Sutatta Supatharawanich1, Nattee Narkbunnam1, Nassawee Vathana1, Chayamon Takpradit1, Kamon Phuakpet1, Bunchoo Pongtanakul1, Sasima Tongsai2, Phakatip Sinlapamongkolkul3, Popchai Ngamskulrungroj4, Wanatpreeya Phongsamart5, Kleebsabai Sanpakit1 and Jassada Buaboonnam1.
1 Division of
Hematology and Oncology, Department of Pediatrics, Faculty of Medicine,
Siriraj Hospital, Mahidol University, Bangkok, Thailand.
2 Office for Research and Development, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand.
3 Department of Pediatrics, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand.
4 Department of Microbiology, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand.
5
Division of Infectious Diseases, Department of Pediatrics, Faculty of
Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Correspondence to: Jassada Buaboonnam, MD, Associate Professor of
Pediatrics Division of Hematology and Oncology, Department of
Pediatrics Faculty of Medicine, Siriraj Hospital, Mahidol University 2
Wanglang Road, Bangkok Noi Bangkok
10700, Thailand. Tel:+66 2 419 5971. Fax: +66 2 866 3021 E-mail:
onco008@yahoo.com
Published: July 1, 2021
Received: January 31, 2021
Accepted: June 2, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021039 DOI
10.4084/MJHID.2021.039
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Although
the outcomes of childhood leukemia and severe aplastic anemia (SAA)
have improved, infectious complications are still the major concern.
Particularly worrisome are invasive fungal diseases (IFDs), one of the
most common causes of infectious-related deaths in patients with
prolonged neutropenia. A retrospective study was conducted of IFDs in
pediatric patients with newly diagnosed or relapsed acute leukemia, or
with SAA, at Siriraj Hospital, Mahidol University, Thailand. There were
241 patients: 150 with acute lymphoblastic leukemia (ALL), 35 with
acute myeloid leukemia (AML), 31 with relapsed leukemia, and 25 with
SAA. Their median age was 5.4 years (range, 0.3–16.0 years). The
overall IFD prevalence was 10.7%, with a breakdown in the ALL, AML,
relapsed leukemia, and SAA patients of 8%, 11.4%, 19.3%, and 16%,
respectively. Pulmonary IFD caused by invasive aspergillosis was the
most common, accounting for 38.5% of all infection sites. Candidemia
was present in 34.6% of the IFD patients; Candida tropicalis
was the most common organism. The overall case-fatality rate was 38.5%,
with the highest rate found in relapsed leukemia (75%). The incidences
of IFDs in patients with relapsed leukemia and SAA who received fungal
prophylaxis were significantly lower than in those who did not (P
= N/A and 0.04, respectively). IFDs in Thai children with hematological
diseases appeared to be prevalent, with a high fatality rate. The usage
of antifungal prophylaxes should be considered for patients with SAA to
prevent IFDs.
|
Introduction
The
outcomes of pediatric leukemia have drastically improved over the past
decade, with 5-year overall survival (OS) rates of 80%–90%[1,2] and 70%[3,4]
for acute lymphoblastic leukemia (ALL) and acute myeloid leukemia
(AML), respectively. However, owing to prolonged neutropenia secondary
to chemotherapy, infectious complications such as invasive fungal
diseases (IFDs) may occur and become a significant cause of death in
such patients, particularly in cases of AML.[5,6]
Apart from directly causing morbidity and mortality, IFDs might delay
the treatment of leukemia and ultimately have an impact on disease-free
survival. Likewise, other diseases manifesting with prolonged
neutropenia, such as severe aplastic anemia (SAA), might be at risk of
IFDs. The incidence of IFDs in children has been reported to vary with
the underlying disease, ranging from 8.4% to 20% in AML and from 10% to
11% in ALL,[7-9] whereas the IFD incidence of SAA in both children and adults is between 8% and 21%.[10,11]
Apart from pre-existing disease, environmental factors (such as the
construction of the hospital) and changes in clinical practice (such as
the use of antibiotic prophylaxis) may be risk factors for IFDs. These
may cause variations in IFD rates between countries.[12]
However, there are scarce data on the incidence and outcomes of IFDs in
Thai children with leukemia and SAA. Investigation of these two aspects
in such patients may guide physicians in preventing IFDs and improving
IFD treatment. The current research aimed to retrospectively study the
prevalence and outcomes of IFDs in pediatric leukemia and SAA and to
determine the risk factors for IFDs in such patients.
Patients and Methods
A
retrospective review was conducted on all children aged younger than 16
years, diagnosed with ALL, AML, and SAA between 2009 and 2019 at
Siriraj Hospital, Mahidol University. The demographic data collected
comprised sex, age, diagnosis of disease, and risk classification. The
clinical factors analyzed were the presence of central venous line, the
duration of neutropenia, the genetic polymorphism of TPMT and NUDT15 (only for ALL), diagnosis of IFD, antifungal prophylaxis, antifungal treatment, and organ involvement.
The
ALL and AML diagnoses were established by cell morphology, flow
cytometry, and cytogenetics, while SAA diagnoses were based on
Camitta’s criteria.[13] Cytogenetics and chromosomal
breakage studies were done to exclude congenital bone marrow syndrome.
The incidences of IFDs during the conditioning regimen and after
allogeneic stem cell transplantation were excluded.
Antifungal
prophylaxis (AFP) was not routinely prescribed for patients with ALL,
except for those treated with infant or relapsed protocol. Patients
with AML diagnosed before 2014 received AFP at physician discretion,
whereas all patients diagnosed after 2014 received AFP according to the
consensus of the Thai Pediatric Oncology Group 2016 guidelines.[14]
The SAA patients received AFP at physician discretion; after 2017, all
SAA patients received AFP since an institute guideline had been
implemented. Itraconazole (ITRA) solution or fluconazole was deemed the
first-line AFP, whereas posaconazole suspension was considered as an
alternative for those aged > 13 years. Therapeutic drug monitoring
was performed for those receiving posaconazole suspension and ITRA to
maintain a trough serum of more than 0.7 mcg/ml and 0.5–4 mcg/ml,
respectively. The diagnoses of IFD were categorized as “proven”,
“probable”, and “possible”, using the criteria of the Fungal Infections
Cooperative Group and the National Institute of Allergy and Infectious
Diseases Mycoses Study Group Consensus Group.[15] Our
research was approved by the Ethics Committee of the Siriraj
Institutional Review Board, Faculty of Medicine, Siriraj Hospital,
Mahidol University, Bangkok, Thailand (Si299/2562).
Descriptive
statistics were used to detail the demographic and clinical
characteristic data. Medians and ranges were calculated for continuous
data, while numbers and percentages were used to describe categorical
data. Pearson’s chi-squared test, Yates’ continuity correction, or
Fisher’s exact test was used to compare the proportions between groups
for categorical data, and the Mann–Whitney U test was used to compare
medians for continuous data. To check for normal distribution of data,
the Shapiro–Wilk test was performed. Simple and multiple binary
logistic regression analyses were used to assess factors associated
with IFDs. The magnitude and direction of association between the
factors and the IFDs were identified using odds ratio (OR) with 95%
confidence interval (95% CI). A P
value < 0.05 was considered statistically significant. All analyses
were performed using PASW Statistics for Windows (version 18.0; SPSS
Inc., Chicago, IL, USA).
Results
In
all, 241 patients were enrolled. Their median age at diagnosis of
hematological disease was 5.4 years (range, 0.3–16.0). The patient and
clinical characteristics data are detailed in Table 1.
Probable and proven IFDs were diagnosed in 26 patients, whereas
possible IFDs were diagnosed in 27. The overall IFD prevalence was
10.7%. The median duration of neutropenia before the diagnosis was 18.5
days (range, 0 to 121). The prevalences of IFDs in the ALL, AML,
relapsed leukemia, and SAA patients were 8%, 11.4%, 19.3%, and 16%,
respectively. Of all IFDs in both ALL and AML, most were diagnosed
during the induction phase (11 patients [91.7%] and two patients [50%],
respectively).
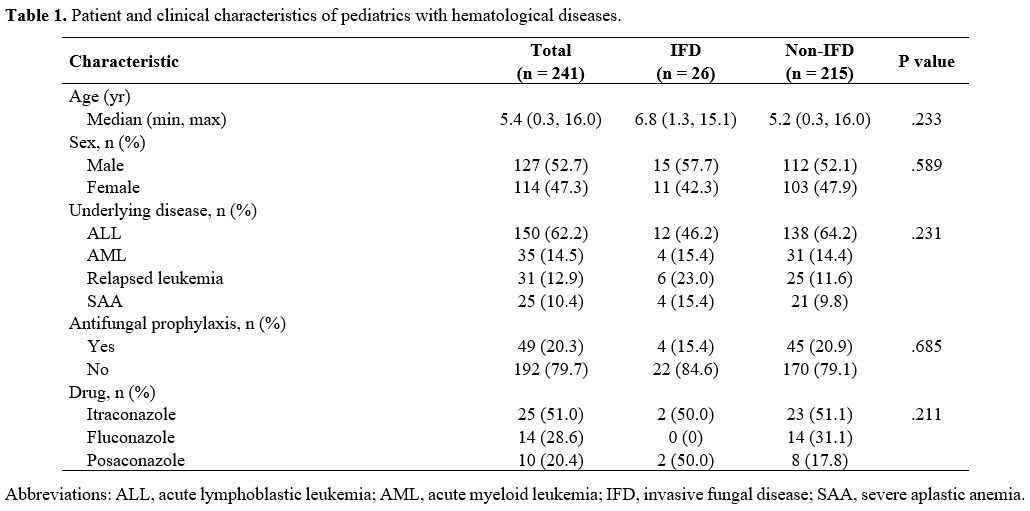 |
Table
1. Patient and clinical characteristics of pediatrics with hematological diseases.
|
Of all the IFD patients, proven IFD and probable IFDs were found in 13 (50%) and 13 (50%), respectively.
Among those with invasive candidiasis, Candida tropicalis was the most commonly identified organism. Table 2
lists the identified organisms and their sites of infection. Ten
patients (38.5%) had pulmonary IFD, nine (34.8%) had fungemia, four
(15.4%) had hepatosplenic IFD, and three (11.5%) had paranasal sinus
IFD. All ten patients with pulmonary IFD were diagnosed with invasive
pulmonary aspergillosis (IPA). Of all 27 possible IFD patients,
twenty-six were provisionally diagnosed with pulmonary IFD with the
negativity of serum galactomannan; one patient had hepatosplenic IFD
without mycological evidence.
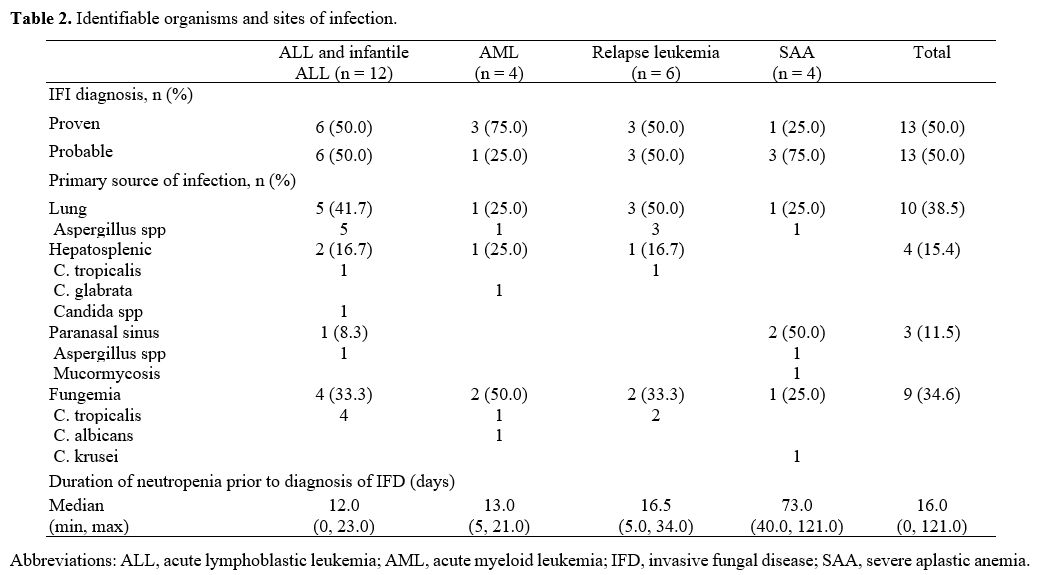 |
Table
2. Identifiable organisms and sites of infection.
|
Antifungal
treatment was prescribed for all 26 IFD patients, whereas 5 patients
received combined medical and surgical intervention. The overall
case-fatality rate of those with IFDs was 38.5%. The case-fatality
rates for ALL, AML, relapsed leukemia, and SAA patients were 8.3%,
25.0%, 83.3%, and 75.0%, respectively.
With regard to the ALL patients, the NCI risk classification, the presence of central venous line, and NUDT 15 and TPMT
polymorphisms were not factors associated with their IFDs. As to the
AML patients, Down syndrome, central venous line, and prolonged
neutropenia of more than one month were not associated with the IFDs.
In the aplastic anemia group, ANC < 200/mm3
and duration of treatment of more than three months were not associated
with the IFDs. The independent factors associated with IFDs for
patients with proven IFD, probable IFD, and possible IFD are presented
in Supplemental Table 1.
The types of AFP are summarized in Table 3.
ITRA was the most commonly prescribed AFP for patients with ALL, AML,
and relapsed leukemia (62.8%); fluconazole was the most common for
patients with SAA (64%). The median duration of fungal prophylaxis was
158 days, ranging from 10 days to 1,102 days. As to relapsed leukemia
and SAA, the IFD prevalence of patients receiving AFP was significantly
lower than that for those who were not administered an AFP (P = N/A and 0.04, respectively). With ALL and AML, the IFD prevalences of the two groups were not significantly different (P = 0.33 and 0.74, respectively).
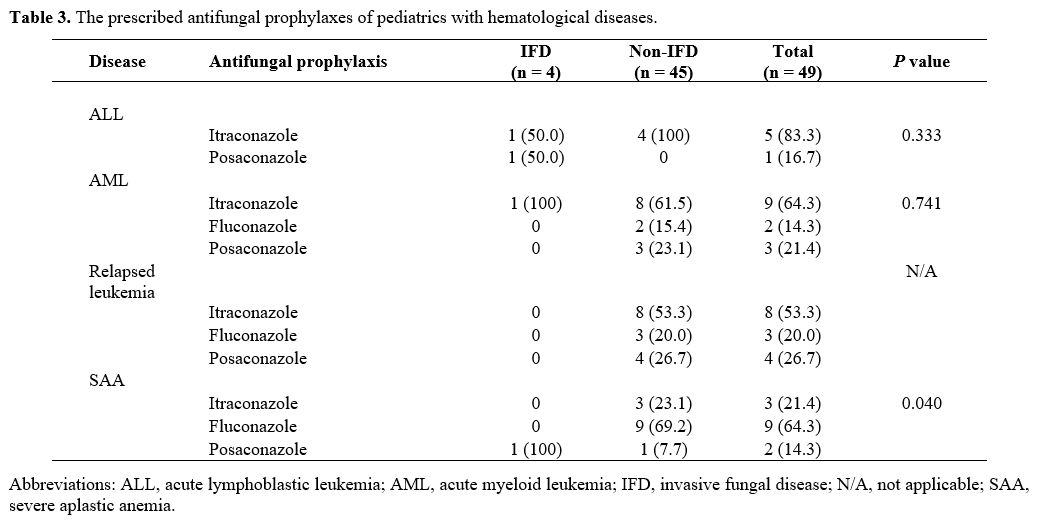 |
Table
3. The prescribed antifungal prophylaxes of pediatrics with hematological diseases.
|
Discussion
In
the present study, the prevalences of IFDs in pediatric ALL and AML
patients (8% and 11.4%, respectively) were higher than the values
reported by other pediatric studies, especially those conducted in
nontemperate zone countries.[5,16]
Environmental and geographical factors might be plausible causes
leading to the increased incidence of IFDs in tropical countries.[17]
Antifungal prophylaxis practice also affects IFD prevalence.
Furthermore, the current work found that the prevalence in individuals
with relapsed leukemia was higher than in non-relapsed patients.
Prolonged exposure to myelotoxic agents causing prolonged
immunosuppression in both humoral- and cell-mediated immunity may
heighten the risk of IFDs in relapsed patients.[18,19]
In our study, the occurrence of IFDs was common during the initial
treatment of both the ALL and AML patients. Therefore, clinical
suspicion of an IFD should be taken into consideration even if patients
have initially received induction therapy. The prevalence of IFDs in
pediatric SAA patients has been reported to range between 8% and 22%,[20,21] whereas that in adult SAA patients in Thailand was 21.2%,[11] compared with 8% in nontemperate countries.[22]
Likewise, the reasons mentioned above might plausibly explain the
higher incidence of IFDs (approximately 16%) revealed by our
investigation. This finding may highlight the role of IFD prophylaxis
for SAA patients.
Candida infections were the most frequently
identifiable cause of the IFDs in this study, which is concordant with
other published pediatric studies.[23] C. tropicalis
was the most common pathogen in the present work. This species appears
to be prevalent in Asia, particularly in the tropical regions of Asia,
such as India and Thailand.[24,25] In contrast, C. albicans has been reported to be the most common organism in most parts of Southwest Asia[26,27] and non-Asian regions.[28,29] Research has also found that the incidences of azole-resistant strains of C. tropicalis have increased,[30] and that the azole resistance of C. tropicalis is approximately ten times that of C. albicans (20.8% vs. 2.3%).[31,32] Hence, conducting a sensitivity test may play a pivotal role in determining the appropriate treatment for patients.
IPA
was the most common IFD in our study, and that corresponds with the
trends of increasing incidence rates observed by other investigations.[23,33]
Our work found that IPA accounted for 16% of the IFD cases, and that
may underscore the roles of clinical and radiographical monitoring as
well as serum galactomannan testing in individuals with a suspected
IFD. The proportion of possible IFDs in the current investigation was
50.9%; most of those cases had the clinical manifestations of a
pulmonary infection in the absence of mycological criteria. Since the
sensitivity of the serum galactomannan test is 70%,[34]
the true prevalence of the IFDs-especially pulmonary IFD-in our study
might be higher than we have reported. Further investigation might be
warranted to improve the diagnostic yield for such cases.
Among
the SAA patients of the present study, the proportion of cases with
mold infections was higher than of those with candida infections, and
that is in accord with the prevalence rates found in adult studies;[10,11]
data for children are scarce. Although recent guidelines recommend
using antimold azole or echinocandin for AML, relapsed leukemia, and
high-risk ALL,[35] there are currently no
recommendations for using an AFP for the treatment of SAA. In our
study, the IFD incidence in SAA was high; however, the incidence for
the SAA patients given an AFP was significantly lower than that for the
patients without an AFP. Therefore, it is recommended that the usage of
an AFP with antimold activity, such as ITRA, should be expanded to
include patients with SAA, especially in a tropical country such as
Thailand.
The reported case-fatality rates of IFDs in children
with hematological malignancies have varied between 20% and 50%, with
the prevalences appearing to depend upon the site of involvement,
pre-existing disease, and organism type.[8,36,37]
Although the current work demonstrated case-fatality rates of 15% to
30% for newly diagnosed leukemia and SAA patients, the mortality was as
high as 90% for the relapsed leukemia patients. Host factors (severe
immunocompromised status, prolonged antimicrobial use, and severe
damage of the mucosal membrane) may exacerbate the risk of IFDs in
cases of relapse. Thus, individuals with relapsed leukemia who are
clinically suspected of an IFD may need early and intensive treatment
to prevent morbidity and mortality.
Other clinical factors have
been reported to present a risk of IFDs, such as NCI risk
classification, the presence of Down syndrome,[38] the usage of a central venous catheter,[39] and the duration of neutropenia.[40] The genetic polymorphism of genes involving thiopurine metabolism, such as NUDT15, is associated with an increased risk of infection in ALL patients.[41]
In our investigation, the aforementioned clinical factors were not
statistically associated with IFDs. Our univariate analysis found that
when possible IFDs were included, age at diagnosis of > 4 years,
relapsed leukemia, AML, and SAA were independent risk factors for IFDs.
In our subsequent multivariate analysis, very severe aplastic anemia
(ANC < 200 mm3) was associated
with an increased risk of IFDs. Therefore, patients with very severe
aplastic anemia should be made aware of the possibility of developing
an IFD, and an AFP should be initiated while the diagnosis is being
established.
This study had limitations that need to be mentioned.
Firstly, given the nature of retrospective studies, there might be a
risk of missing or incomplete data. Secondly, the pediatric population
in this study was drawn from a national tertiary referral hospital;
therefore, the data may not be fully generalized to other populations.
In
conclusion, the prevalence of IFDs in Thailand appeared higher than the
rates reported for other countries. The usage of an AFP should be
considered to prevent mortality in SAA patients as well as AML and
relapsed leukemia patients.
Acknowledgments
The
authors gratefully acknowledge Mrs. Sam Ormond from the Clinical
Research Centre, Faculty of Medicine, Thammasat University and Mr.
David Park for editorial assistance.
References
- Takahashi H, Kajiwara R, Kato M, et al. Treatment
outcome of children with acute lymphoblastic leukemia: the Tokyo
Children's Cancer Study Group (TCCSG) Study L04-16. International
Journal of Hematology. 2018;108(1):98-108. https://doi.org/10.1007/s12185-018-2440-4 PMid:29589281
- Larsen
EC, Devidas M, Chen S, et al. Dexamethasone and High-Dose Methotrexate
Improve Outcome for Children and Young Adults With High-Risk B-Acute
Lymphoblastic Leukemia: A Report From Children's Oncology Group Study
AALL0232. J Clin Oncol. 2016;34(20):2380-2388. https://doi.org/10.1200/JCO.2015.62.4544 PMid:27114587 PMCid:PMC4981974
- Tsukimoto
I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive
use of cytarabine improves the outcome in childhood acute myeloid
leukemia: the AML99 trial from the Japanese Childhood AML Cooperative
Study Group. J Clin Oncol. 2009;27(24):4007-4013. https://doi.org/10.1200/JCO.2008.18.7948 PMid:19620491
- Reedijk
AMJ, Klein K, Coebergh JWW, et al. Improved survival for children and
young adolescents with acute myeloid leukemia: a Dutch study on
incidence, survival and mortality. Leukemia. 2019;33(6):1349-1359. https://doi.org/10.1038/s41375-018-0314-7 PMid:30568171
- Lehrnbecher
T, Schöning S, Poyer F, et al. Incidence and Outcome of Invasive Fungal
Diseases in Children With Hematological Malignancies and/or Allogeneic
Hematopoietic Stem Cell Transplantation: Results of a Prospective
Multicenter Study. Frontiers in Microbiology. 2019;10(681). https://doi.org/10.3389/fmicb.2019.00681 PMid:31040830 PMCid:PMC6476895
- Wang
SS, Kotecha RS, Bernard A, et al. Invasive fungal infections in
children with acute lymphoblastic leukaemia: Results from four
Australian centres, 2003-2013. Pediatric Blood & Cancer.
2019;66(10):e27915. https://doi.org/10.1002/pbc.27915
- Castagnola
E, Rossi MR, Cesaro S, et al. Incidence of bacteremias and invasive
mycoses in children with acute non-lymphoblastic leukemia: results from
a multi-center Italian study. Pediatr Blood Cancer.
2010;55(6):1103-1107. https://doi.org/10.1002/pbc.22750 PMid:20680968
- Mor
M, Gilad G, Kornreich L, et al. Invasive fungal infections in pediatric
oncology. Pediatric Blood & Cancer. 2011;56(7):1092-1097. https://doi.org/10.1002/pbc.23005 PMid:21319281
- Kaya
Z, Gursel T, Kocak U, et al. Invasive fungal infections in pediatric
leukemia patients receiving fluconazole prophylaxis. Pediatr Blood
Cancer. 2009;52(4):470-475. https://doi.org/10.1002/pbc.21868 PMid:19058205
- Valdez
JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality
and improved survival in severe aplastic anemia in the past two
decades. Clinical infectious diseases : an official publication of the
Infectious Diseases Society of America. 2011;52(6):726-735. https://doi.org/10.1093/cid/ciq245 PMid:21367725 PMCid:PMC3106262
- Lertpongpiroon
R, Rattarittamrong E, Rattanathammethee T, et al. Infections in
patients with aplastic Anemia in Chiang Mai University. BMC Hematology.
2018;18(1):35. https://doi.org/10.1186/s12878-018-0129-9 PMid:30534380 PMCid:PMC6280474
- Bajpai
VK, Khan I, Shukla S, et al. Invasive Fungal Infections and Their
Epidemiology: Measures in the Clinical Scenario. Biotechnology and
Bioprocess Engineering. 2019;24(3):436-444. https://doi.org/10.1007/s12257-018-0477-0
- Guinan EC. CLINICAL ASPECTS OF APLASTIC ANEMIA. Hematology/Oncology Clinics of North America. 1997;11(6):1025-1044. https://doi.org/10.1016/S0889-8588(05)70481-0
- Group TTPO. National protocol for the treatment of childhood cancers 2016. Bangkok: The Thai Society of Hematology.
- De
Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive
fungal disease from the European Organization for Research and
Treatment of Cancer/Invasive Fungal Infections Cooperative Group and
the National Institute of Allergy and Infectious Diseases Mycoses Study
Group (EORTC/MSG) Consensus Group. Clin Infect Dis.
2008;46(12):1813-1821. https://doi.org/10.1086/588660 PMid:18462102 PMCid:PMC2671227
- Rosen
GP, Nielsen K, Glenn S, et al. Invasive Fungal Infections in Pediatric
Oncology Patients: 11-Year Experience at a Single Institution. Journal
of Pediatric Hematology/Oncology. 2005;27(3). https://doi.org/10.1097/01.mph.0000155861.38641.ca PMid:15750444
- Tang
J-L, Kung H-C, Lei W-C, et al. High Incidences of Invasive Fungal
Infections in Acute Myeloid Leukemia Patients Receiving Induction
Chemotherapy without Systemic Antifungal Prophylaxis: A Prospective
Observational Study in Taiwan. PloS one. 2015;10(6):e0128410-e0128410. https://doi.org/10.1371/journal.pone.0128410 PMid:26061179 PMCid:PMC4462587
- Romani L. Cell mediated immunity to fungi: a reassessment. Medical Mycology. 2008;46(6):515-529. https://doi.org/10.1080/13693780801971450 PMid:19180748
- van
Tilburg CM, van Gent R, Bierings MB, et al. Immune reconstitution in
children following chemotherapy for haematological malignancies: a
long-term follow-up. Br J Haematol. 2011;152(2):201-210. https://doi.org/10.1111/j.1365-2141.2010.08478.x PMid:21114483
- Samanta
A, Chandra J, Kaur R, et al. Clinical Profile and Microbiologic
Spectrum of Febrile Neutropenic Episodes in Children With Severe
Aplastic Anemia. J Pediatr Hematol Oncol. 2020;42(3):193-197. https://doi.org/10.1097/MPH.0000000000001631 PMid:32209945
- Quarello
P, Saracco P, Giacchino M, et al. Epidemiology of infections in
children with acquired aplastic anaemia: a retrospective multicenter
study in Italy. Eur J Haematol. 2012;88(6):526-534. https://doi.org/10.1111/j.1600-0609.2012.01770.x PMid:22381133
- Valdez
JM, Scheinberg P, Nunez O, et al. Decreased Infection-Related Mortality
and Improved Survival in Severe Aplastic Anemia in the Past Two
Decades. Clinical Infectious Diseases. 2011;52(6):726-735. https://doi.org/10.1093/cid/ciq245 PMid:21367725 PMCid:PMC3106262
- Pana
ZD, Roilides E, Warris A, et al. Epidemiology of Invasive Fungal
Disease in Children. J Pediatric Infect Dis Soc.
2017;6(suppl_1):S3-s11. https://doi.org/10.1093/jpids/pix046 PMid:28927200 PMCid:PMC5907880
- Tan
BH, Chakrabarti A, Li RY, et al. Incidence and species distribution of
candidaemia in Asia: a laboratory-based surveillance study. Clinical
Microbiology and Infection. 2015;21(10):946-953. https://doi.org/10.1016/j.cmi.2015.06.010 PMid:26100373
- Awasthi
AK, Jain A, Awasthi S, et al. Epidemiology and microbiology of
nosocomial pediatric candidemia at a northern Indian tertiary care
hospital. Mycopathologia. 2011;172(4):269-277. https://doi.org/10.1007/s11046-011-9431-9 PMid:21533904
- Al
Thaqafi AH, Farahat FM, Al Harbi MI, et al. Predictors and outcomes of
Candida bloodstream infection: eight-year surveillance, western Saudi
Arabia. Int J Infect Dis. 2014;21:5-9. https://doi.org/10.1016/j.ijid.2013.12.012 PMid:24468816
- Kmeid
J, Jabbour JF, Kanj SS. Epidemiology and burden of invasive fungal
infections in the countries of the Arab League. J Infect Public Health.
2020;13(12):2080-2086. https://doi.org/10.1016/j.jiph.2019.05.007 PMid:31248814
- Cleveland
AA, Farley MM, Harrison LH, et al. Changes in incidence and antifungal
drug resistance in candidemia: results from population-based laboratory
surveillance in Atlanta and Baltimore, 2008-2011. Clinical infectious
diseases : an official publication of the Infectious Diseases Society
of America. 2012;55(10):1352-1361. https://doi.org/10.1093/cid/cis697 PMid:22893576 PMCid:PMC4698872
- Santolaya
ME, Alvarado T, Queiroz-Telles F, et al. Active surveillance of
candidemia in children from Latin America: a key requirement for
improving disease outcome. Pediatr Infect Dis J. 2014;33(2):e40-44. https://doi.org/10.1097/INF.0000000000000039 PMid:23995591
- Arastehfar
A, Daneshnia F, Hafez A, et al. Antifungal susceptibility, genotyping,
resistance mechanism, and clinical profile of Candida tropicalis blood
isolates. Medical Mycology. 2019;58(6):766-773. https://doi.org/10.1093/mmy/myz124 PMid:31828316 PMCid:PMC7398758
- Pham
LTT, Pharkjaksu S, Chongtrakool P, et al. A Predominance of Clade 17
Candida albicans Isolated From Hemocultures in a Tertiary Care Hospital
in Thailand. Frontiers in Microbiology. 2019;10(1194). https://doi.org/10.3389/fmicb.2019.01194 PMid:31258518 PMCid:PMC6587676
- Tulyaprawat
O, Pharkjaksu S, Chongtrakool P, et al. An Association of an eBURST
Group With Triazole Resistance of Candida tropicalis Blood Isolates.
Frontiers in Microbiology. 2020;11(934). https://doi.org/10.3389/fmicb.2020.00934 PMid:32508774 PMCid:PMC7248567
- Apsemidou
A, Petridis N, Vyzantiadis T-A, et al. Invasive Aspergillosis in
Children: Update on Current Guidelines. Mediterranean journal of
hematology and infectious diseases. 2018;10(1):e2018048-e2018048. https://doi.org/10.4084/mjhid.2018.048 PMid:30210741 PMCid:PMC6131109
- Pfeiffer
CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a
galactomannan assay: a meta-analysis. Clin Infect Dis.
2006;42(10):1417-1427. https://doi.org/10.1086/503427 PMid:16619154
- Lehrnbecher
T, Fisher BT, Phillips B, et al. Clinical Practice Guideline for
Systemic Antifungal Prophylaxis in Pediatric Patients With Cancer and
Hematopoietic Stem-Cell Transplantation Recipients. Journal of clinical
oncology : official journal of the American Society of Clinical
Oncology. 2020;38(27):3205-3216. https://doi.org/10.1200/JCO.20.00158 PMid:32459599 PMCid:PMC7499615
- Kobayashi
R, Kaneda M, Sato T, et al. The clinical feature of invasive fungal
infection in pediatric patients with hematologic and malignant
diseases: a 10-year analysis at a single institution at Japan. J
Pediatr Hematol Oncol. 2008;30(12):886-890. https://doi.org/10.1097/MPH.0b013e3181864a80 PMid:19131772
- Pana
ZD, Seidel D, Skiada A, et al. Invasive mucormycosis in children: an
epidemiologic study in European and non-European countries based on two
registries. BMC infectious diseases. 2016;16(1):667-667. https://doi.org/10.1186/s12879-016-2005-1 PMid:27832748 PMCid:PMC5105268
- Nucci
M, Anaissie E. How we treat invasive fungal diseases in patients with
acute leukemia: the importance of an individualized approach. Blood.
2014;124(26):3858-3869. https://doi.org/10.1182/blood-2014-04-516211 PMid:25339358
- Gamaletsou
MN, Walsh TJ, Zaoutis T, et al. A prospective, cohort, multicentre
study of candidaemia in hospitalized adult patients with haematological
malignancies. Clin Microbiol Infect. 2014;20(1):O50-57. https://doi.org/10.1111/1469-0691.12312 PMid:23889746
- van
de Peppel RJ, Dekkers OM, von dem Borne PA, et al. Relapsed and
secondary disease drive the risk profile for invasive aspergillosis
prior to stem cell transplantation in patients with acute myeloid
leukemia or myelodysplastic syndrome. Med Mycol. 2014;52(7):699-705. https://doi.org/10.1093/mmy/myu036 PMid:25049037
- Qian
J, Jiang C, Wenji Q, et al. Inherited NUDT15 Variants Substantially
Increased Infection and Related Medical Cost in Children with Acute
Lymphoblastic Leukemia. Blood. 2018;132(Supplement 1):320-320. https://doi.org/10.1182/blood-2018-99-114400
Supplementary Data
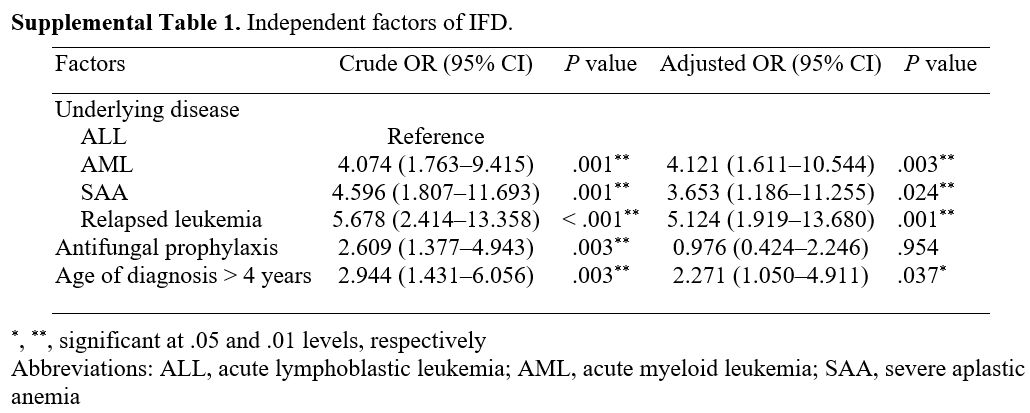 |
Supplemental Table
1. Independent factors of IFD.
|
[TOP]



