neurotoxicity. In the following days the
clinical conditions of the child improved, with a decrease in CK,
CK-MB, AST, ALT and troponin values; she was weaned from mechanical
ventilation after 25 days. In the following weeks the girl presented a
progressive clinical improvement with complete normalisation of the
neuromuscular disease in about two months. The girl was treated with
sirolimus (ongoing), and low dose of prednisone (0,2 mg/kg/die)
gradually tapered off over nine months. Currently, after 15 months, the
girl is asymptomatic in very good general conditions without any
neuromuscular alteration. The values of CK-MB, AST, ALT and troponin
are average while CK persists slightly abnormal (1.5 times above the
normal upper range).
Discussion
Polymyositis is a sign of cGVHD[1,10,12-15] although not a frequent manifestation, the incidence being approximately 3.4-7.7%.[1,5]
Host-reactive donor lymphocytes are the cells responsible for muscle
damage involving preferentially and symmetrically the proximal muscle
groups. Most of the cases reported in the literature concern adults[1,17] and only a few cases are described in children.[10-13]
This case is the earliest age of onset reported, and from a literature
review it is the only one diagnosed among paediatric allogeneic HSCT
performed from 2012 to 2018. It is interesting to point out that this
case occurred in a patient with HLA allele mismatch on loci A, DRB1,
DPB1 that induced to potentiate the regimen of GVHD prophylaxis with
the use of post-transplant cyclophosphamide. Clinical features are
similar to those of idiopathic polymyositis, involving bilateral
muscular weakness of proximal muscles while lower extremities are less
frequently involved.[6] Muscular pain is not always present whilst heart involvement has been reported.[1,5] In our patient, as reported in literature[6,14]
the symptoms appeared after the tapering or withdrawal of
immunosuppression therapy. Laboratory tests show elevated CK (5-50
times above normal) in most patients, as in our case, however CK
may be normal or slightly increased in clinically stable patients.[5,6] Unlike idiopathic polymyositis the presence of myositis-specific antibodies is rarely positive.[6] EMG shows the typical myopathic pattern5 although in some cases it may be normal,[14]
as described in our patient. GVHD-related polymyositis has been
reported to respond to corticosteroids alone or in combination with
cyclosporine, mycophenolate mofetil, methotrexate or cyclophosphamide.[14]
Due to the rarity of this manifestation and the complexity of
differential diagnosis, biopsy has a key role in confirming the
diagnosis. The typical histopathology examination showed segmental
muscle fibre necrosis, muscle fibre regeneration and mononuclear cell
inflammation. Generally, immunohistochemistry shows cytotoxic CD8+ T
donor cell infiltration of endomysium and CD4+ and CD8+ T cell
infiltration of the perimysium.[5] We found
B-lymphocytes (CD20+ cells) infiltration in the muscle that correlated
with the abnormally high number of CD20+ cells on peripheral blood.
This finding suggested a treatment based on the combination of
high-dosed methylprednisolone together with rituximab, whereas
tacrolimus was replaced by sirolimus because of some anecdotal cases of
tacrolimus-related myositis.[16] A biopsy with B-cell
infiltration has already been described in literature, and the therapy
with rituximab has been shown to be effective in adult patients.[17-19]
This case of cGVHD-related-polymyositis is the first described in a
pediatric patient who was successfully treated with rituximab. A lower
dose was used, if compared to other cases (105 mg/m2 versus 375 mg/m2),[20] thus reducing the possible risks due to immunosuppression.
Conclusions
In
conclusion, in a patient undergoing HSCT with myalgia and muscle
weakness even without other signs of cGVHD, it is crucial to exclude a
myopathy secondary to steroid treatment, a myasthenia gravis or a viral
myopathy.[20] The cGVHD-related polymyositis is a rare condition, but it has to be suspected and confirmed with EMG and muscle biopsy.[20]
This condition can be treated, in addition to steroid therapy, with
rituximab, in particular if the muscle biopsy demonstrates B-cell
infiltration.
References
- Ahn JS, Cho SH, Kim YK, Yang DH, Bae WK, Shim HJ et
al. Polymyositis and myocarditis after donor lymphocyte infusion. Int J
Hematol. 2009 Jul;90(1):113-116. Epub 2009 May 27. Blood. 2002 Aug
15;100(4):1192-200. https://doi.org/10.1007/s12185-009-0332-3 PMid:19472035
- Zecca
M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C, Fagioli F et
al. Po.AIEOP-BMT Group. Italian Association for Pediatric Hematology
and Oncology-Bone Marrow Transplant. Chronic graft-versus-host disease
in children: incidence, risk factors, and impact on outcome. Blood.
2002 Aug 15;100(4):1192-200. https://doi.org/10.1182/blood-2001-11-0059 PMid:12149197
- Bertaina
A, Zecca M, Buldini B, Sacchi N, Algeri M, Saglio F et al. Unrelated
donor vs HLA-haploidentical α/β T-cell- and B-cell-depleted HSCT in
children with acute leukemia. Blood. 2018 Dec 13;132(24):2594-2607.
Epub 2018 Oct 22. https://doi.org/10.1182/blood-2018-07-861575 PMid:30348653
- Sharaf
N, Prayson RA. Relapsing polymyositis in chronic graft versus host
disease. J Clin Neurosci. 2014 Nov;21(11):1964-5. https://doi.org/10.1016/j.jocn.2014.03.025 PMid:24980629
- Grauer
O, Wolff D, Bertz H, Greinix H, Kühl JS, Lawitschka A et al.
Neurological manifestations of chronic graft-versus-host disease after
allogeneic haematopoietic stem cell transplantation: report from the
Consensus Conference on Clinical Practice in chronic graft-versus-host
disease. Brain. 2010 Oct;133(10):2852-65. https://doi.org/10.1093/brain/awq245 PMid:20846944
- Koeppen
S1, Thirugnanasambanthan A, Koldehoff M. Neuromuscular complications
after hematopoietic stem cell transplantation. Support Care Cancer.
2014 Sep;22(9):2337-41 https://doi.org/10.1007/s00520-014-2225-0 PMid:24682581
- Lee
SM1, Sumegi J, Villanueva J, Tabata Y, Zhang K, Chakraborty R, Sheng X,
Clementi R, de Saint Basile G, Filipovich AH. Patients of African
ancestry with hemophagocytic lymphohistiocytosis share a common
haplotype of PRF1 with a 50delT mutation. J Pediatr. 2006
Jul;149(1):134-7. https://doi.org/10.1016/j.jpeds.2006.03.003 PMid:16860143
- Benezech
S, Walzer T, Charrier E, Heidelberg D, De Saint-Basile G, Bertrand Y,
Belot A. Late-onset hemophagocytic lymphohistiocytosis with
neurological presentation. Clin Case Rep. 2017 Sep 12;5(11):1743-1749.
doi: 10.1002/ccr3.1135. eCollection 2017 Nov. https://doi.org/10.1002/ccr3.1135 PMid:29152263 PMCid:PMC5676276
- Locatelli
F, Jordan MB, Allen CE, Cesaro S, Rizzari C, Rao A et al. Safety and
Efficacy of Emapalumab in Pediatric Patients with Primary
HemophagocyticLymphohistiocytosis. Blood 2018 132:LBA-6). https://doi.org/10.1182/blood-2018-120810
- Pier
N, Dubowitz V. Chronic graft versus host disease presenting with
polymyositis. Br Med J (Clin Res Ed). 1983 Jun 25;286(6383):2024. https://doi.org/10.1136/bmj.286.6383.2024 PMid:6409214 PMCid:PMC1548493
- Anderson
BA, Young PV, Kean WF, Ludwin SK, Galbraith PR, Anastassiades TP.
Polymyositis in chronic graft vs host disease. A case report. Arch
Neurol. 1982 Mar;39(3):188-90. https://doi.org/10.1001/archneur.1982.00510150058015 PMid:7039565
- Tse
S, Saunders EF, Silverman E, Vajsar J, Becker L, Meaney B. Myasthenia
gravis and polymyositis as manifestations of chronic
graft-versus-host-disease. Bone Marrow Transplant. 1999
Feb;23(4):397-9. https://doi.org/10.1038/sj.bmt.1701575 PMid:10100585
- Klein
R1, Franck P, Ehl S, Schmitt-Graeff A, Duffner U, Niemeyer CM.
Polymyositis-an unusual presentation of cGvHD in children. Pediatr
Transplant. 2007 Mar;11(2):225-7. https://doi.org/10.1111/j.1399-3046.2006.00615.x PMid:17300507
- Maillard-Lefebvre
H, Morell-Dubois S, Lambert M, Charlanne H, Launay D, Hachulla E et al.
Graft-versus-host disease-related polymyositis. Clin Rheumatol. 2010
Apr;29(4):431-33. https://doi.org/10.1007/s10067-009-1350-5 PMid:20069327
- Michelis
FV, Bril V, Lipton JH. A case report and literature review of chronic
graft-versus-host disease manifesting as polymyositis. Int J Hematol.
2015 Jul;102(1):144-6. Epub 2015 Mar 3. Review. https://doi.org/10.1007/s12185-015-1768-2 PMid:25732066
- Orlandi
V, Campieri C, Mosconi G, D'Arcangelo GL, Feliciangeli G, Scolari MP et
al.Tacrolimus-associated myositis: a case report in a renal transplant
patient.Transplant Proc. 2004 Apr;36(3):708-10. https://doi.org/10.1016/j.transproceed.2004.03.018 PMid:15110639
- Williams
KM, Ostrow LW, Loeb DM, Chung T, Cohn RD, Corse AM et al.
Immunohistochemistry of affected tissue may guide cGVHD treatment
decisions. Bone Marrow Transplant. 2012 May;47(5):731-3. https://doi.org/10.1038/bmt.2011.164 PMid:21927032 PMCid:PMC4251459
- Teshima
T, Nagafuji K, Henzan H, Miyamura K, Takase K, Hidaka M et al.
Rituximab for the treatment of corticosteroid-refractory chronic
graft-versus-host disease. Int J Hematol. 2009 Sep;90(2):253-260. https://doi.org/10.1007/s12185-009-0370-x PMid:19543951
- Von
Bonin M, Oelschlägel U, Radke J, Stewart M, Ehninger G, Bornhauser M et
al. Treatment of chronic steroid-refractory graft-versus-host disease
with low-dose rituximab. Transplantation. 2008 Sep 27;86(6):875-9. https://doi.org/10.1097/TP.0b013e318183f662 PMid:18813113
- Jagasia
MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW et al. National
Institutes of Health Consensus Development Project on Criteria for
Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014
Diagnosis and Staging Working Group report. Biol Blood Marrow
Transplant. 2015 Mar;21(3):389-401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001 PMid:25529383 PMCid:PMC4329079