 |
Table
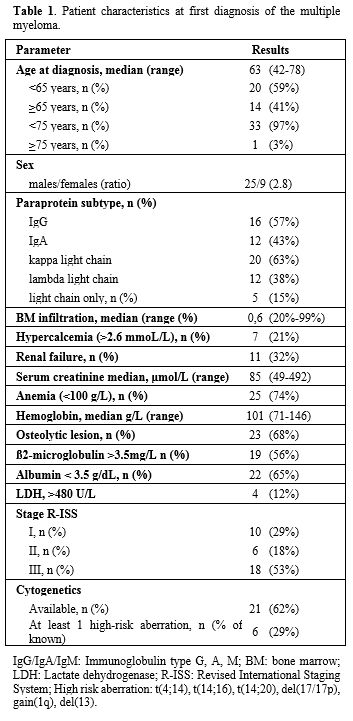
1. Patient characteristics at first diagnosis of the multiple myeloma. |
Prior therapies including daratumumab. Among the 34 patients fulfilling the criteria of three treatment lines, including PI, IMID, and anti-CD38 treatment, and effectively receiving subsequent therapy line(s), the median number of previous lines was 4.5 (range 2-12 lines). 24 (55%) patients had four or more prior therapy lines, mainly because anti-CD38 treatment was first given late in these patients. HDCT and ASCT were performed in 31 (91%) patients. The prior treatment lines are summarized in Table 2. 14 (40%) patients were quad-refractory, thus refractory to bortezomib, lenalidomide, carfilzomib, and pomalidomide, and 13 (37%) patients were penta-refractory, thus refractory also to daratumumab.
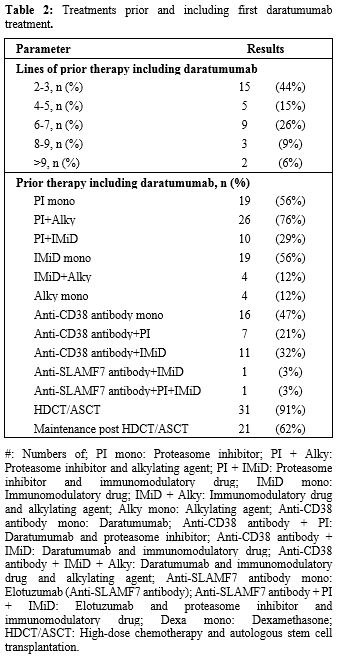 |
Table 2. Treatments prior and including first daratumumab treatment. |
First treatment line after inclusion. The median interval from the initial diagnosis to the first treatment after fulfilling the study criteria was 67 months (range 19 to 189 months). 11 (32%) patients received one subsequent treatment line, 13 (38%) patients received two subsequent treatment lines, and 8 (24%) patients received three or more lines of treatment (Table 3). The most frequent treatment line was IMID/dexamethasone in 11 (32%) patients, followed by PI/dexamethasone in 10 (29%) patients, alkylating agents in 9 (26%) patients, daratumumab combined with a PI in 6 (18%) patients, and PI combined with IMID in 6 (18%) patients. Six (18%) patients received HDCT/ASCT during relapse treatment.
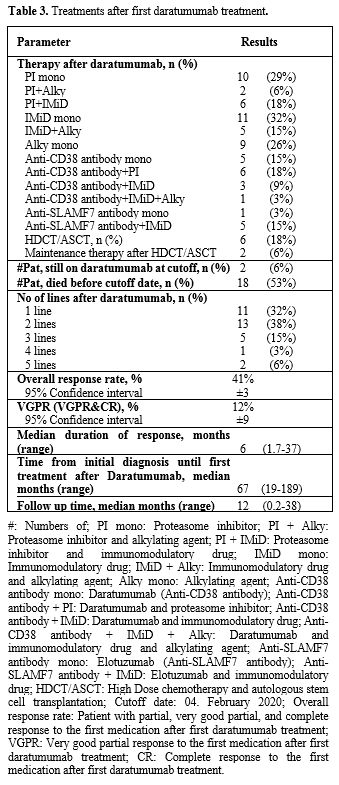 |
Table 3. Treatments after first daratumumab treatment. |
The ORR to the first treatment after study inclusion was 41%, with a median duration of response of 5 months (range 1 to 37 months). 12% of the patients had an excellent partial response or better, with a median duration of this response of 8 months (range 3 to 37 months). So far, 33 (59%) patients have died, all due to disease progression.
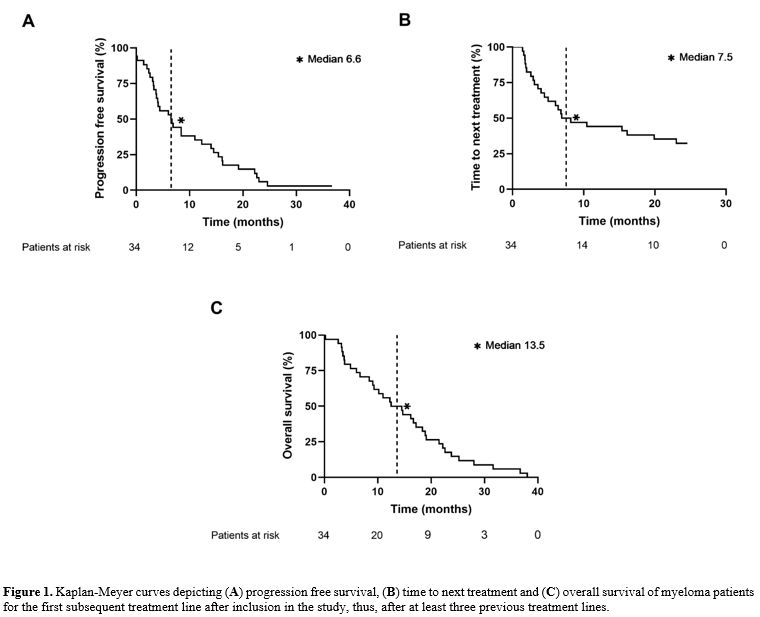
Outcome. The median PFS after the first treatment line after inclusion in the study was 6.6 months (range, 0 to 36.6 months; Figure 1A). For the patients with two or more further treatment lines, the median PFS was 6.6 months (range, 0 to 24.5 months) compared to median PFS of 5 months (range, 0.1 to 36.6 months) for those with only one further line. The median TTNT between the first and the second treatment line was 7.5 months (range 1.4-24.6 months) for the patients with effectively at least two further lines of treatment (Figure 1B). The median OS of the cohort was 13.5 months (range, 0.1 to 38.0 months) after starting the first line of treatment within the study (Figure 1C). For patients with two or more further treatment lines, the OS was 15.6 months (range, 3.5 to 38) compared to 7.5 months (range, 0.1 to 36.6 months) for the patients with only one further treatment line.