Antonio Leone1, Nicola Carlo Bianco2, Giulia D’Ambra2, Salvatore Lucchesi2, Elisa La Rosa2, Amato Infante1, Daniele Perla1 and Consolato Gullì1.
1 Department
of Radiological and Hematological Sciences, Fondazione Policlinico
Universitario A. Gemelli, IRCCS, Università Cattolica del Sacro Cuore,
Largo A. Gemelli 1, 00168 Rome, Italy.
2 Department of
Radiological and Hematological Sciences, Università Cattolica del Sacro
Cuore, Largo A. Gemelli 1, 00168 Rome, Italy.
Correspondence to:
Antonio Leone, MD. Department of Radiological and Hematological
Sciences, Fondazione Policlinico Universitario A. Gemelli, IRCCS.
Università Cattolica del Sacro Cuore, Largo A. Gemelli, 1,00168 Rome,
Italy. Tel: +39-06- 30156054, Fax: +39-06-35501928. E-mail:
a.leonemd@tiscali.it. ORCID:
http://orcid.org/0000-0003-3669-6321
Published: July 1, 2022
Received: March 24, 2022
Accepted: June 17, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022055 DOI
10.4084/MJHID.2022.055
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objective:
Diagnosing diabetes-related foot osteomyelitis is sometimes a challenge
for clinicians since it may occur without local or systemic signs of
infection. Thus, the primary purpose of this article was to evaluate
the role of progressive radiographic changes in diagnosing diabetic
foot osteomyelitis.
Materials and Methods:
A retrospective review of databases of our Institution was performed to
identify all long-standing diabetic foot patients who underwent two
radiographic examinations spaced no more than five weeks apart and a
subsequent magnetic resonance (MR) examination from November 2015 to
November 2020. A total of 46 patients (32 men, 14 women; mean age, 57.3
years) were identified.
Results:
serial radiographs showed 89% sensitivity, 38% specificity, 80%
diagnostic accuracy, 87% positive predictive value (PPV), 43% negative
predictive value (NPV) to diagnose osteomyelitis (P value <
0,05). Bone destruction was the most reliable radiographic sign
with 89% sensitivity, 88% specificity, 89% diagnostic accuracy, 97%
PPV, 64% NPV (P value < 0,05).
Conclusion: Progressive bony changes detected by serial radiographs are a useful tool to diagnose diabetic foot osteomyelitis.
|
Introduction
Osteomyelitis
is the most common long-term complication of diabetic foot. Infective
diabetic foot complications generally begin with a neuropathic ulcer
which often develops on the plantar surface of the toes, the metatarsal
heads, and the calcaneus. A prompt diagnosis of infective diabetic foot
conditions is pivotal in the patients' management, treatment, and
prognosis. Diagnostic evaluation starts clinically, based on the
patient's history and physical examination. International Working Group
on the Diabetic Foot and Infectious Diseases Society of America
proposed a clinical classification system to define the presence and
severity of an infection of the diabetic foot.[1,2]
Blood tests are widely available and easily obtained as part of the
diagnostic work-up. White blood cell count and inflammatory serum
biomarkers (C-reactive protein, erythrocyte sedimentation rate, and
procalcitonin) are considered the most useful.[1-3]
The "probe-to-bone" (PTB) test is a minimally invasive examination that
explores a foot ulcer with a sterile blunt metal probe; the test is
positive if the probe reaches the bone. However, its accuracy depends
on the operator's experience, pre-test likelihood of infection, and
ulcer's location.[1,2,4] Bone biopsy,
providing a histopathologic and microbiologic evaluation of the
specimen, is the reference standard to diagnose osteomyelitis and
determine the causative pathogen.[1,2]
Nevertheless, it is an invasive procedure requiring time and experience.[1,2]
The culture of soft tissue specimens may be an alternative diagnostic
approach. Still, it shows a relatively low concordance with bone
biopsy, with the risk of missing pathogens or contaminating the sample.[1,2,5]
In this scenario, imaging is an additional, complementary, and less
invasive diagnostic tool. Radiography should be the first imaging
modality when diabetic foot osteomyelitis is suspected due to its low
cost and wide availability.[2,6,7] It is well known that radiography lacks sensitivity in early infection;[3,6,8]
the clinical manifestations of osteomyelitis can precede corresponding
bone radiographic changes (such as demineralization, bone destruction,
and periosteal reaction) by up to 4 weeks.[7,9-12]
Furthermore, these radiographic changes can be caused by neuropathic osteoarthropathy.[13-15]
However, even when not diagnostic, it provides information on bone
structure, alignment, and anatomic details of the area of interest and
any pre-existing condition that could be misinterpreted in subsequent
MR examinations.[6,16] If suspicion
of bone infection remains despite an initial negative radiographic
examination, repeating radiography in a few weeks can either exclude or
suggest the diagnosis of osteomyelitis (if progressive bony changes are
evident).[7,9,14] Several authors[2,7,9,10,14,17-19]
considered a possible role for serial radiographs in this clinical
scenario. However, to the best of our knowledge, we are unaware of any
studies of the role of serial radiographs in this setting; no studies
compared the diagnostic accuracy of serial radiographs to MR imaging,
which is currently considered the imaging modality of choice for
diagnosing osteomyelitis in the diabetic foot.[7,20] Therefore, this study's primary purpose was to evaluate serial radiographs' role in diagnosing diabetic foot osteomyelitis.
Materials and Methods
Our
institutional review board approved this retrospective
single-institution study and waived the informed consent requirement.
We performed a computerized database search of our Institution's
radiological records from November 2015 to November 2020; the keywords
were diabetic foot, neuropathic osteoarthropathy, and osteomyelitis. A
total of 133 long-standing diabetic foot patients were identified. For
these patients, the inclusion criteria were: (a) patients had to have
undergone two radiographic examinations spaced between 14 and 35 days
at our Institution for suspected bone and soft tissue infections; (b)
Patients had to have undergone subsequent magnetic resonance (MR)
imaging, including unenhanced T1-weighted, fluid-sensitive and
gadolinium-enhanced sequences within two weeks after radiography. The
exclusion criteria were: (a) presence of other relevant diseases other
than diabetes; (b) no serial radiographs or time interposed between two
radiographs for more than 35 days; (c) no MR imaging performed; (d) MR
imaging performed without intravenous contrast agent; (e) MR imaging
spaced more than two weeks from the second radiograph. Inclusion and
exclusion criteria are summarized in the patients' flow diagram (Figure 1).
Only those patients who met all inclusion criteria for the study were
recruited. Patients' demographic and clinical data were collected
through our Institution's medical record database. Age, sex, diabetes
type, time since diabetes diagnosis, anatomic distribution of
osteomyelitis, and microbiological findings, when present, were
recorded.
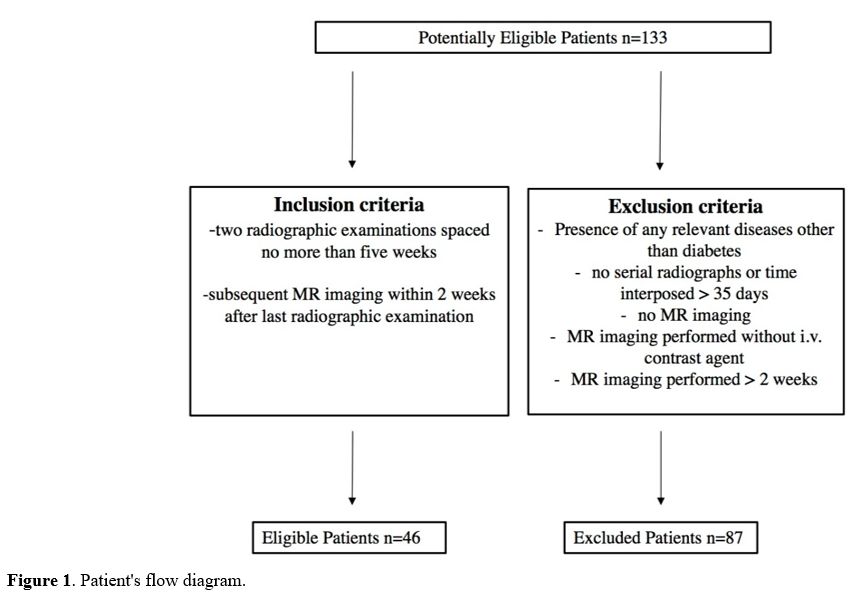 |
Figure
1. Patient's flow diagram.
|
Radiographic
examinations were performed, including three standard views of the foot
(lateral, anteroposterior, and medial oblique); four parameters were
analyzed: periosteal reaction, osteopenia, gas in the soft tissues, and
bone destruction. In addition, the accuracy of radiographic images was
evaluated by comparing them with MR imaging. MR images were obtained
with one of two 1.5 superconducting systems (Signa Excite GE Medical
Systems, Milwaukee, Wis) and Magneton Avanto, Siemens Healthcare,
Erlangen, Germany. Both scanners were equipped with a dedicated coil,
and the patient was lying supine with the knees bent at 35°. Two
radiologists (30 and 3 years of clinical experience in muscle-skeletal
radiology, respectively) reviewed all the radiographic and MR images in
consensus.
Statistical Analysis.
The sample is described in its clinical and demographic characteristics
using descriptive statistics techniques: categorical variables are
expressed as absolute frequencies and percentages. Quantitative
variables are summarized with mean and standard deviation, if normally
distributed, as median and interquartile range, if not normally
distributed. Normality was checked with the Kolmogorov-Smirnov test.
Chi-squared and parametric/not-parametric tests were applied according
to quantitative variables' normal/not-normal distribution.
Sensitivity,
specificity, positive predictive value (PPV), negative predictive value
(NPV), and diagnostic accuracy were evaluated and compared between
subgroups. Data were analyzed with dedicated software (SPSS for
Windows, version 25.0; IBM, Chicago, IL, USA). The data were considered
statistically significant at P < .05.
Results
Forty-six
patients with two serial radiographs spaced between 4 and 5 weeks and
subsequent MR imaging (performed within 15 days) were included. The
mean age was 57.3 years (range 32-84 years; standard deviation 13.6).
In addition, 32 (70%) patients were male, and 14 (30%) were female. In
addition, 41 patients (90%) had type 2 diabetes, and 5 had type 1
diabetes (10%). Mean duration of diabetes was 16.1 years (standard
deviation 9). Osteomyelitis was located in the forefoot, midfoot, and
hindfoot for 82%, 7%, and 11% of patients, respectively. Of the 46
patients, microbiological analysis was available in 26. Gram-positive
bacteria were the most common organisms (n=22, 86%). Gram-negative
bacteria were present in 4 cases (14%). The most common bacterium was
methicillin-sensitive S. aureus (MSSA) (n=7, 28%). The clinical,
demographic, and microbiological characteristics of patients are
summarized in Table 1. The
mean time between radiographs was 29 days (standard deviation of 6.6).
The mean time between the second radiograph and MR imaging was 8 days
(standard deviation 3.7). Time interposed between serial radiographs
and second radiographic examination and MR imaging showed no
statistical correlation with MR findings of osteomyelitis.
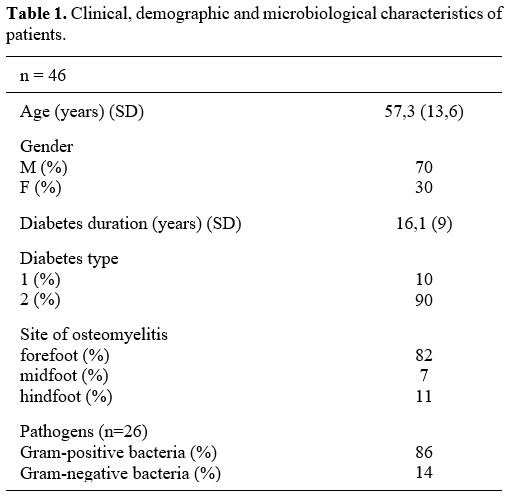 |
Table 1. Clinical, demographic and microbiological characteristics of patients.
|
Based
on radiographic findings, bone destruction was seen in 35 patients,
osteopenia in 16 patients, gas in the soft tissues in 10, and
periosteal reaction in 6. The presence of at least one radiographic
finding was seen in 39 patients (84.7%), showing 89% sensibility, 38%
specificity, the accuracy of 80%, a positive predictive value (PPV) of
87%, negative predictive value (NPV) 43% (p <0,05) and there was no
statistical correlation with age or sex. Among radiographic signs, bone
destruction showed 89% sensibility, 88% specificity, 89% accuracy, 97%
VPP, 64% VPN (p < 0.001). Other radiographic findings, isolated or
in combination, showed no statistical correlation. Data are summarized
in table 2.
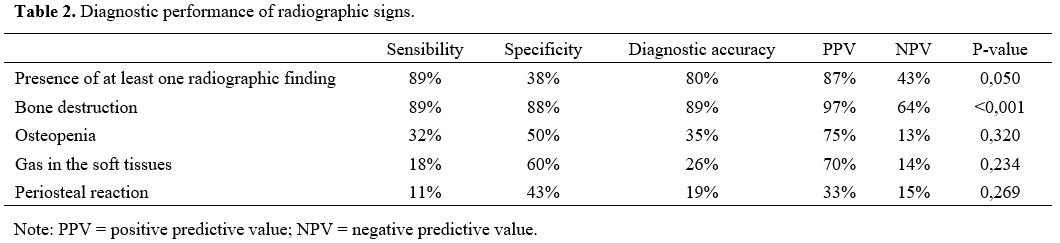 |
Table 2. Diagnostic performance of radiographic signs.
|
Discussion
Diabetes-related
foot osteomyelitis is a common clinical problem and almost invariably
originates from an infected foot ulcer in adjacent soft tissue. The
importance of correct diagnosis cannot be understated since
osteomyelitis complicates treatment and is associated with more
operations, limb amputation, and prolonged use of antibiotics.
Diagnosis of osteomyelitis is sometimes challenging for clinicians
since osteomyelitis may occur in the absence of local or systemic signs
of infection because of the frequent presence of peripheral neuropathy
or vascular insufficiency, especially in chronic infections.[21] In agreement with previous studies in the literature,[22-27] most patients of our population (Table 1)
had long-standing diabetes (16.1 years), with the prevalence of type 2
(90%), and the forefoot was the most involved anatomic site (82%).
Moreover, in our cohort of patients, most pathogens were Gram-positive
bacteria, and Meticillin-Sensitive Staphylococcus Aureus was the most
common, according to literature data.[23,29] In fact, it is well known that S. Aureus is a bacterium frequently involved in skin, soft tissue, bone, and joint infection.[29,30]
Diagnosis
of osteomyelitis relies on clinical data and laboratory tests
supplemented by various imaging modalities such as radiography and MR
imaging.[1] Radiography is the first-line imaging modality when diabetic foot osteomyelitis is suspected.[16,31,32] However, this imaging modality has low sensitivity and specificity for detecting acute osteomyelitis.[6,17] In a metanalysis by Dinh et al.,[17] radiography showed 54% pooled sensitivity, and 68% pooled specificity; a more recent metanalysis[33]
showed 68.9% sensitivity and 77.9% specificity. Sensibility is low
because bony abnormalities can be detected on radiographs only in the
late stage of osteomyelitis when at least half of the bone has been
destroyed;[7,10,34]
the timing of radiographs in relation to the chronicity of the ulcer is
reported as another parameter that affects sensibility.[11,33]
In addition, reported specificity is low because differentiating
infectious from noninfectious bone disorders may be difficult with
radiographs; osteomyelitis can overlap with other common diabetic foot
conditions, such as fractures and Charcot neuropathic osteoarthropathy,
especially without adequate clinical data.[13-15] MR
imaging is the modality of choice for assessing diabetic foot
osteomyelitis, with a sensitivity of 90% and specificity of 83%.[1,20]
In this setting, several authors[1,7,9,10,14,17-19]
proposed serial radiographs as an additional diagnostic tool to detect
diabetic foot osteomyelitis. However, to the best of our knowledge, no
previous studies examined the diagnostic performance of serial
radiographs. Sensibility and PPV for radiography in detecting diabetic
foot osteomyelitis range from 28% to 75%.[17,32,34]
In our experience, serial radiographs showed higher sensibility and PPV
(89% and 97%, respectively). However, bone abnormalities can take at
least 2-4 weeks to manifest on radiographs.[7,9,10-12]
Time interposed between serial radiographs (14-35 days) may allow bone
changes to progress enough to be observed at the second examination (Figure 2);
furthermore, comparing radiographs can help assess the evolution of
pre-existing findings. However, within the selected time range, we did
not find any exact timing associated with a better diagnostic
performance. In accordance with Konarzewska et al.,[10]
our results may demonstrate that the continued absence of any bony
abnormality on serial radiographs probably excludes osteomyelitis.
Specificity and NPV for serial radiographs were 38% and 43%,
respectively, lower than those reported in the literature for a single
radiograph (pooled specificity 68%).[17,33]
These data may be explained by the low inner specificity of
radiographical findings, which are more frequently identified by serial
radiographs than by a single radiograph. In fact, bone destruction and
osteopenia in the atrophic form of Charcot neuropathic osteoarthropathy
or periosteal reaction, also following fractures, cannot be reliably
distinguished, even when their progression over time is considered.[1,2,7,11,14]
Furthermore, the radiographic technique also plays a role; serial
radiographs should be performed with the same technical parameters, as
different kVp and mAs values may lead to false positives.[11]
Among the four radiographic signs evaluated, bone destruction is the
most reliable sign in the diagnosis of diabetic foot osteomyelitis.
This finding is in agreement with Alvaro-Alfonso et al.[35]
These authors were the first to stratify different signs in a single
radiographic examination performed for suspected diabetic foot
osteomyelitis, showing 76% sensibility and 45% specificity for
bonedestruction. However, our results with serial radiographs
demonstrated both higher sensibility (89%) and (88%) specificity for
this sign (Figure 3). Other
radiographic signs, alone or in association, showed lower diagnostic
performance, and data were not statistically significant.
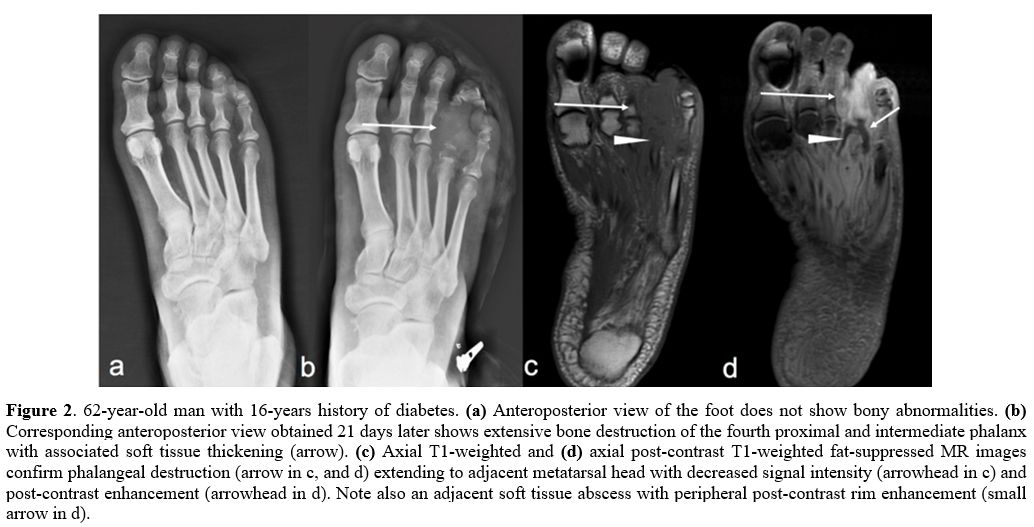 |
Figure 2. 62-year-old man with 16-years history of diabetes. (a) Anteroposterior view of the foot does not show bony abnormalities. (b)
Corresponding anteroposterior view obtained 21 days later shows
extensive bone destruction of the fourth proximal and intermediate
phalanx with associated soft tissue thickening (arrow). (c) Axial T1-weighted and (d)
axial post-contrast T1-weighted fat-suppressed MR images confirm
phalangeal destruction (arrow in c, and d) extending to adjacent
metatarsal head with decreased signal intensity (arrowhead in c) and
post-contrast enhancement (arrowhead in d). Note also an adjacent soft
tissue abscess with peripheral post-contrast rim enhancement (small
arrow in d). |
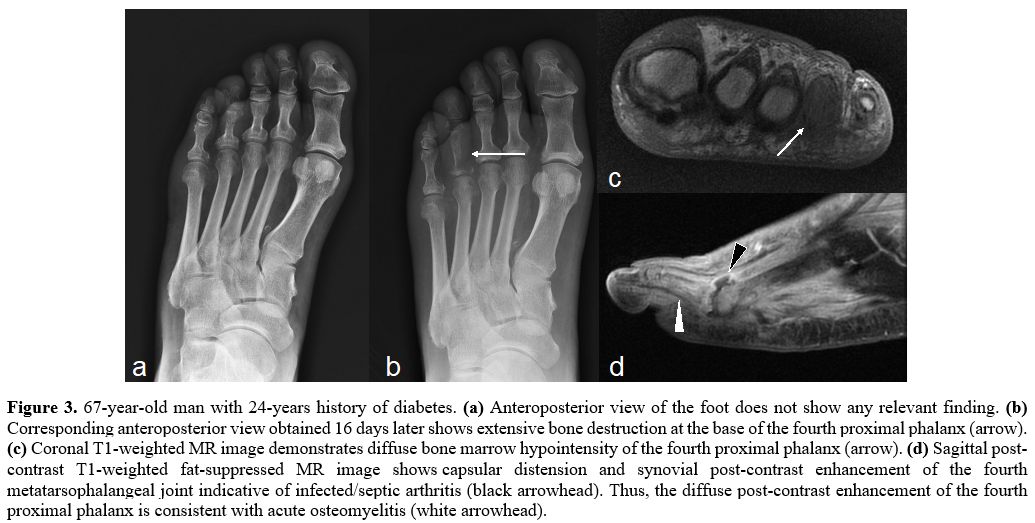 |
Figure 3. 67-year-old man with 24-years history of diabetes. (a) Anteroposterior view of the foot does not show any relevant finding. (b)
Corresponding anteroposterior view obtained 16 days later shows
extensive bone destruction at the base of the fourth proximal phalanx
(arrow). (c) Coronal T1-weighted MR image demonstrates diffuse bone marrow hypointensity of the fourth proximal phalanx (arrow). (d)
Sagittal post-contrast T1-weighted fat-suppressed MR image shows
capsular distension and synovial post-contrast enhancement of the
fourth metatarsophalangeal joint indicative of infected/septic
arthritis (black arrowhead). Thus, the diffuse post-contrast
enhancement of the fourth proximal phalanx is consistent with acute
osteomyelitis (white arrowhead). |
This
study has several drawbacks. First, it has a retrospective design, and
the small population sampling may be prone to selection bias. Second,
although radiographic findings were compared with MR imaging (the
imaging modality of choice in this setting), we could not confirm data
with bone biopsy for all patients. Finally, patients underwent
radiography with the clinical suspicion of osteomyelitis; however,
accurate information about the clinical signs and symptoms was
frequently lacking, though the appropriateness and detail of
radiographic referral request play an important role in the image
interpretation.
Conclusions
Detecting
bone destruction with serial radiographs may be an additional
diagnostic tool when diabetic foot osteomyelitis is suspected. However,
further studies are required before the true management value of serial
radiographs can be determined.
References
- Lipsky BA, Senneville É, Abbas ZG et al;
International Working Group on the Diabetic Foot (IWGDF). Guidelines on
the diagnosis and treatment of foot infection in persons with diabetes
(IWGDF 2019 update). Diabetes Metab Res Rev. 2020 Mar;36 Suppl 1:e3280 https://doi.org/10.1002/dmrr.3280 PMCid:PMC7154668
- Lipsky
BA, Berendt AR, Cornia PB et al.; Infectious Diseases Society of
America. 2012 Infectious Diseases Society of America clinical practice
guideline for the diagnosis and treatment of diabetic foot infections.
Clin Infect Dis. 2012 Jun;54(12):e132-73. https://doi.org/10.1093/cid/cis346 PMid:22619242
- Lauri
C, Leone A, Cavallini M, Signore A, Giurato L, Uccioli L. Diabetic Foot
Infections: The Diagnostic Challenges. J Clin Med. 2020 Jun
8;9(6):1779. https://doi.org/10.3390/jcm9061779 PMid:32521695 PMCid:PMC7355769
- Lam
K, van Asten SA, Nguyen T, La Fontaine J, Lavery LA. Diagnostic
accuracy of probe to bone to detect osteomyelitis in the diabetic foot:
a systematic review. Clin Infect Dis. 2016;63:944-948. https://doi.org/10.1093/cid/ciw445 PMid:27369321
- Prompers
L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals
with diabetic foot ulcers: focus on the differences between individuals
with and without peripheral arterial disease. The EURODIALE study.
Diabetologia. 2008;51:747-755. https://doi.org/10.1007/s00125-008-0940-0 PMid:18297261 PMCid:PMC2292424
- Leone
A, Vitiello C, Gullì C, Sikora AK, Macagnino S, Colosimo C. Bone and
soft tissue infections in patients with diabetic foot. Radiol Med.
2020;125(2):177-187. https://doi.org/10.1007/s11547-019-01096-8 PMid:31650327
- Senneville
EM, Lipsky BA, van Asten SAV, Peters EJ. Diagnosing diabetic foot
osteomyelitis. Diabetes Metab Res Rev. 2020 Mar;36 Suppl 1:e3250 https://doi.org/10.1002/dmrr.3250
- Butalia
S, Palda VA, Sargeant RJ, Detsky AS, Mourad O. Does this patient with
diabetes have osteomyelitis of the lower extremity? JAMA 2008;
299(7):806-813. https://doi.org/10.1001/jama.299.7.806 PMid:18285592
- Markanday
A. Diagnosing diabetic foot osteomyelitis: narrative review and a
suggested 2-step score-based diagnostic pathway for clinicians. Open
Forum Infect Dis. 2014 Aug 7;1(2):ofu060. https://doi.org/10.1093/ofid/ofu060 PMid:25734130 PMCid:PMC4281812
- Konarzewska
A, Korzon-Burakowska A, Rzepecka-Wejs L, Sudoł-Szopińska I, Szurowska
E, Studniarek M. Diabetic foot syndrome: Charcot arthropathy or
osteomyelitis? Part I: Clinical picture and radiography. J Ultrason.
2018 Mar;18(72):42-49. doi: 10.15557/JoU.2018.0007. https://doi.org/10.15557/JoU.2018.0007 PMid:29844940 PMCid:PMC5911718
- Aragón-Sánchez
J. Seminar review: A review of the basis of surgical treatment of
diabetic foot infections. Int J Low Extrem Wounds. 2011
Mar;10(1):33-65. https://doi.org/10.1177/1534734611400259 PMid:21444608
- Nawaz
A, Torigian DA, Siegelman ES, Basu S, Chryssikos T, Alavi A. Diagnostic
performance of FDG-PET, MRI, and plain film radiography (PFR) for the
diagnosis of osteomyelitis in the diabetic foot. Mol Imaging Biol. 2010
Jun;12(3):335-42. https://doi.org/10.1007/s11307-009-0268-2 PMid:19816744
- Linsley,
J., & Reel, S. (2021). The appropriateness of X-ray referrals of
osteomylelitis and its timely management with antibiotics: a service
evaluation. The Diabetic Foot Journal, 24(3), 10-15
- Lee YJ, Sadigh S, Mankad K, Kapse N, Rajeswaran G. The imaging of osteomyelitis. Quant Imaging Med Surg. 2016 Apr;6(2):184-98. https://doi.org/10.21037/qims.2016.04.01 PMid:27190771 PMCid:PMC4858469
- Lipsky BA. Osteomyelitis of the foot in diabetic patients. Clin Infect Dis. 1997 Dec;25(6):1318-26. https://doi.org/10.1086/516148 PMid:9431370
- Vopat
ML, Nentwig MJ, Chong ACM, Agan JL, Shields NN, Yang SY. Initial
Diagnosis and Management for Acute Charcot Neuroarthropathy. Kans J
Med. 2018 Nov 29;11(4):114-119. https://doi.org/10.17161/kjm.v11i4.8709 PMid:30937152 PMCid:PMC6276967
- Dinh
MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination
and imaging tests for osteomyelitis underlying diabetic foot ulcers:
meta-analysis. Clin Infect Dis. 2008 Aug 15;47(4):519-27. https://doi.org/10.1086/590011 PMid:18611152 PMCid:PMC7450707
- Schinabeck
MK, Johnson JL. Osteomyelitis in diabetic foot ulcers. Prompt diagnosis
can avert amputation. Postgrad Med. 2005 Jul;118(1):11-5. https://doi.org/10.3810/pgm.2005.07.1678 PMid:16106914
- Tran
K, Mierzwinski-Urban M. Serial X-Ray Radiography for the Diagnosis of
Osteomyelitis: A Review of Diagnostic Accuracy, Clinical Utility,
Cost-Effectiveness, and Guidelines [Internet]. Ottawa (ON): Canadian
Agency for Drugs and Technologies in Health; 2020 Mar 13.
- Donovan
A, Schweitzer ME. Use of MR imaging in diagnosing diabetes-related
pedal osteomyelitis. Radiographics. 2010 May;30(3):723-36. https://doi.org/10.1148/rg.303095111 PMid:20462990
- Edmonds M. Double trouble: infection and ischemia in the diabetic foot. Int J Low Extrem Wounds 2009;8:62-63. https://doi.org/10.1177/1534734609337930 PMid:19443892
- Faglia
E, Clerici G, Caminiti M, Curci V, Somalvico F. Influence of
osteomyelitis location in the foot of diabetic patients with
transtibial amputation. Foot Ankle Int. 2013 Feb;34(2):222-7. https://doi.org/10.1177/1071100712467436 PMid:23413061
- King
CM, Castellucci-Garza FM, Lyon L, Doyle MD, Nimick C, Williams ML.
Microorganisms Associated With Osteomyelitis of the Foot and Ankle. J
Foot Ankle Surg. 2020 May-Jun;59(3):491-494. https://doi.org/10.1053/j.jfas.2019.08.032 PMid:32354506
- Ledermann
HP, Morrison WB, Schweitzer ME. MR image analysis of pedal
osteomyelitis: distribution, patterns of spread, and frequency of
associated ulceration and septic arthritis. Radiology. 2002
Jun;223(3):747-55. https://doi.org/10.1148/radiol.2233011279 PMid:12034944
- Moura
Neto A, Zantut-Wittmann DE, Fernandes TD, Nery M, Parisi MC. Risk
factors for ulceration and amputation in diabetic foot: study in a
cohort of 496 patients. Endocrine. 2013 Aug;44(1):119-24 https://doi.org/10.1007/s12020-012-9829-2 PMid:23124278
- Oyibo
SO, Jude EB, Tarawneh I, Nguyen HC, Armstrong DG, Harkless LB, Boulton
AJ. The effects of ulcer size and site, patient's age, sex and type and
duration of diabetes on the outcome of diabetic foot ulcers. Diabet
Med. 2001 Feb;18(2):133-8 https://doi.org/10.1046/j.1464-5491.2001.00422.x PMid:11251677
- Mehmood
K, Akhtar ST, Talib A, Abbasi B,
Siraj-ul-Salekeen, Naqvi IH. Clinical profile and management
outcome of diabetic foot ulcers
in a tertiary care hospital. J Coll Physicians Pak. 18, 408-412 (2008)
- Lauterbach S, Kostev K, Becker R.
Characteristics of diabetic patients visiting a podiatric practice in
Germany. J. Wound Care 19, 140-144 (2010) https://doi.org/10.12968/jowc.2010.19.4.140 PMid:20379125
- Macdonald
KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot
infections: a meta-analysis. BMC Infect Dis. 2021 Aug 9;21(1):770. https://doi.org/10.1186/s12879-021-06516-7 PMid:34372789 PMCid:PMC8351150
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998 Aug 20;339(8):520-32 https://doi.org/10.1056/NEJM199808203390806 PMid:9709046
- Hochman
MG. Connolly C. (2018) Imaging of Infection in the Diabetic Foot. In:
Veves A., Giurini J., Guzman R. (eds) The Diabetic Foot. Contemporary
Diabetes. Humana, Cham. https://doi.org/10.1007/978-3-319-89869-8_5
- Internal
Clinical Guidelines team. Diabetic Foot Problems: Prevention and
Management. London: National Institute for Health and Care Excellence
(UK); 2015 Aug.
- Llewellyn A, Kraft J,
Holton C, Harden M, Simmonds M. Imaging for detection of osteomyelitis
in people with diabetic foot ulcers: A systematic review and
meta-analysis. Eur J Radiol. 2020 Oct;131:109215. https://doi.org/10.1016/j.ejrad.2020.109215 PMid:32862106
- Crim JR, Seeger LL. Imaging evaluation of osteomyelitis. Crit Rev Diagn Imaging. 1994;35(3):201-56.
- Álvaro-Afonso
FJ, Lázaro-Martínez JL, García-Morales E, García-Álvarez Y,
Sanz-Corbalán I, Molines-Barroso RJ. Cortical disruption is the most
reliable and accurate plain radiographic sign in the diagnosis of
diabetic foot osteomyelitis. Diabet Med. 2019 Feb;36(2):258-259. https://doi.org/10.1111/dme.13824 PMid:30246491
[TOP]




