Fang Jin1,2#,Dongli Li1,2#, Chenxin Yan3, Weihua Zou4 and Fuchu Qian1,2.
1 Department of Precision Medicine, Huzhou Central Hospital, Affiliated Central Hospital Huzhou University, Huzhou, China.
2 Department of Precision Medicine, Affiliated Huzhou Hospital Zhejiang University School of Medicine, Huzhou, China.
3 Shulan International Medicine College of Zhejiang Shuren University, Hangzhou, China.
4 Department of Laboratory Medicine, Huzhou Central Hospital, Affiliated Central Hospital Huzhou University, Huzhou, China.
# These authors contributed equally to this work.
Correspondence to:
Prof. Fuchu Qian, Department of Precision Medicine, Huzhou Central
Hospital, Affiliated Central Hospital Huzhou University, No.1558
Sanhuan North Road, Huzhou, 313000, Zhejiang Province, The People's
Republic of China. Tel: (+86)-572-2819062. E-mail:
qfc313009@126.com
Published: September 1, 2022
Received: November 30, 2021
Accepted: August 1, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022061 DOI
10.4084/MJHID.2022.061
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Chronic
liver diseases such as chronic hepatitis B, cirrhosis, and liver cancer
caused by hepatitis B virus (HBV) infection pose a serious threat to
public health. Mother-to-child transmission is one of the main routes
of HBV dissemination.[1] So far, immunoprophylaxis is an important strategy for preventing and controlling HBV infection.[2]
In
China, the chronic HBV infection rate in children gradually decreased
due to the universal implementation of the hepatitis B vaccine strategy
since 1992.[3] However, some studies have reported
that mutations within the major hydrophilic region (MHR) region within
the surface gene of HBV result in immune escape and contribute to
immunoprophylaxis failure.[4,5] Moreover, the genotype distribution is unequal in different areas in China.[6]
The
HBV genotypes and surface gene mutation among children born after the
universal HBV vaccination program have not been investigated in Huzhou,
China. Thus, the present study was made up to delineate the molecular
characteristics in vaccinated children with HBsAg positive in this area.
Materials and Methods
In
the present study, 58 children vaccinated with HBsAg positive from the
serum HBsAg screening were enrolled among 7342 children in the Huzhou
Central Hospital. The study was approved by the ethics committee of
Huzhou Central Hospital in accordance with the ethical guidelines of
the Declaration of Helsinki.
Routine HBV serological markers,
alanine aminotransferase (ALT), and aspartate aminotransferase (AST)
were detected in the Department of Laboratory Medicine. HBV DNA was
quantified, and the HBV surface gene region was amplified and sequenced
as previously described methods.[7] Genotyping of HBV was performed by using an online tool (https://www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi). Serotype was determined as previously described.[8]
In addition, the amino acid (AA) substitutions in the surface gene
region were analyzed in comparison to the standard reference HBV
isolates obtained from GenBank (Genotype B: AB073846, AB602818, D00329;
Genotype C: AB014381, AY123041, X04615) by using MEGA 7.0 software.
Results
Fifty-six samples were sequenced successfully. The characteristics of the HBV-infected children are described in Table 1.
Among these children, 45 cases were infected with HBV genotype B, and
11 were infected with HBV genotype C. There were no significant
differences between children HBV infected with genotypes B and C at
demographic and virological characteristics. Furthermore, three
serotypes were found in the present study: adw (42), adr (11), and ayw
(3).
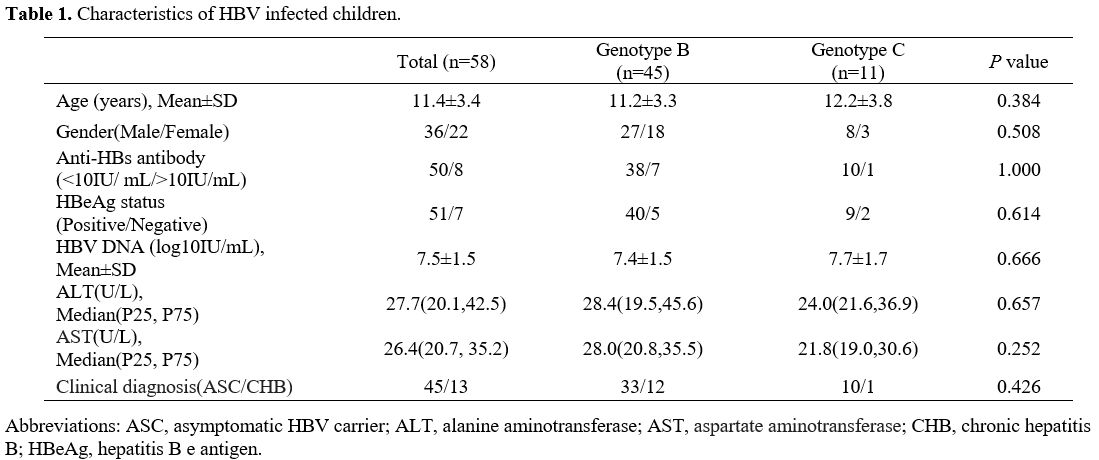 |
Table
1. Characteristics of HBV infected children. |
Eighteen
AA substitution sites in the MHR region were identified in 21 of 56
children (37.5%). Among these sites, 12 AA substitutions were found
within the 'a' determinant. The mutation rate in the 'a' determinant
region was 28.6% (16/56); these substitutions included K122R, I126T,
Q129H, P142H, and T143M (Table 2).
It's worth noting that eight children HBsAb positive (>10 mIU/mL)
were infected with HBV; seven of these eight children (87.5%) were
>9 years old. Among these HBV isolates, four (50%, 4/8) showed AA
substitutions (P127T, T140I, T143M, G145R) within the 'a' determinant.
When
we compared mutations within MHR between genotype B and genotype C, we
found mutations in 33.3% of (15/45) children infected with genotype B
and in 27.2% (3/11) children infected with genotype C. However, no
statistical difference was found (P
= 0.702). Furthermore, frequencies of mutations in different regions
between these two genotypes had no statistical differences (P>0.05) (Table 2).
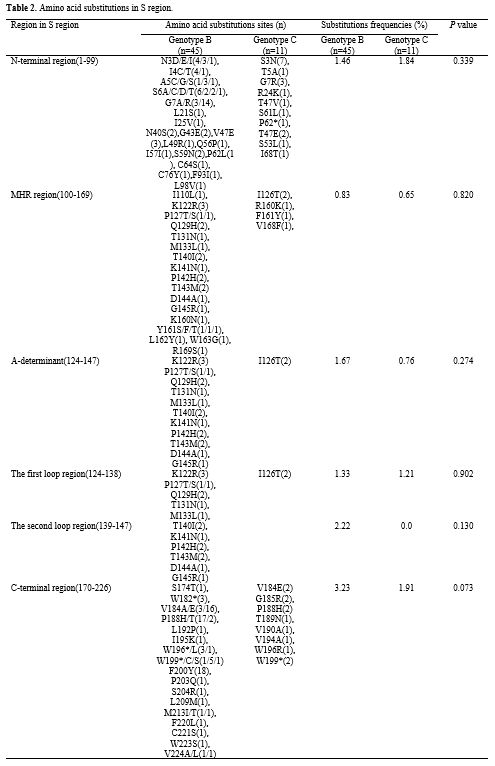 |
Table 2. Amino acid substitutions in S region. |
The characteristics were compared between the children with and without MHR mutations (Table 3).
Most children in the group without MHR mutations were boys (71.4%,
35/35), whereas, in the group with MHR mutations, only 47.6 % (10/21)
of children were boys. Additionally, no statistical differences were
observed in the other factors, including AST, ALT levels; HBeAg status;
and proportion of genotype B between the two groups (P > 0.05).
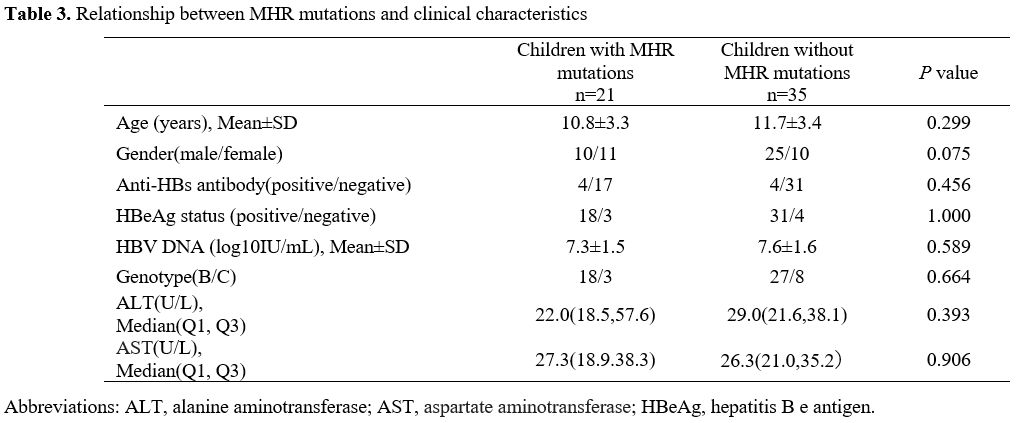 |
Table 3. Relationship between MHR mutations and clinical characteristics |
Discussion
The
present results showed that the predominant HBV serotypes and genotypes
were adw and B among children in the Huzhou area. It differed from our
previous study, which showed that the distribution of HBV genotype B
was in 43.5% (78/179) of HBV-infected adults in the same area.[9]
Considering the children in this study were all vaccinated and adults
in our previous study were all not vaccinated, we assume that the B
genotype of HBV may be more able to infect children vaccinated. Zheng
et al.[10] reported that most HBV infections in
vaccinated Chinese blood donors were genotype B, which supports this
suppose. On the contrary, genotype C may lead to a higher rate of HBV
breakthrough infection than genotype B, as reported in a Taiwan study.[11] However, more evidence must be collected to clarify the correlation between HBV genotype and HBV infection after vaccination.
MHR
region is the main B-cell epitope, which may affect antibody
immunogenicity. The 'a' determinant within MHR is the determinant
antigen, and the target for the neutralizing antibody produced after
the vaccine, many AA substitutions in the 'a' determinant affect the
binding of neutralizing antibodies.[12] In the
present study, 21 of 56 children (37.5%) were found to have AA
substitutions in MHR. Furthermore, 28.6% (16/56) of children harbored
mutations in the 'a' determinant. Many previous studies confirmed that
mutations in MHR, especially within the 'a' determinant, contributed to
immune escape of vaccine.[13,14] Therefore, present
findings suggest that the risk of transmission of mutant HBV still
exists in the Huzhou area. However, to our knowledge, the K141N and
P142H substitution found in this study was not mentioned previously; if
these two mutations could lead to vaccine immune escape, further
investigation needs.
The most common immune escape mutant G145R/A
is in the second loop of the 'a' determinant. However, the G145R
mutation was only found in one child in this study, indicating this
mutation is not common in Huzhou. Of note, the proportion of girls
(52.4%, 11/21) in children with MHR mutations was higher than that in
children without MHR mutations (28.6%, 10/35). However, the difference
was not statistically significant (P=0.075) due to the relative sample
size. However, few studies focus on this issue. Whether girls are more
susceptible to HBV infection with mutant HBV deserves further
investigation.
Anti-HBs can neutralize the HBsAg and eliminate the
HBV infection, a protective marker in vaccine recipients. But in the
present study, eight children with positive level anti-HBs
(<100mIU/mL) were infected with HBV, suggesting that presence of
low-level anti-HBs could not completely prevent HBV infection.
Furthermore, seven of eight children (87%) were older than nine years;
this result was consistent with other studies, which revealed that the
anti-HBs levels gradually decreased with age in some vaccinated
children.[15-17] Previous studies have shown that people with anti-HBs remain at risk of HBV infection.[18-20]
Another research reported that the incidence of occult HB infection
(OBI) in infants with low anti-HBs (<100mIU/mL) was significantly
higher than that in non-vaccinate infants, indicating the occurrence of
OBI in infants may be due to the limited neutralizing capacity provided
by low anti-HBs titers.[21] Therefore, we recommend
that it is necessary to monitor and strengthen immunization in children
with low-level anti-HBs to reduce the risk of HBV infection.
In
summary, the present findings suggest that genotype B is the
predominant genotype in children. It may be associated with the threat
of HBV infection in vaccinated children, MHR mutations, and decreased
levels anti-HBs in Huzhou. Further long-term prospective observation
and functional analysis of mutant HBV strains in vitro and in vivo
experiments are needed to confirm the findings in the present study.
Nevertheless, the results may help a different vaccine improvement
strategy, prevention, and control of HBV infection in children.
Acknowledgments
This work was supported by Huzhou Municipal Science and Technology Bureau [grant number. 2020GY02].
References
- Pan CQ, Duan Z P,
Bhamidimarri KR et al. An
algorithm for risk assessment and intervention of mother to child
transmission of hepatitis B virus. Clinical Gastroenterology and
Hepatology. The Official Clinical Practice Journal of the
American
Gastroenterological Association. 2012, 10, 452-459. https://doi:10.1016/j.cgh.2011.10.041
PMid:22079509
- Jia
J D, Hou JL, Wei L, Zhuang H. [Highlights of the guidelines of
prevention and treatment for chronic hepatitis B (2019 version)].
Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese
Journal of Hepatology. 2020, 28, 21-23. https://doi:10.3760/cma.j.issn.1007-3418.2020.01.006
PMID:32023693
- Cui
F. Shen L, Li L et al. Prevention of Chronic Hepatitis B after 3
Decades of Escalating Vaccination Policy, China. Emerging Infectious
Diseases. 2017, 23, 765-772. https://doi.org/10.3201/eid2305.161477
PMid:28418296 PMCid:PMC5403029
- Coleman PF. Detecting
hepatitis B surface antigen mutants. Emerging Infectious Diseases.
2006, 12, 198-203. https://doi.org/10.3201/eid1203.050038
PMid:16494742 PMCid:PMC3293431
- Harrison
TJ, Hopes EA, Oon CJ, Zanetti AR, Zuckerman AJ. Independent emergence
of a vaccine-induced escape mutant of hepatitis B virus. Journal of
Hepatology. 1991, 13 Suppl 4, S105-107. https://doi.org/10.1016/0168-8278(91)90037-C
PMID:1726588
- Li
HM, Wang JQ, Wang R et al. Hepatitis B virus genotypes and genome
characteristics in China. World Journal of Gastroenterology. 2015, 21,
6684-6697. https://doi.org/10.3748/wjg.v21.i21.6684
PMid:26074707 PMCid:PMC4458779
- Qian
F, Zou W, Jin F, Li D, Shen Y. Prevalence of Potential Resistance
Related Variants Among Chinese Chronic Hepatitis B Patients Not
Receiving Nucleos(T)ide Analogues. Infection and Drug Resistance. 2020,
13, 2407-2416. https://doi.org/10.2147/IDR.S249476
PMid:32765014 PMCid:PMC7381783
- Velkov
S, Protzer U, Michler T. Global Occurrence of Clinically Relevant
Hepatitis B Virus Variants as Found by Analysis of Publicly Available
Sequencing Data. Viruses. 2020, 12. https://doi.org/10.3390/v12111344
PMid:33238650 PMCid:PMC7700573
- QIAN Fuchu, QIN Jiqu, YANG Shuixin, DAI Licheng, CHEN Zuo,
Distribution of Hepatitis B Virus Genotypes in the Patients from Huzhou
Area. Zhejiang J Prev Med (chinese). 2009, 21(11), 6-14.
- Zheng,
X.; Ye, X.; Du, P., et al. High prevalence of anti-hepatitis B core
antigen in hepatitis B virus-vaccinated Chinese blood donors suggests
insufficient protection but little threat to the blood supply,
Transfusion. 2015, 55, 890-897 https://doi.org/10.1111/trf.12902
PMid:25363504
- Wen,
W. H.; Chen, H. L.; Ni, Y. H., et al. Secular trend of the viral
genotype distribution in children with chronic hepatitis B virus
infection after universal infant immunization, Hepatology (Baltimore,
Md). 2011, 53, 429-436. https://doi.org/10.1002/hep.24061
PMid:21274864
- Huang,
Y.; Wang, B.; Peng, Z.; Tang, N.; Chen, W. Hepatitis B virus surface
gene mutants in immunoprophylaxis-failed infants from Southern China,
Journal of Medical Virology. 2019, 91, 1069-1075. https://doi.org/10.1002/jmv.25430
PMid:30761578
- Coppola,
N.; Onorato, L.; Minichini, C., et al. Clinical significance of
hepatitis B surface antigen mutants, World Journal of Hepatology. 2015,
7, 2729-2739. https://doi.org/10.4254/wjh.v7.i27.2729
PMid:26644816 PMCid:PMC4663392
- Hsu,
H. Y.; Chang, M. H.; Ni, Y. H., et al. Chronologic changes in serum
hepatitis B virus DNA, genotypes, surface antigen mutants and reverse
transcriptase mutants during 25-year nationwide immunization in Taiwan.
Journal of Viral Hepatitis. 2017, 24, 645-653. https://doi.org/10.1111/jvh.12687
PMid:28182307
- Lin,
X.; Yang, J.; Lu, H., et al. Minimization of hepatitis B infection
among children in Jiangsu, China, 12years after integration of
hepatitis B vaccine into the expanded program on immunization. Vaccine.
2016, 34, 6458-6463. https://doi.org/10.1016/j.vaccine.2016.11.022
PMid:27866767
- Yue,
X.; Ge, C.; Zhuge, S., et al. Changes and analysis of anti-HBs titres
after primary immunization in 1- to 16-year-old Chinese children: A
hospital-based study. Journal of Viral Hepatitis. 2018, 25, 373-380. https://doi.org/10.1111/jvh.12818
PMid:29091317
- Zhu,
Q.; Shao, X.; Chen, S., et al. Epidemiological serosurvey of hepatitis
B virus among children aged 1-14 years in Guangdong Province, China.
International Journal of Infectious Diseases: IJID: Official
Publication of the International Society for Infectious Diseases. 2018,
71, 25-29. https://doi.org/10.1016/j.ijid.2018.01.027
PMid:29408358
- Wang,
Z.; Zeng, J.; Li, T., et al. Prevalence of hepatitis B surface antigen
(HBsAg) in a blood donor population born prior to and after
implementation of universal HBV vaccination in Shenzhen, China. BMC
Infectious Diseases. 2016, 16, 498. https://doi.org/10.1186/s12879-016-1834-2
PMid:27647214 PMCid:PMC5028969
- Ye,
X.; Li, T.; Xu, X., et al. Characterisation and follow-up study of
occult hepatitis B virus infection in anti-HBc-positive qualified blood
donors in southern China. Blood transfusion.
2017, 15, 6-12. https://doi:10.2450/2016.0268-15
PMID:27416568 PMCID:PMC5269423.
- Diarra B, Yonli AT, Sorgho PA, Compaore TR,
Ouattara AK, Zongo WA, Tao I, Traore L, Soubeiga ST, Djigma FW,
Obiri-Yeboah D, Nagalo BM, Pietra V, Sanogo R, Simpore J. Occult
hepatitis B virus Infection and associated genotypes among
HBsAg-negative subjects in Burkina Faso. Mediterr J Hematol Infect Dis.
2018 Jan 1;10(1):e2018007. https://doi.org/10.4084/MJHID.2018.007
- Zhou,
S.; Li, T.; Allain, J. P., et al. Low occurrence of HBsAg but high
frequency of transient occult HBV infection in vaccinated and
HBIG-administered infants born to HBsAg positive mothers, Journal of
Medical Virology. 2017, 89, 2130-2137. https://doi.org/10.1002/jmv.24861
PMid:28543299
[TOP]


