Kanchan Mishra, Avani Shah, Krima Patel, Kanjaksha Ghosh and Sumit Bharadva
Surat Raktadan Kendra
& Research Centre, 1st Floor, Khatodara Health Centre, Near
ChosathJoganiya Mata Mandir, UdhanaMagdalla Road, Khatodara –
394210, Surat (Gujarat), India
Correspondence to: Dr. Sumit Bharadva, MD, MD IHBT (Director), Surat
Raktadan Kendra & Research Centre (Regional Blood Transfusion &
Research Centre) 1st Floor, Khatodara Health Centre, UdhanaMagdalla
Road, Khatodara – 395002, Surat (Gujarat), India Email:
sumit_bharadva@hotmail.com
Published: July 1, 2020
Received: April 7, 2020
Accepted: June 6, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020038 DOI
10.4084/MJHID.2020.038
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Multitransfused β-thalassemia major patients are always at high risk of
having Transfusion Transmitted Infections (TTIs). This study was aimed
to determine the seroprevalence of HBsAg, Anti-HIV-1/2, and Anti-HCV
among these patients and to correlate the same with NAT testing.
Methods:
A total of 196 patients with β-thalassemia were included in the study.
Patients were screened for the presence of viral markers by
third-generation ELISA test as well as for viral DNA/RNA by NAT
test.
Results:
Among 196 multi-transfused Beta-thalassemia patients, the
seroprevalence of anti-HCV was very high 100 (51.1%), however,
anti-HIV1/2 was 6 (3.1%), and HBsAg were 3 (1.5%). Surprisingly similar
patterns were observed in the prevalence of molecular markers, as
HCV-RNA were 66 (33.7%) of the patients along with HIV-1 RNA were 8
(4.1%), and HBV-DNA were 5 (2.5%) patients. Overall eight (4.1%)
patients were found to have coinfections, where two were positive for
HBsAg/anti-HCV by ELISA along with 3 (1.5%) were positive for
HBV-DNA/HCV-RNA, 1 (0.5%) was positive for HIV-RNA/HBV-DNA, and 2 (1%)
had coinfection of HIV-RNA/ HCV RNA by NAT testing
Conclusion:
The prevalence of HCV infection among multi-transfused β-thalassemia
patients is significantly higher than that of the HBV and HIV
infections. This scenario should be controlled and monitored by doing
regular follow-up testing schedules of such patients and also the
administration of the booster dose of the HBV vaccine along with HCV treatment with antiviral DAAs..
|
Introduction
Thalassemia
major patients have a high prevalence of transfusion-transmitted
infections, mainly as a consequence of viral infections acquired
through blood transfusion. Thalassemia is one of the common genetic
conditions prevalent in India. It is estimated that there are about
65,000-67,000 Beta-thalassemia patients in India, with around
9,000-10,000 cases added every year, with an estimated incidence of 2
per 1,000 births and a carrier frequency of 3-4%, it constitutes a
significant health burden.[1]
Thalassemia is a
term for a group of disorders in which there are reduced levels of
hemoglobin, decreased red blood cell production, and anemia.
Furthermore, severe anemia can cause serious, even life-threatening
complications if left untreated.[2,3] Therefore
individuals diagnosed with beta-thalassemia undergo lifelong blood
transfusion to maintain standard hemoglobin (Hb) levels for their
health management.[4] Hepatitis-B virus (HBV),
Hepatitis-C virus (HCV) and Human immunodeficiency virus (HIV) are
three most common chronic viral pathogens among multitransfused
thalassemia major individuals4 as all these viruses can transmit
through blood transfusion apart from other routes.
Coinfections of HIV/HCV, HIV/HBV, and HBV/HCV in thalassemia patients are associated with reduced survival.[5]
Therefore coinfection is common in people with high exposure to blood
and blood products. The main concern with HIV/HCV coinfection is that
it can lead to more severe liver diseases and increased the risk of
progression to liver cancer, especially in immunocompromised
thalassemic patients.[6] There are several reports
related to HIV/HCV coinfection among thalassemic patients; however
there is no report related to HBV/HCV coinfection that can lead to
liver cirrhosis and hepatocellular carcinoma (HCC), and no report
related to HIV/HBV from different parts of India.[7,8]
Blood
units are screened with assays of steadily increasing sensitivity for
Hepatitis-B surface antigen (HBsAg) since 1971, against HIV since 1989
and against HCV since 2001.[9] Indeed, the risk of
being infected by a contaminated blood unit today is orders of
magnitude lower when compared to thirty years ago, due to continuous
improvement and implementation of donor selection, sensitive screening
tests. However, HCV is still a significant problem in patients with
thalassemia.
Despite the enormous burden of thalassemia in India,
a survey of blood transfusion practices noted that testing for
transfusion-transmitted infections is unsatisfactory and poorly
regulated in most blood banks, both private and government, throughout
India.[10,11] This results in a continuing risk of
transmission of infectious agents in thalassemia children receiving
multiple blood transfusions.
There is a scarcity of literature
emphasizing the magnitude of transfusion-associated viral hepatitis in
thalassemics in Western India. The need to explore the burden of
transfusion-mediated infections in these patients cannot be
overemphasized. Therefore this study was aimed to determine the
prevalence of anti-HCV, HBsAg, and anti-HIV-1 and viral RNA/DNA
positivity of HBV, HCV, and HIV-1in thalassemic
children. Moreover, we believe that this is the first work that
shows the prevalence of HBV, HCV, and HIV-1 in thalassemia patients
using both sero-molecular markers in Western India.
Materials and Methods
This
study was approved by the Institutional Ethics Committee (IEC) and
conducted in 2015. The study group included 196 beta-thalassemia Major
children who are taking regular blood units for transfusion from Surat
Raktadan Kendra & Research Centre (SRKRC). Blood samples of these
patients were obtained from the serology Department of SRKRC prior to
taking their informed consent having information like age, sex,
address, history of the previous transfusions, total number of
transfusions to date, the age when the first transfusion was given, etc.
Serum and Plasma samples.
Blood sample of all the patients was collected in 2 ml EDTA and plain
tubes; plasma and serum were separated by centrifugation at room
temperature, labeled appropriately in two aliquots, and stored at -30°C
in a deep freezer, till the tests for TTI serology as well as NAT was
performed.
Serological assay.
All the serum samples of 196 patients were screened for anti-HIV-1/2,
HBsAg, and ant-HCV using third-generation ELISA kits. For anti-HCV, SD
HCV ELISA 3.0 test system (Boi SD standard diagnosis Pvt. Ltd, India);
for anti-HIV-1/2, Microlisa (J. Mitra& Co. Pvt. Ltd, India) and for
HBsAg, SD HBV ELISA 3.0 test system (Boi SD standard diagnosis Pvt.
Ltd, India) was used.
Nucleic Acid Amplification (NAT).
All plasma samples of 196 patients were screened for research purposes
for the viral genome of HBV, HCV, and HIV-1 with a commercially
available RT-PCR kit (Altona Diagnostics GmbH, Germany). The PCR was
performed on an ABI Prism 7500 Real-Time PCR System (Thermo Fisher,
USA).
i. Extraction of the viral genome:
HBV-DNA, HCV-RNA, and HIV-1 RNA were extracted from plasma samples with
the use of Chemagic Prepito-D automated nucleic acid extractor
(PerkinElmer, USA), in combination with reagents/buffers of the Prepito
Viral DNA/RNA Kit.
ii. Amplification of viral genome by Real-Time-PCR:
HBV-DNA and HIV and HCV-RNA were amplified by RealStar HBV PCR Kit 1.0,
RealStar HCV RT-PCR Kit 1.0, and Real-Star HIV RT-PCR Kit 1.0 (Altona
Diagnostics GmbH, Germany) as described in the manufacturer’s protocol.
The PCR was performed on an ABI Prism 7500 Real-Time PCR System (Thermo
Fisher, USA).
Statistical software.
The data were subjected to statistical analysis using SPSS version 10.0
software. Mean and standard deviations were computed. For discrete
variables, the Chi-square test was applied to determine the association
between two variables. A student’s test was done to compare the mean of
two groups. A significant difference was accepted at p = 0.05.
Results
A
total of 196 patients of thalassemia patients were included in this
study. Amongst them, 133 (67.8%) were males, and 63 (32.14%) were
females with the age group between five years to fifteen years (Table 1).
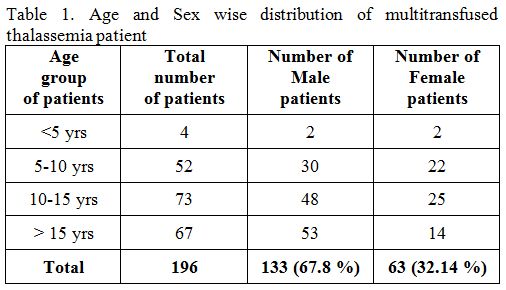 |
Table 1. Age and Sex wise distribution of multitransfused thalassemia patient. |
Hepatitis B Virus (HBV).
Out of 196 multitransfused thalassemia patients, the prevalence of
HBsAg positivity and HBV-DNA positivity were 1.5% (3/196) and 2.5%
(5/196) respectively, in which three male patients were HbsAg positive
and four male and one female patient were HBV-DNA positive (Table 2A). Every sample that positive for HBsAg was also positive for HBV-DNA, and there were two samples solely positive for HBV-DNA.
Hepatitis C Virus (HCV).
Out of 196 multitransfused thalassemia patients, the prevalence of
anti-HCV positivity and HCV-RNA positivity was 51% (100/196), and 33.7%
(66/196) (Table 2B), in which
28 females and 72 males patients were anti-HCV positive and 13 females
and 53 males patients were HIV-RNA positive. On the contrary, two
samples that were positive for anti-HCV but found negative for HCV-RNA.
In HCV seropositive samples, the positive rate of HCV-RNA was 64%
(64/100).
Human Immunodeficiency Virus (HIV).
Out of 196 multitransfused thalassemia patients, the prevalence of
anti-HIV positivity and HIV-RNA positivity were 3.1% (6/196) and 4.1%
(8/196), respectively (Table 2C),
in which five males and one female patient were anti-HIV positive, and
five males and three females were HIV-RNA positive. However, two
ant-HIV positive samples were not found positive for HIV-RNA. On the
other hand, two samples were solely positive for HIV-RNA.
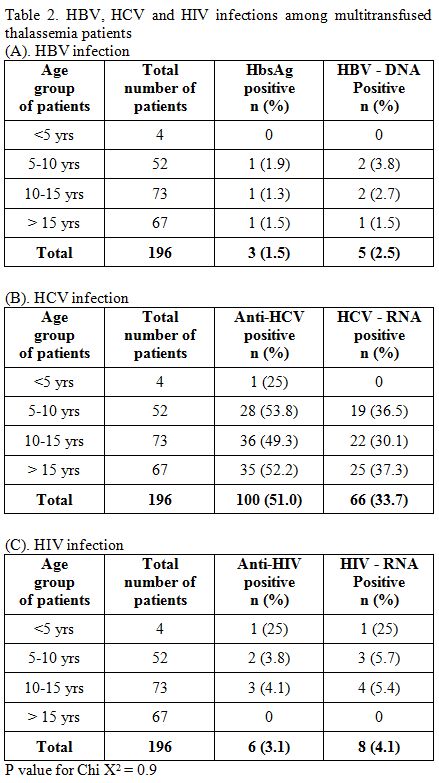 |
Table
2. HBV, HCV and HIV infections among multitransfused thalassemia patients. |
Coinfections among multitransfused thalassemia patients.
Amongst 196 multitransfused thalassemia patients, we found 2 (1.0%)
coinfections cases of HBV and HCV by ELISA test. Furthermore there were
6 (3.0%) cases of co-infections by NAT testing, in which 3 (1.5%) were
HBV/HCV, 1 (0.5%) were HIV/HBV and 2 (1%) were HIV/HCV.
Discussion
The
β-thalassemia is the most common inherited hemoglobin disorder in the
Indian subcontinent, with an uneven distribution among the different
endogenous populations. Furthermore, hemoglobinopathies cases are more
in Gujarat compared to other Indian states. Earlier we have reported a
high prevalence of β-thalassemia trait (BTT) and sickle cell trait
(SCT) in South Gujarat.[12,13,14]
Conventional
treatment of patients suffering from β-thalassemia is based on adequate
and safe blood transfusions and receiving regular iron-chelation
therapy from early childhood, all of which improve the quality of life
and survival of patients.[15] On the other hand,
blood transfusions expose the patients to the risk of acquiring
transfusion-transmissible infections (TTIs). The possibility of
acquiring TTIs is associated with the number of units transfused;
therefore, the infection rate of TTIs increases with age in subsequent
years.[16] Therefore, each blood transfusion/blood
unit composes a chance for acquiring TTI, and as the number of blood
transfusion increases, the higher the risk of exposure. Furthermore,
since thalassemia patients need multiple transfusions, it is logical
that the provision of blood may not be prompt in every visit in a
single center. As a result, most of the thalassemia patients had
received blood units from multiple hospitals or blood banks within or
outside the state.
In the present study, seropositivity for TTIs
was 57% (109/196). HIV seropositivity was 3.1%, HBV was 1.5%, and HCV
was 51%. Different studies from all over India reported the highest
TTIs transmission rates for HCV, ranging from 2.2-44%, followed by HBV
ranging from 1.2-7.4% and HIV ranging from 0-9%.[17,18,19]
The prevalence of anti-HCV in multiple-transfused patients is confirmed to be high.[19-21] A three-year prospective study from India by Choudhury et al., 2001,[19]
observed that anti-HCV prevalence in the same number of thalassemia
major patients was 23%, 30.7%, and 35.9% each year, respectively. The
present study showed comparable results as described by Choudhury et
al. 2001,[19] and Mukherjee et al., 2017.[22] Furthermore anti-HCV seropositivity in our patients (51%) was comparable among multitransfused patients in Jordan (40%),[23] Egypt (45% to 76%),[24] Iran (44.7%),[25] and Pakistan (51.3%).[26]
In
our study, the seropositivity of HBsAg among thalassemic children was
1.5%. Our result was comparable to a study in India, where the
prevalence rate of HBsAg was ranged from 1.2-7.4%.[22]
However, the prevalence rate is still high compared to other Asian
countries, such as Turkey and Malaysia, which reported lower HBsAg
seroprevalence rates of 0.75% and 1%, respectively.[27,28]
Differences in the prevalence of TTIs amongst thalassemic could be
related to geographical differences in the prevalence of the viral
infections among blood donors, the nature of blood donors, whether
replacement or voluntary and most important the nature of care
individual thalassemics receives.
In India, it is mandatory to
screen donated blood for anti-HIV 1 and 2 (since 1991), anti-HCV (since
2001), and HBsAg (along with malaria and syphilis) became mandatory
since 2002. Since then, the risk has been limited to the blood units
collected during the “window period”. However, TTIs can still occur
from blood units negative for the markers for these infections, as
reported by different investigators and international studies.[17,18] Since HBV and HCV are transmissible by the parenteral route and may be found not only in blood but also in other body fluids.
Nucleic acid testing (NAT) is widely recommended for the screening of the donor’s blood. Makroo et al., 2008,[29] and our two recent studies; Mishra et al., 2016,[30] and Ghosh et al., 2017A,[31]
have shown that blood units negative for HBsAg, anti-HIV, and anti-HCV
have 1:1807, 1:15906 and 1:39761 NAT positivity rate and that the
majority of the positivity was due to Hepatitis-B virus, underlining
the need for HBV vaccination in thalassemic patients. Moreover, HBV
infection can be prevented in these patients, as a very effective HBV
vaccine is available; therefore, all patients who require multiple
transfusions should be vaccinated right from the beginning.
In
the present study, 77% of cases positive for anti-HCV were between 10
to 15 years of age; they have been receiving transfusions before 2001
when screening for anti-HCV became mandatory. There is a reduction in
the development of anti-HCV post-2001, but it has not been eliminated.
The causes of high anti-HCV prevalence may be due to donors being
usually asymptomatic in early stages, despite being screened for
anti-HCV, possibly due to missing early window period infections. Most
of the cases of positive anti-HCV had received transfusions before
anti-HCV become mandatory therefore patients have more chances of
developing anti-HCV due to the cumulative increase in the number of
transfusions.[15]
In the present study
prevalence of HIV-RNA positive was higher (4.1%) while Anti-HIV
positive was (3.15). Moreover, two samples were solely positive for
HIV-RNA; it is due to immunosuppression with decreased production of
antibodies, or the window period of a recent infection.[17,18]
Though two ant-HIV positive samples were not found positive for
HIV-RNA, the primary purpose of ant-HIV screening tests is to risk
factors for disease in large numbers of individuals, thereby initially
ant-HIV positive samples should be verified by a second repeat testing.
In this study amongst 196 multi-transfused thalassemia patients,
there were six cases of coinfections by NAT testing (two coinfections
HIV-RNA/HCV-RNA, one coinfection HIV-RNA/HBV-DNA and three coinfections
of HBV-DNA/HCV-RNA), wherein there were two coinfections cases of
HBsAg/anti-HCV by ELISA. Coinfection is consequently common in people
with high exposure to blood and blood products.[32] The primary concern of coinfection is that it can lead to more severe liver diseases in multitransfused patients.[6]
NAT
testing was introduced in the developed countries in the late 1990s and
early 2000s and presently, around 33 countries in the world have
implemented NAT for HIV;[33,34] however, NAT is not as
yet a mandatory screening test in India. We have implemented NAT
testing at our center from April 2013; however, the implementation of
NAT technology is still limited at different centers in India; this
might contribute to the high infectious marker positivity observed in
the present study. Blood screening using NAT can reduce the window
periods of HIV, HBV and HCV infections substantially.[30,31,34,35]
Various studies from India reported a combined NAT yield (NAT
positive/Seronegative) for HIV, HBV and HCV is high as compared to that
reported from other developed countries.[29,36] These elevated yields of NAT suggest a higher prevalence of TTIs in India, highlighting the need for NAT in our country.
A
vaccine for hepatitis-B is available and should be given to recipients,
especially to multitransfused patients, before transfusion.
Furthermore, a high prevalence of HCV in multi-transfused patients of
Beta-thalassemia necessitates better methods of screening like NAT in
the facilities. Periodic monitoring of multi-transfused subjects
through hemovigilance should be made a part of the blood safety
programs. Furthermore, HCV infection leads to liver fibrosis,
cirrhosis, and hepatocellular carcinoma (HCC).[37]
Until couple of years ago, the recommended therapy for HCV treatment
consisted of chelation therapy along with pegylated-interferon alpha
plus ribavirin, a therapy with significant side effects.[38]
More recently, the use of Direct-acting Antiviral Agents (DAAs) gave a
breakthrough and demonstrated to be appropriate in HCV management in
patients with thalassemia disease for whom previous regimens gave
restrictions.[38] DAAs has led to a real HCV eradication with negative viremia and sustained viral response between 90 and 98%.[39]
Thus treatments with DAAs, with adequate iron chelation, and
non-invasive monitoring liver status is recommended to prevent
cirrhosis and HCC in thalassemia patients.[40]
However it remains the big crisis of the costs of DAAs therapies,
treatment regimens are very expensive, and this can limit their
application, and lengthen the time for the global eradication of HCV.
Transfusion
Transmitted Infections mainly occur in patients who are dependent on
blood transfusion like thalassemia major, sickle cell disease, chronic
renal failure, etc. Such patients should be encouraged to stick to one
center for their blood unit requirement for transfusion, although it is
logical that the provision of blood may not be prompt in every visit.
Therefore all sectors need to strict donor screening with a mandatory
screening of blood products against the TTIs and control the quality of
blood donors, along with the use of modern molecular biology techniques
such as NAT for the screening of blood units, and bringing awareness in
the community will surely help in reducing the problem statement.
Furthermore, periodic screening of multi-transfused subjects through
hemovigilance should be made a part of the blood safety programs.
Conclusions
Despite
the standard procedures followed by blood banks to ensure blood safety,
HBV, HCV, and HIV present a significant challenge in the management of
thalassemia patients. There is still a severe risk for HCV infection.
On the contrary, there is a minor risk for HBV infection in patients
with thalassemia. Administering HBV vaccine along with HCV treatment
with DAAs, in conjunction with adequate iron chelation, ensuring the
immune status, and monitoring hepatitis markers might considerably
minimize the incidence of viral hepatitis among them.
Acknowledgments
The authors thank all the technicians of TTI department of the SRKRC for doing ELISA testing of these multitransfused patients.
References
- Jaiswal SP, Chitnis DS, Jain AK, et al. Prevalence
of hepatitis viruses among multi-transfused homogenous thalassaemia
patients. Hepatol Res. 2001; 19:247-53. https://doi.org/10.1016/S1386-6346(00)00102-9
- Cappellini MD, Piga A. Current status in iron chelation in hemoglobinopathies. CurrMol Med. 2008; 8(7):663-74. https://doi.org/10.2174/156652408786241438 PMid:18991652
- Shah
A, Patel P, Patel K, et al. Comparison of serology and molecular
detection of common red cell antigens in multitransfused thalassemia
major and sickle cell anemia patients. Transfus Apher Sci. 2019 Jul
9:102599. https://doi.org/10.1016/j.transci.2019.06.026 PMid:31326292
- Makroo
RN, Arora JS, Chowdhry M, et al. Red cell alloimmunization and
infectious marker status (human immunodeficiency virus, hepatitis B
virus and hepatitis C virus) in multiply transfused thalassemia
patients of North India. Indian J Pathol Microbiol. 2013; 56(4):378-83.
https://doi.org/10.4103/0377-4929.125295 PMid:24441225
- Mohammadi
M, Talei G, Sheikhian A, et al. Survey of both hepatitis B virus
(HBsAg) and hepatitis C virus (HCV-Ab) coinfection among HIV positive
patients. Virol J. 2009; 6: 202. https://doi.org/10.1186/1743-422X-6-202 PMid:19922624 PMCid:PMC2785785
- Anbazhagan
GK, Sridharan K, Thirunalasundari T. Prevalence Pattern of Blood Borne
Hepatitis Group of Viruses in Liver Disease Patients. World J Medi.
Sci. 2007; 2:01.01.
- Sadhukhan, Provash.
HIV-HCV Co-Infection among Multitransfused Thalassemic Individuals-A
Review. J Hum Virol Retrovirol. 2016, 3(4): 00103. https://doi.org/10.15406/jhvrv.2016.03.00103
- Saha
K, Firdaus R, Santra P, et al. Recent pattern of Coinfection amongst
HIV seropositive individuals in tertiary care hospital, Kolkata. Virol
J. 2011; 8:116. https://doi.org/10.1186/1743-422X-8-116 PMid:21396133 PMCid:PMC3066117
- Narayan S. Microbes and blood transfusion. Indian J Med Microbiol. 2001;19:119-126.
- Kapoor
D, Saxena R, Sood B, Sarin SK. Blood transfusion practices in India:
results of a national survey. Indian J Gastroenterol. 2000;19(2):64-67.
- Shrivastava
M, Kumar S, Navaid S, et al.A Cross-Sectional Study on Burden of
Hepatitis C, Hepatitis B, HIV and Syphilis in Multi-Transfused
Thalassemia Major Patients Reporting to a Government Hospital of
Central India. Indian J Hematol Blood Transfus. 2015;31(3):367-73. https://doi.org/10.1007/s12288-014-0462-5 PMid:26085723 PMCid:PMC4465515
- Patel
A, Shah A, Sorathiya S, Gupte S. Hemoglobinopathies in South Gujarat
population and incidence of anemia in them. Indian J Human Genet. 2012;
18 (3): 294-98. https://doi.org/10.4103/0971-6866.107979 PMid:23716936 PMCid:PMC3656517
- Bhukhanvala
D, Italia K, Sawant P, et al. Molecular characterization of
β-thalassemia in four communities in South Gujarat-codon 30 (G→A) a
predominant mutation in the Kachhiya Patel community. Ann Hematol.
2013; 92(11):1473-6. https://doi.org/10.1007/s00277-013-1777-2 PMid:23665927
- Bhukhanval
D, Sorathiya S, Surve R, et al. Hemoglobin variants in Muslim community
in South Gujarat, Western India. Int J Lab Hematol. 2014; 36(1): 15-7. https://doi.org/10.1111/ijlh.12123 PMid:23795655
- Jain
R, Perkins J, Johnson ST, et al. A prospective study for prevalence
and/or development of transfusion-transmitted infections in multiply
transfused thalassemia major patients. Asian J Transfus Sci.
2012;6(2):151-154. https://doi.org/10.4103/0973-6247.98919 PMid:22988380 PMCid:PMC3439754
- Atwa
ZT, Abdel Wahed WY. Transfusion transmitted infections in frequently
transfused thalassemic children living in Fayoum Governorate, Egypt:
Current prevalence and risk factors. J Infect Public Health. 2017;
10(6):870-874. https://doi.org/10.1016/j.jiph.2017.02.012 PMid:28292647
- Singh
H, Pradhan M, Singh RL, et al. High frequency of hepatitis B virus
infection in patients with â-thalassemia receiving multiple
transfusions. Vox Sang. 2003; 84(4):292-9. https://doi.org/10.1046/j.1423-0410.2003.00300.x PMid:12757503
- Karimi
M, Mohammadi F, Behmanesh F, et al. Effect of combination therapy of
hydroxyurea with l-carnitine and magnesium chloride on hematologic
parameters and cardiac function of patients with beta-thalassemia
intermedia. Eur J Haematol. 2010; 84(1):52-8. https://doi.org/10.1111/j.1600-0609.2009.01356.x PMid:19799627
- Choudhury N, Phadke S. Transfusion transmitted diseases. Indian J Pediatr 2001;68:951-8. https://doi.org/10.1007/BF02722595 PMid:11758132
- Patel
A, Goswami H. A retrospective study for prevalence of
transfusion-transmitted infections in multiply transfused thalassemia
major pediatric patients. Internet J Sci Res. 2014; 3:283-5.
- Chakrabarti
S, Pradhan P, Roy A, et al. Prevalence of anti HCV, HBsAg and HIV
antibodies in high risk recipients of blood and blood products. Indian
J Public Health. 2006; 50(1):43-4.
- Mukherjee
K, Bhattacharjee D, Chakraborti G. Prevalence of hepatitis B and
hepatitis C virus infection in repeatedly transfused thalassemics in a
tertiary care hospital in eastern India. Int J Res Med Sci.
2017;5(10):4558-4562. https://doi.org/10.18203/2320-6012.ijrms20174596
- Al-Sheyyab
M, Batieha A, El-Khateeb M. The prevalence of hepatitis B, hepatitis C
and human immune deficiency virus markers in multi-transfused patients.
J Trop Pediatr. 2001; 47(4):239-42. https://doi.org/10.1093/tropej/47.4.239 PMid:11523766
- El-Faramawy
AA, El-Rashidy OF, Tawfik PH, Hussein GH. Transfusion transmitted
hepatitis: where do we stand now? A one center study in Upper Egypt.
Hepat Mon. 2012 Apr; 12(4):286-91. https://doi.org/10.5812/hepatmon.852 PMid:22690237 PMCid:PMC3360939
- Hassanshahi
G, Arababadi MK, Assar S, et al.Post-transfusion-transmitted hepatitis
C virus infection: a study on thalassemia and hemodialysis patients in
southeastern Iran. Arch Virol. 2011; 156:1111-1115. https://doi.org/10.1007/s00705-011-0950-y PMid:21340738
- Shahid
S, Ahmad I, Khan M, Khalid F, Ahmad S, Bashir A. Prevalence of HBsAg
and anti-HCV antibodies in poly-transfused ß-thalassaemia major
children in Lahore. Biomedica. 2013; 29:230-3.
- Lee
W.S., C.M. Teh, L.L. Chan. Risks of seroconversion of hepatitis B,
hepatitis C and human immunodeficiency viruses in children with
multitransfused thalassaemia major. J Paediatr Child Health. 2005;
41(5-6): 265-268. https://doi.org/10.1111/j.1440-1754.2005.00608.x PMid:15953326
- Ocak
S, Kaya H, Cetin M, Gali E, Ozturk M. Seroprevalence of hepatitis B and
hepatitis C in patients with thalassemia and sickle cell anemia in a
long-term follow-up. Arch Med Res. 2006; 37 (7): 895-898. https://doi.org/10.1016/j.arcmed.2006.04.007 PMid:16971232
- Makroo
RN, Choudhury N, Jagannathan L, et al. Multicenter evaluation of
individual donor nucleic acid testing (NAT) for simultaneous detection
of human immunodeficiency virus -1 & hepatitis B & C viruses in
Indian blood donors. Indian J Med Res. 2008; 127:140-147.
- Mishra
KK, Trivedi A, Sosa S, Patel K, Ghosh K. NAT positivity in seronegative
voluntary blood donors from western India. Transfus Apher Sci. 2017;
56(2):175-178. https://doi.org/10.1016/j.transci.2016.11.003 PMid:28041821
- Ghosh
K, Mishra KK, Trivedi A, Sosa S, Patel K. Assessment of semi-automated
nucleic acid testing programme in a Regional Blood Transfusion Centre.
Br J Biomed Sci. 2017-A: 74(1): 42-47. https://doi.org/10.1080/09674845.2016.1220708 PMid:27996693
- Biswas
A, Gupta D, Ghosh M, Datta A, Gupta N, et al. HIV-HCV Co-Infection
among Multitransfused Thalassemic Individuals-A Review. J Hum Virol
Retrovirol. 2016; 3(4): 00103. https://doi.org/10.15406/jhvrv.2016.03.00103
- Roth
WK, Busch MP, Schuller A, et al. International survey on NAT testing of
blood donations: expanding implementation and yield from 1999 to 2009.
Vox Sang. 2012; 102:82-90. https://doi.org/10.1111/j.1423-0410.2011.01506.x PMid:21933190
- Ghosh
K, Mishra K. Nucleic acid amplification testing in Indian blood banks:
A review with perspectives. Indian J Pathol Microbiol. 2017-B;
60(3):313-318. https://doi.org/10.4103/IJPM.IJPM_361_16 PMid:28937364
- Soldan
K, Davison K, Dow B: Estimates of the frequency of HBV, HCV, and HIV
infectious donations entering the blood supply in the United Kingdom,
1996 to 2003. Euro Surveill. 2005; 10:9-10. https://doi.org/10.2807/esm.10.02.00520-en PMid:29183543
- Chatterjee
K, Coshic P, Borgohain M, et al. Individual donor nucleic acid testing
for blood safety against HIV-1 and hepatitis B and C viruses in a
tertiary care hospital. Natl Med J India. 2012; 25:207-209.
- De
Sanctis V., Soliman A.T., Daar S et al. A Concise Review on the
Frequency, Major Risk Factors and Surveillance of Hepatocellular
Carcinoma (HCC) in β-Thalassemias: Past, Present and Future
Perspectives and the ICET-A Experience. Mediterr J Hematol Infect Dis.
2020; 12(1): e2020006. https://doi.org/10.4084/mjhid.2020.006 PMid:31934316 PMCid:PMC6951357
- Zachou
K., Arvaniti P.,.Gatselis N.K Azariadis K., Papadamou G., Rigopoulou
E., Dalekos G.N. Patients with Haemoglobinopathies and Chronic
Hepatitis C: A Real Difficult to Treat Population in 2016? Mediterr J
Hematol Infect Dis. 2017; 9(1): e2017003. https://doi.org/10.4084/mjhid.2017.003 PMid:28101309 PMCid:PMC5224816
- Maffei
L, Sorrentino F, Caprari P, et al. HCV Infection in Thalassemia
Syndromes and Hemoglobinopathies: New Perspectives. Front. Mol. Biosci.
2020; 7:7. https://doi.org/10.3389/fmolb.2020.00007 PMid:32118034 PMCid:PMC7025587
- Kamal
S, Abdelhakam S, Ghoraba D, et al. The Course of Hepatitis C Infection
and Response to Anti-viral Therapy in Patients with Thalassemia Major
and Hepatitis C Infection: A Longitudinal, Prospective Study. Mediterr
J Hematol Infect Dis. 2019; 11(1): e2019060. https://doi.org/10.4084/mjhid.2019.060 PMid:31700585 PMCid:PMC6827603
TOP]

