Ixazomib
is an orally available proteasome inhibitor, which leads to cell growth
arrest and apoptosis via interfering with protein degradation.[1]
An analysis of the safety and tolerability of ixazomib, in combination
with lenalidomide (Revlimid) and dexamethasone (IRd) for multiple
myeloma, illustrated that cutaneous and subcutaneous adverse events
were the most common cause of dose reduction.[2]
Furthermore, there have been case reports of Ixazomib-induced cutaneous
necrotizing vasculitis. Here, we report the case of a 70-year-old male
patient with IgM multiple myeloma who developed a rash after ixazomib
treatment and developed into skin exfoliation after adding lenalidomide.
He
was diagnosed with IgM-κ MM in October 2020 with complaints of waist
pain. The initial workup revealed multiple bone destructions in the
thoracolumbar vertebrae and appendages, bilateral ribs, and shoulder
blades. Peripheral blood showed hemoglobin of 121 g/L, platelets of
131×109/L, white blood cell count of 5.49×109/L,
serum creatinine of 96 μmol/L, and serum calcium of 2.78 mmol/L. Serum
protein electrophoresis and immunofixation showed an IgM-kappa
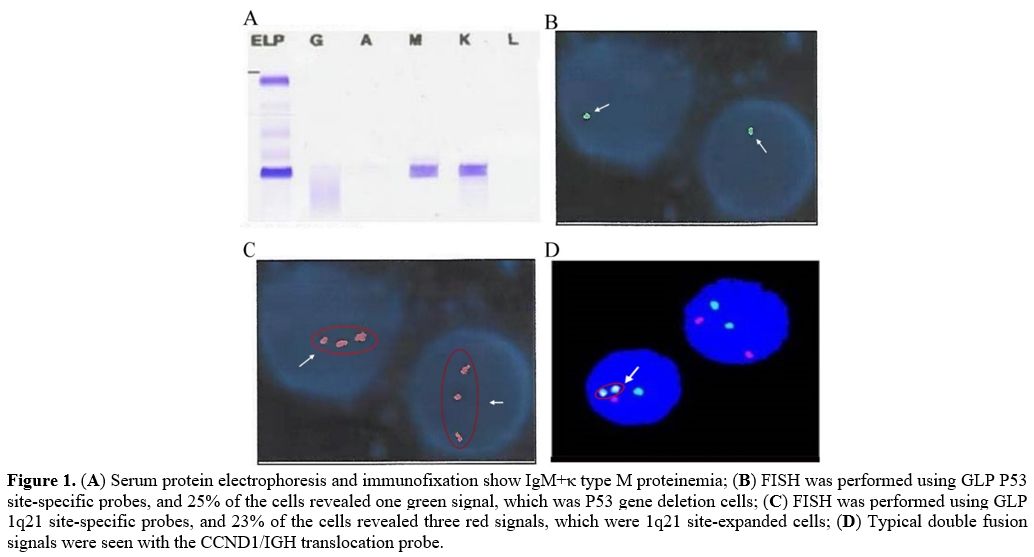
monoclonal protein (Figure 1A)
with an M protein of 70.6 g/L. Serum kappa-light chain levels were
elevated (1261 mg/L), and the kappa/lambda ratio was 157.3267. Urinary
kappa light chains were 4225 mg/L, compared with lambda light chains of
5.9 mg/L. A bone marrow aspirate confirmed the presence of 60% plasma
cells and a normal karyotype, 46, XY. Fluorescence in situ
hybridization (FISH) analysis of the specimen showed immunoglobulin
heavy chain (IGH) translocations t(11;14), del(17p), and 1q21
gain/amplification (Figure 1B, C, D). Finally, the patient was diagnosed with multiple myeloma IgM+κ type (D-S stage III, ISS stage II, R-ISS stage III).
Considering
the obvious symptoms of bone pain, the most common nonhematologic
adverse reaction of bortezomib was peripheral neuropathy, and neuralgia
side effects may exacerbate patient suffering. When the creatinine
clearance rate was approximately 49 ml/(min*1.73 m2),
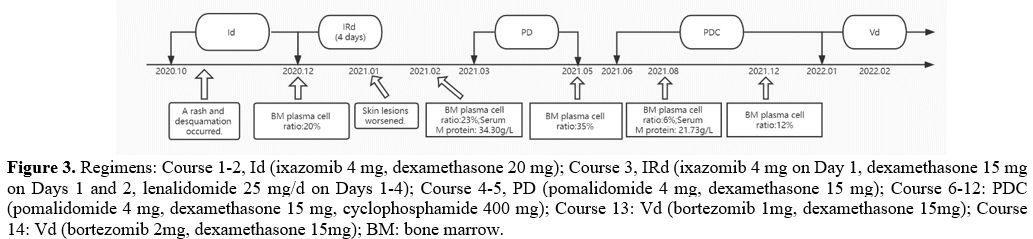
bortezomib and lenalidomide were abandoned. Then, the patient started
therapy with ixazomib 4 mg on Days 1, 8, and 15 plus dexamethasone 20
mg on Days 1, 8, 15, and 22. After some time on this regimen, he
developed some rashes but did not pay attention to them. After two
cycles of chemotherapy were completed, the creatinine clearance rate
returned to normal, which allowed the use of lenalidomide. In pursuit
of more effective treatment, an IRd regimen (ixazomib 4 mg on Days 1,
8, and 15 plus dexamethasone 15 mg on Days 1, 2, 8, 9, 15, 16, 22, and
23 with lenalidomide 25 mg/d) was planned to be implemented.
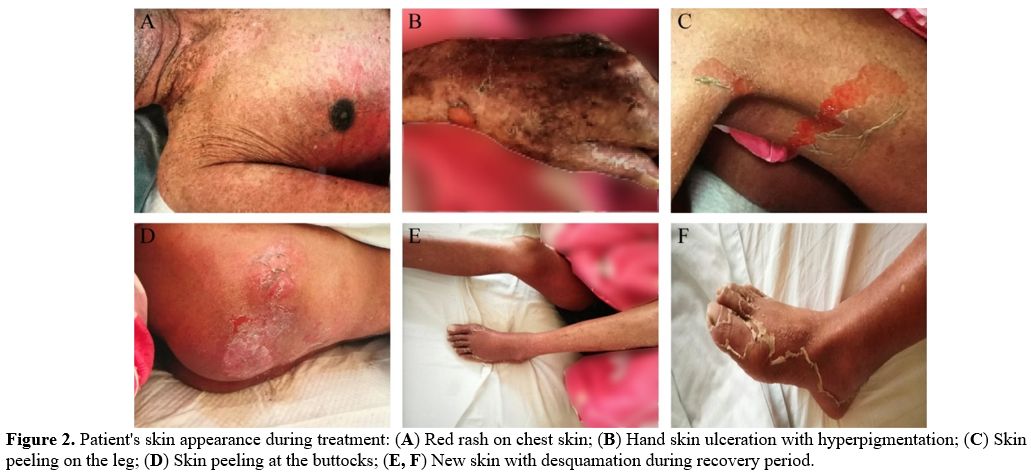
Unfortunately, erythema and desquamation symptoms were further
aggravated after four days of chemotherapy (Figure 2 A, B, C, D),
which forced us to stop the treatment. In addition, a skin biopsy could
not be performed due to severe skin lesions and exudation. After
specialist symptomatic treatment, the skin peeling and exudation
recovered (Figure 2 E, F).
Because we were unsure which drug caused the skin peeling, we could no
longer allow the patient to take ixazomib or lenalidomide continuously.
Therefore, the treatment regimen was changed to pomalidomide and
dexamethasone (PomDex) in the next cycle, with anti-platelet
aggregation to prevent thromboembolism. During these two cycles, he did
not have other skin lesions. In May 2021, bone marrow aspiration
confirmed that abnormal proliferation of plasma cells increased to 35%.
With a suboptimal response to PomDex, the addition of cyclophosphamide
was expected to improve the response and outcomes further. In January
2022, we tried to add bortezomib from 1mg to 2mg, closely monitoring
the skin reactions. The patient did not experience rash or skin peeling
again. The treatment and review results of the patient are summarized
in Figure 3.
According
to the updated cytogenetic risk stratification criteria (including 2016
IMWG and 2018 mSMART 3.0), high-risk cytogenetic abnormalities (HRCAs)
are defined as 1q amplification, del (17p), t (4; 14), t (14; 16) and t
(14; 20). Recently, several foreign studies have proposed that a
combination of various adverse prognostic factors leads to a worse
prognosis and survival.[3] An unsatisfactory drug effect cannot exclude the existence of poor prognostic markers.
The cutaneous side effect profile of ixazomib remains to be documented. Kumar et al.[2]
discussed the safety of ixazomib in patients with previously untreated
myeloma. 17% of patients had skin and subcutaneous tissue disorders,
including rash maculopapular, rash, rash pruritic, erythema, skin
exfoliation, etc. In this case, the rash was considered due to
ixazomib, the only new medication before the patient's skin
manifestations. However, exacerbation of the rash caused by
lenalidomide cannot be ruled out. The rash was observed with IRd,
reflecting the overlapping character of the toxicity that has been seen
with ixazomib alone[2] and with lenalidomide.[4,5]
The rash observed with IRd typically ranges from limited erythematous,
macular, and/or papular lesions that could be pruritic over a few body
areas to a more generalized eruption predominantly on the trunk or
extremities.[6] Most lenalidomide-related rashes are
of mild-to-moderate severity and present as patchy, raised macular skin
lesions, sometimes with localized urticaria and/or pruritus.[5] However, serious dermatological reactions, including Stevens-Johnson syndrome (SJS)[7,8] and toxic epidermal necrolysis (TEN),[8,9]
have been reported with lenalidomide. For the aggravation of skin
lesions that occurred 4 days after starting IRd, the cutaneous adverse
reactions of lenalidomide may have a certain boosting effect, with
ixazomib playing a leading role.
Both ixazomib and bortezomib are
proteasome inhibitors, but cross-allergic reactions may not necessarily
occur, which has a clinical significance for drug use. Also, it is
important to monitor the skin response after treatment with IRd.
Recognizing and identifying cutaneous adverse events allows for early
intervention and management while achieving continuous and effective
treatment.