Ibrahim Halil Acar1, Sebnem Izmir Guner2*, Muzeyyen Aslaner Ak3, Mesut Gocer4, Erman Ozturk5, Figen Atalay6, Gulden Sincan7, Aysun Sentürk Yikilmaz8, Omer Ekinci9, Idris Ince10, Emine Gulturk11, Nazli Demir12, Ali Dogan13, Yildiz Ipek14 and Birol Guvenc1.
1 Adana Cukurova University, Faculty of Medicine, Hematology Department, Adana, Turkey
2 Istanbul Gelisim University, Memorial Sisli Hospital Hematology&Bone Marrow Transplantation Unite, Istanbul, Turkey
3 Zonguldak Bulent Ecevit University, Faculty of Medicine, Hematology Department, Zonguldak, Turkey
4 Health Sciences University, Antalya Training and Research Hospital, Hematology Clinic, Antalya, Turkey
5 Istanbul Medeniyet University, Faculty of Medicine, Hematology Department, Istanbul, Turkey
6 Baskent University, School of Medicine, Department of Hematology, Ankara, Turkey
7 Atatürk University, Faculty of Medicine, Department of Hematology, Erzurum, Turkey
8 Denizli Goverment Hospital, Hematology Clinic, Denizli, Turkey.
9 Medicana International Istanbul, Adult Hematology and Bone Marrow Transplantation Center, Istanbul, Turkey.
10 Dr. Ersin Arslan Training and Research Hospital, Department of Hematology, Gaziantep, Turkey.
11 Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Hematology Clinic, Istanbul, Turkey.
12 Sisli Hamidiye Etfal Training and Research Hospital, Hematology Clinic, Istanbul, Turkey.
13 Van Yuzuncu Yil University, Faculty of Medicine, Department of Hematology, Van, Turkey.
14 Istanbul Kartal Dr. Lutfi Kirdar City Hospital, Hematology Clinic, Istanbul, Turkey.
Correspondence to:
Sebnem Izmir Guner, Istanbul Gelisim University, Memorial Sisli
Hospital Hematology&Bone Marrow Transplantation Unite, Istanbul,
Turkey. Address: Kaptan Pasa, Kaptan Pasa Mah. Piyale Pasa Bulv,
Okmeydanı Cd. No: 4, 34384/Sisli/Istanbul. Tel. +90 532 614 84 98.
E-mail:
sebnemizmirguner@gmail.com
Published: November 1, 2022
Received: May 23, 2022
Accepted: October 11, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022074 DOI
10.4084/MJHID.2022.074
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objectives:
Patients with hematological malignancies have a high risk of mortality
from coronavirus disease 2019 (COVID-19). This study aimed to
investigate the impact of COVID-19 on mortality rates in patients with
various hematological malignancies and to determine risk factors
associated with all-cause mortality.
Methods:
A multicenter, observational retrospective analysis of patients with
hematological malignancies infected with COVID-19 between July 2020 and
December 2021 was performed. Demographic data, clinical
characteristics, and laboratory parameters were recorded. Patients were
grouped as non-survivors and survivors. All-cause mortality was the
primary outcome of the study.
Results:
There were 569 patients with a median age of 59 years. Non-Hodgkin
lymphoma (22.0%) and multiple myelomas (18.1%) were the two most
frequent hematological malignancies. The all-cause mortality rate was
29.3%. The highest mortality rates were seen in patients with acute
myeloid leukemia (44.3%), acute lymphoid leukemia (40.5%), and
non-Hodgkin lymphoma (36.8%). The non-survivors were significantly
older (p<0.001) and had more comorbidities (p<0.05). In addition,
there were significantly more patients with low lymphocyte percentage
(p<0.001), thrombocytopenia (p<0.001), and high CRP (p<0.001)
in the non-survived patients. Age ≥ 65years (p=0.017), cardiac
comorbidities (p=0.041), and continuation of ongoing active therapy for
hematological cancer (p<0.001) were the independent risk factors for
the prediction of mortality.
Conclusions:
In patients with hematological malignancies, coexistent COVID-19 leads
to a higher mortality rate in elderly patients with more comorbidities.
Acute myeloid and lymphoid leukemia and non-Hodgkin lymphoma have the
highest mortality rates. Older age, cardiac diseases, and continuation
of ongoing active therapy for hematological cancer are the independent
risk factors for mortality in hematological malignancy patients with
COVID-19.
|
Introduction
Following
the declaration of COVID-19 as a global pandemic in March 2020 by the
World Health Organization, several studies reported that the corrupted
immunity seen in patients with COVID-19 infection causes poor outcomes
in patients using chemotherapy, radiotherapy, and systemic
immunosuppressive treatment.[1,2] It has been reported that the overall mortality rate of cancer patients with COVID-19 infection could be as much as 40%.[1,3-5]
Hematological disorders such as myeloproliferative disorders, leukemia,
lymphomas, and myelodysplastic syndromes are among the most vulnerable
cohorts for COVID-19, considering the worse outcomes.[2,4,6-8] The development of severe infections leads to worsening clinical outcomes in these patients.[2,9,10]
Hematological malignancies have been responsible for more severe
clinical conditions due to COVID-19 infection than solid organ tumors.[11]
In
patients with hematological malignancies, such as leukemia, lymphoma,
and myeloma, humoral and cellular immunosuppression can be a leading
factor for the significantly increased mortality rates following
COVID-19 infection.[1] The immunosuppressive treatment
modalities like chemotherapeutic agents or autologous or alloge¬neic
hematopoietic stem cell transplantation may aggravate this situation.[1,10]
Although there is a significant concern about the increased morbidity
and mortality risk due to COVID-19 infection, each hematological
malignancy does not pose the same risk.[9,12]
Previous
studies have documented various high-risk factors for mortality,
including the type of malignancy, advanced disease, comorbidity, age
> 60 years, need for intensive care unit admission, and recent
systemic chemotherapy in hematological cancer patients infected with
COVID-19.[2,5,9,13]
Because of the methodological heterogeneity, the reported outcomes show
remarkable variations. However, determination of the prognostic factors
for mortality of COVID-19 in hematological malignancy patients based on
real-time data may be used to perform impactful decisions.[14] That way, risk assessment and decision-making for effective supportive care would be possible.
This
study aimed to evaluate the impact of COVID-19 on the mortality rates
in patients with various hematological malignancies and determine the
risk factors associated with all-cause mortality.
Materials and Methods
Study.
This study was a multicenter, observational retrospective analysis of
patients with hematological malignancies who had been diagnosed with
COVID-19 between July 2020 and December 2021. Fourteen tertiary centers
specialized in hematological diseases throughout Turkey participated in
the study. The local ethical committee approved the study
(Institutional Review Board of Memorial Sisli Hospital, Istanbul,
Turkey, Jul 28, 2020, Number: 003). The researchers agreed to apply the
principles of the Helsinki Declaration. However, written consent could
not be taken due to the study's retrospective design and the unanimity
of data.
Patients.
The patients with newly diagnosed hematological malignancies or ongoing
treatment/follow-up were evaluated. The specific hematological
malignancies were Hodgkin and non-Hodgkin lymphoma, multiple myeloma,
leukemia (acute myeloid, acute and chronic lymphoid, chronic myeloid,
chronic myelomonocytic, hairy cell), myelodysplastic syndromes,
polycythemia vera, essential thrombocytopenia, myelofibrosis,
histiocytosis, and mastocytosis.[7] The severity and
the treatment modality (home isolation, outpatient, and inpatient
treatment) of COVID-19 and the remission status of the hematological
malignancies were not considered for the inclusion of the cases. The
diagnosis of COVID-19 was performed and proved via a positive
qualitative real-time reverse transcriptase-polymerase chain reaction
(RT-PCR) on the nasal and oropharyngeal swab samples. The inclusion
criteria were as follows: age over 18 years, diagnosis of hematological
malignancy before COVID-19, and RT-PCR proved diagnosis of COVID-19.
The patients with clinical findings suspicious of COVID-19 but without
positive RT-PCR (clinical diagnosis) and presumed second primary
cancers were excluded. A standardized protocol for diagnosing and
treating COVID-19 based on the Turkish Ministry of Health Guidelines
was used for all patients.[15]
Variables.
A Microsoft Excel spreadsheet format was used in collecting and
recording the related data. All centers entered their data into this
predetermined sheet and electronically submitted it in an anonymized
form to the study's principal investigator/data processor. The entries
were checked for duplicated data.
Demographic data (age, sex) and
clinical characteristics (admission symptoms, severe obesity,
comorbidities) were recorded. The body mass index value equal to or
higher than 40 kg/m2 was defined as class III obesity.[16]
The patients' presenting symptoms at the time of admission for COVID-19
were also searched and recorded. The type of baseline hematological
cancer and past and ongoing treatment details were collected using the
hospital information system of each center and the patient's medical
files. The ongoing treatment was defined as having the treatment within
30 days before COVID-19 diagnosis.[5,17]
The
laboratory parameters at the diagnosis of COVID-19 [leukocyte and
platelet counts, lymphocyte percentage, C-reactive protein (CRP), and
D-dimer] were investigated and categorized as low, normal, or high
using each laboratory's lower and upper limits. In addition, we
categorized the treatment modalities for hematological malignancies as
conventional chemotherapy, targeted therapies (small-molecule
inhibitors and monoclonal antibodies), immunotherapy (checkpoint
inhibitors), and immunomodulatory.[14] Any change in the chemotherapy protocols associated with COVID-19 was noted.
The
antiviral drugs, broad-spectrum antibiotics, antifungal and
antimalarial medications, glucocorticoids, immune-modulating agents
(Interleukin-6 and Janus kinase inhibitors), immune plasma therapy, and
other medical modalities for COVID-19 were recorded. In addition, the
adverse effects due to COVID-19 treatment were noted.
Statistical analysis.
All-cause mortality was the primary outcome of this study; therefore,
the patients were grouped as the non-survivors and survivors. We
compared the groups regarding demographic and clinical characteristics;
however, the factors impacting the development of all-cause mortality
were also analyzed.
For descriptive statistics, mean ± standard
deviation was used to present continuous data with normal distribution.
A Median with minimum-maximum values was applied for continuous
variables without normal distribution. Numbers and percentages were
used for categorical variables. The Shapiro-Wilk and Kolmogorov-Smirnov
tests analyzed the normal distribution of the numerical variables. Q-Q
plots and histograms also checked the normal distribution pattern.
The
Mann-Whitney U test compared two independent groups for the variables
without normal distribution. The Pearson Chi-Square, Fisher's Exact,
and Fisher Freeman Halton tests were used to compare the differences
between categorical variables in 2x2 and RxC tables.
Binary
logistic regression was performed to analyze the factors that impact
the development of mortality. In addition, statistically or clinically
significant factors regarding hematological malignancies in the
univariate analysis were included in the multivariate analysis.
For
statistical analysis, IBM SPSS Statistics V.21 was used. All
statistical analyses determined the significance level (p-value) at
0.05.
Results
There
were 569 patients in the study group with a median age of 59 years
(18-91 years). There was a higher prevalence of elderly (≥65 years)
(61.3%) and male patients (57.6%). Non-Hodgkin lymphoma and multiple
myelomas were the two most common hematological malignancies in 125
(22.0%) and 103 patients (18.1%). Hypertension was the most frequent
comorbidity (28.3%), followed by respiratory diseases (16.5%) and
diabetes mellitus (16.0%) in the study group. The study cohort's
baseline demographic and clinical characteristics are given in Table 1.
 |
Table 1. Demographic and clinical characteristics of the study group. |
One
hundred and sixty-seven patients did not survive, with an all-cause
mortality rate of 29.3%. Considering all cases, 154 cases (92.2%) were
COVID-related mortality. We hospitalized 410 patients (72.1%) for
COVID-19 treatment. The hospitalization rate was significantly higher
in the non-survived group (99.4% vs.60.7%, p<0.001). The highest
mortality rates were seen in patients with acute myeloid leukemia
(44.3%), acute lymphoid leukemia (40.5%), non-Hodgkin lymphoma (36.8%),
and multiple myeloma (31.1%), and myelodysplastic syndromes (29.5%).
The
comparison of the demographic and clinical characteristics of the
patients revealed that the non-survivors were significantly older
(median age 64 vs. 57 years, p<0.001) than the survivors. The
proportion of patients aged 65 years or more was significantly higher
in the non-survived group (p=0.001). Sex distribution was similar in
the groups (p=0.780). Comparing the frequencies of the hematological
malignancies revealed no significant difference in the survived and
non-survived patients (p=0.781). The incidences of hypertension
(p=0.019), respiratory diseases (p=0.047), cardiac diseases (p=0.003),
and chronic renal failure (p=0.026) were significantly higher in
patients who were non-survived. The survival and non-survived patients'
clinical characteristics were similar (Table 1).
The distribution of the presenting symptoms is detailed in Table 2.
Fever (76.3%), fatigue (58.3%), coughing (52.3%), myalgia (48.0%), and
dyspnea (47.3%) were the most common symptoms at the admission of the
patients. There were significant differences in the frequencies of the
admission symptoms between the survivors and non-survivors. The
non-survived patients more frequently have had fever (p<0.001),
fatigue (p=0.032), dyspnea (p<0.001), irritability/confusion
(p=0.006), gastrointestinal symptoms (p=0.045), and chest pain
(p=0.001).
 |
Table 2. COVID-19 symptoms and signs at diagnosis. |
There
were 329 patients (57.8%) in the study group with ongoing active
oncological treatment. We found a significant difference considering
the use of any treatment. The proportion of non-survived patients with
on-therapy was significantly higher than those of the survived patients
(58.9% vs.46.8%, p=0.001). The distribution of the oncological
treatment modalities revealed no significant difference (Table 3).
The oncological treatment was stopped in 299 patients (90.6%) due to
COVID-19. The proportion of patients with ongoing therapy after
COVID-19 was significantly lower in the non-survived patients (p=0.028).
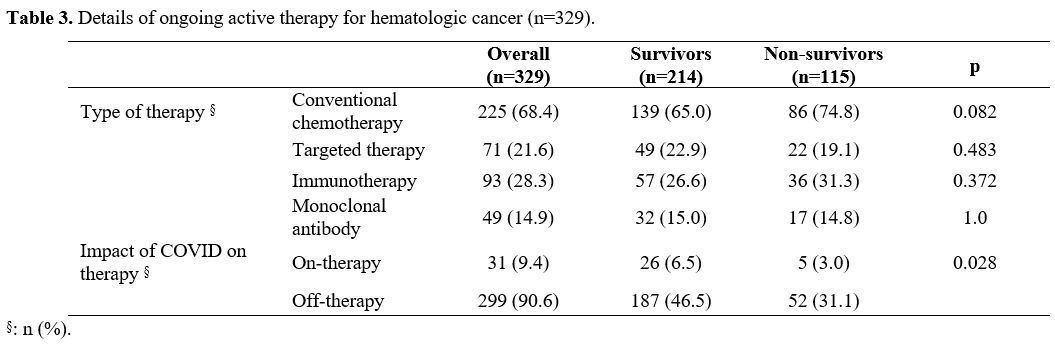 |
Table 3. Details of ongoing active therapy for hematologic cancer (n=329). |
The results of the laboratory investigations at the admission are given in Table 4.
There were significant differences in leukocyte count, lymphocyte
percentage, platelet count, CRP, and D-Dimer between the survived and
non-survived patients. The median values of platelet counts and the
percentage of lymphocytes were significantly lower in the non-survived
patients (p<0.001 and p<0.001, respectively). In addition, we
found significantly higher CRP and D-dimer values in the non-survived
group (p<0.001 and p<0.001).
 |
Table 4. Laboratory investigations of the survived and non-survived patients. |
Table 5
presents the details of the treatment used for COVID-19. 77.9% of the
patients received antiviral medications, and glucocorticoids were used
in 299 patients (52.5%). Other details are summarized in Table 5.
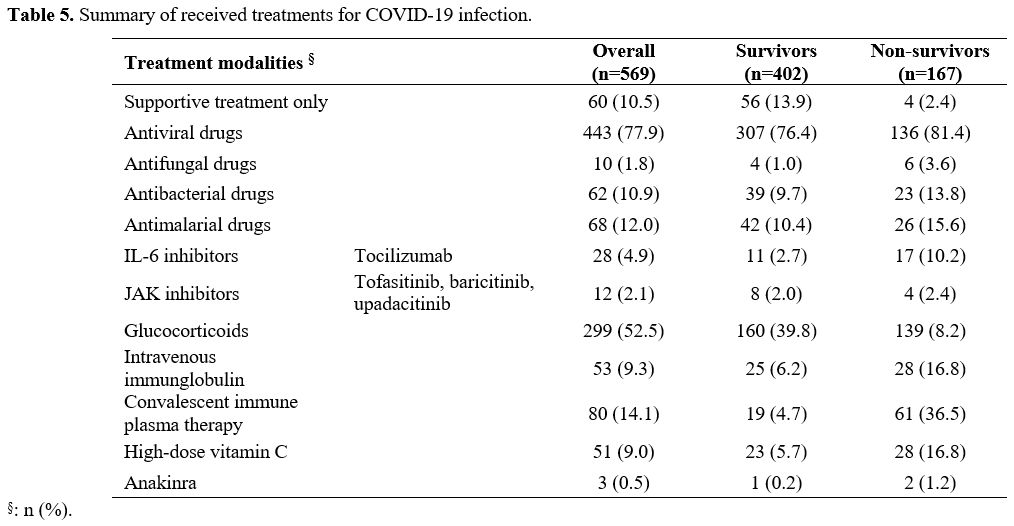 |
Table 5. Summary of received treatments for COVID-19 infection. |
We
detected a total of 84 side effects in the study group.
Nausea/vomiting, elevated liver enzymes, and neutropenia were the most
frequent complications in 23, 18, and 11 patients. The development of
side effects associated with COVID-19 treatment was more frequently
seen in non-survived patients (p<0.001).
Binary logistic
regression analysis revealed that age ≥ 65years (OR=1.669, CI
95%:1.096-2.542, p=0.017), cardiac diseases (OR=1.748, CI
95%:1.024-2.984, p=0.041), and the continuation of ongoing active
therapy for hematological cancer (OR=2.510, CI 95%:1.707-3.691,
p<0.001) were the independent risk factors for the prediction of
mortality in hematological cancer patients with COVID-19 infection (Table 6).
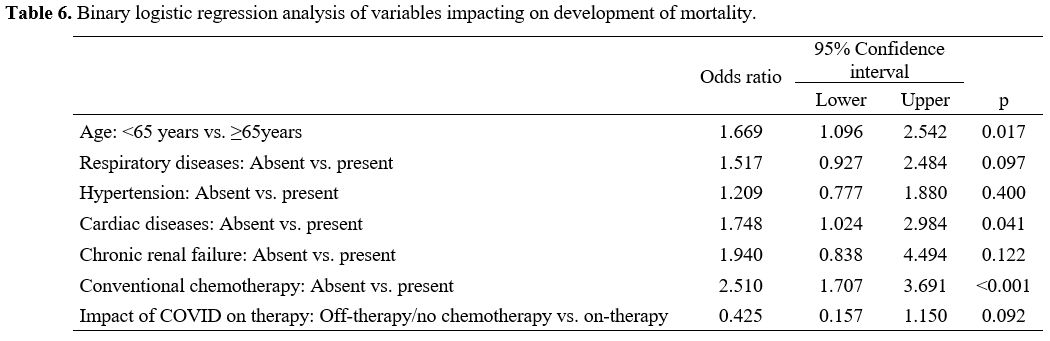 |
Table 6. Binary logistic regression analysis of variables impacting on development of mortality. |
Discussion
This
study presented the outcomes of 569 COVID-19 patients treated due to
hematological malignancies in 14 tertiary centers in Turkey. The
all-cause mortality rate was 29.3% in the study group. The older
patients with comorbidities were the most susceptible group to
mortality. Older age, cardiac comorbidities, and continuation of
ongoing active therapy for hematological cancer during the COVID-19
pandemic were the independent risk factors for mortality in the binary
logistic regression model.
Several studies have focused on the outcomes of COVID-19 in hematological malignancy patients.[5,7,9,13]
The European Hematology Association Survey (EPICOVIDEHA) published the
outcomes of 3801 patients with hematological malignancy. Non-Hodgkin
lymphoma, multiple myeloma, chronic lymphoid leukemia, acute myeloid
leukemia, and myelodysplastic syndromes were more frequent than other
diseases.[2] The frequency rank of the diseases in our
study was almost similar to the findings of this cohort. In
population-based data from the Turkey Ministry of Health, non-Hodgkin
lymphoma was the most frequent malignancy seen in almost one-third of
1480 laboratory-confirmed COVID-19 patients.[18] Other studies documented many COVID-19 cases with non-Hodgkin lymphoma and multiple myeloma.[7,19]
In the EPICOVIDEHA study, the incidence of acute myeloid leukemia was
12.5%, like 13.9% in our study. Although the authors thought this
disease was a rare malignancy compared to the other types, Wood et al.[20]
reported that acute leukemia was the most common type of cancer,
followed by non-Hodgkin lymphoma in the ASH Research Collaborative
COVID-19 Registry for Hematology. So, it should be kept in mind that
the heterogeneity of the hematological diagnoses and their treatment
modalities leads to difficulty in evaluating the outcomes.
In the
studies investigating the mortality rate of hematological malignancy
patients after they were infected with COVID-19, the overall mortality
rates were reported to be up to 40%.[2,5,7,9,17,21,22] Vijenthira et al.[4]
reviewed the outcomes of 3377 patients with hematological malignancies
and COVID-19 in a systematic review and meta-analysis. They found a
higher mortality risk in older and hospitalized patients. Recent cancer
treatment was not associated with mortality. Several authors used
different time points to determine mortality rates ranging from 14 to
45 days leading to conflicting evaluations.[9,13,22] The overall mortality rate in the European Hematology Association Survey was 31.2%.[2]
The current study's all-cause mortality rate was 29.3% during the
in-hospital follow-up period. We think our mortality rate was similar
to the previously published studies. Higher mortality rates have been
explicitly detected in patients with severe COVID-19.[9]
It has also been mentioned that COVID-19 caused higher mortality rates
in patients with hematological cancer than those with solid tumors.[18]
So, the methodological differences, different study date intervals
regarding the various waves of the COVID-19 pandemic, and the
evaluation periods for mortality should be considered when comparing
the outcomes.[13] In light of these data, we may
think that COVID-19 leads to higher mortality rates in patients with
hematological malignancies.[2,5,7,14]
The
possible association between the type of hematological cancer and
mortality is another speculated issue. According to the European
Survey, the highest mortality rates were detected in patients with
acute myeloid leukemia and myelodysplastic syndromes.[2]
In the UK Coronavirus Cancer Monitoring Project (UKCCMP), acute
leukemia and myeloma had the highest mortality compared to the other
hematological cancer types.[14] A subgroup analysis of hematological malignancies was not performed in Vijenthira's review paper.[4] However, the variances according to the type of malignancy have been studied by others.[6]
The relatively lower or higher mortality rates have been reported in
patients with lymphoma or acute myeloid leukemia infected with
COVID-19.[2,6,14]
In the current study, the five diseases with the highest mortality
rates (acute myeloid leukemia, acute lymphoid leukemia, non-Hodgkin
lymphoma, multiple myeloma, and MDS) were similar to the previous
studies. There should be several explanations for the worst outcome and
highest mortality rates in these specific hematological malignancies.
Age, profound immunodeficiency status due to the underlying disease or
its treatment, and any possible delay in the treatment have been
speculated to explain the poor outcomes in these patients.[2,22]
The
impact of hematological cancer treatment and its type is another
conflicting issue. The features of treatment modalities for
hematological malignancies are thought to be associated with the
outcomes of COVID-19.[9] However, in the UKCCMP
cohort, the authors found no association between cytotoxic
chemotherapy, anti-CD20 therapy, and mortality.[14]
Although there were no significant differences in the frequencies of
the cancer types and the ongoing active treatment modalities for
hematological cancer between the survivors and non-survivors, the
continuation of the ongoing active therapy for hematological cancer was
one of the independent risk factors for mortality in our study. It is
not easy to show the exact cause-and-effect relationships in a
retrospective study. Besides, comparing the mortality rates in the
different studies may be problematic regarding the patients' different
demographic and clinical characteristics. So, prospective studies are
needed to overcome the controversies between the studies,
Previous studies reported various risk factors for mortality. Older patients were more susceptible to mortality.[2,13,14,22]
Although different cut-off values to define elderly people have been
used, we may conclude that patients over 65 or 70 have higher mortality
rates. In the present study, older age (≥65 years) was significantly
associated with the development of mortality. We think that as the
patient's age increases, comorbidities and other clinical situations
might reflect the increased mortality risk more appropriately. Age,
comorbidities, neutrophilia, lymphopenia, and high CRP were significant
predictors of mortality in the UKCCMP cohort.[14] Thrombocytopenia was another significant factor associated with higher mortality risk.[24] However, others found no significant impact on mortality of age, sex, comorbidity, leukocyte, and lymphocyte counts.[7]
In the current study, older age, cardiac comorbidities, and
continuation of ongoing active therapy for hematological cancer were
the independent risk factors for mortality in the multivariate model.
It is unsurprising to obtain controversial findings due to the
different patient and tumor characteristics.[22]
Different
treatment modalities based on the severity and remission status of
COVID-19 may impact the outcomes of patients with hematological
malignancies. Although we did not use the severity grading of the
infection, the inclusion criteria for COVID-19 infection were
well-standardized based on the national treatment protocols. Besides,
various factors, including the intensity of immunosuppressive treatment
and the type of hematological cancer and its treatment modalities,
might contribute to the differences in the outcomes of the patients
infected with COVID-19.[9] Several authors also
proposed that in their treatment's pre-induction, induction, and
refractory phases, hematological malignancy patients might have weaker
immunity than those in the maintenance phase.[23]
Azhdari Tehrani et al. found that the pre-induction and induction
phases of the treatment for hematological cancer were significantly
associated with increased mortality for different hematological
cancers.[23] A systematic review and meta-analysis by
Naimi et al. analyzed that the weakening of the immune system is a
common consequence of anti-tumor therapies.[24]
Previous studies showed that anti-tumor therapies during the first 14
days of COVID infection caused poor prognosis in cancer patients.[25,26] Avoidance of the treatment modalities leading to an immunosuppressive status has been recommended.[25]
Although the types of ongoing active therapy for hematological cancer
were not associated with the development of mortality in this patient
group, the continuation of these therapies was the independent risk
factor for mortality. We also could not discriminate between the
different phases of the therapies. So, we think the treatment
strategies for patients with hematological cancer should be tailored
considering the current status of cancer and COVID infection
simultaneously.
The patients' symptoms may show variations
considering the underlying malignancy and COVID-19. The most frequent
symptoms were fever, weakness, cough, and dyspnea.[7,13,20,22,27]
We detected significant differences in the incidences of the admitting
symptoms between the survivors and non-survivors. Fever, fatigue,
dyspnea, irritability/confusion, gastrointestinal complaints, and chest
pain were more frequently seen in the non-survivors. However, the triad
of "malignancy, infection, and treatment" may lead to complexity in
this patient group. So, we may not be sure of the exact role of these
significant symptoms and signs of mortality.
The multicenter
design and large sample size were the study's major strengths.
Nevertheless, retrospective data analysis might be the main limitation
of incomplete data. We could not evaluate the exact reasons for the
mortality attributable and contributable to either hematological
malignancy or COVID-19.[2] The clinical benefits of
the treatment modalities for COVID-19 were not analyzed in this study,
considering the study's retrospective nature. Besides, the side effects
developed during the COVID-19 treatment would be due to the viral
exposure that could not be differentiated using this retrospective
data. Although a predetermined worksheet was used, incomplete data
entry was considered possible.
In conclusion, coexistent COVID-19
was significantly associated with a higher mortality rate in elderly
patients with more comorbidities in patients with hematological
malignancies. Acute myeloid and lymphoid leukemia and non-Hodgkin
lymphoma had the highest mortality rates. Older age, cardiac diseases,
and continuation of ongoing active therapy were the independent risk
factors for mortality in hematological malignancies with COVID-19.
Acknowledgment
All authors would like to thank the colleagues who participated in this study.
References
- Papakonstantinou E, Dragoumani K, Efthimiadou A,
Palaiogeorgou AM, Pierouli K, Mitsis T, Chrousos GP, Bacopoulou F,
Vlachakis D. Haematological malignancies implications during the times
of the COVID-19 pandemic. Oncol Lett. 2021;22(6):856. https://doi.org/10.3892/ol.2021.13117 PMID: 34777590
- Pagano
L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M,
Klimko N, Koehler P, Pagliuca A, Passamonti F, Verga L, Víšek B, Ilhan
O, Nadali G, Weinbergerová B, Córdoba-Mascuñano R, Marchetti M, Collins
GP, Farina F, Cattaneo C, Cabirta A, Gomes-Silva M, Itri F, van Doesum
J, Ledoux MP, Čerňan M, Jakšić O, Duarte RF, Magliano G, Omrani AS,
Fracchiolla NS, Kulasekararaj A, Valković T, Poulsen CB, Machado M,
Glenthøj A, Stoma I, Ráčil Z, Piukovics K, Navrátil M, Emarah Z, Sili
U, Maertens J, Blennow O, Bergantim R, García-Vidal C, Prezioso L,
Guidetti A, Del Principe MI, Popova M, de Jonge N, Ormazabal-Vélez I,
Fernández N, Falces-Romero I, Cuccaro A, Meers S, Buquicchio C, Antić
D, Al-Khabori M, García-Sanz R, Biernat MM, Tisi MC, Sal E, Rahimli L,
Čolović N, Schönlein M, Calbacho M, Tascini C, Miranda-Castillo C,
Khanna N, Méndez GA, Petzer V, Novák J, Besson C, Duléry R, Lamure S,
Nucci M, Zambrotta G, Žák P, Seval GC, Bonuomo V, Mayer J, López-García
A, Sacchi MV, Booth S, Ciceri F, Oberti M, Salvini M, Izuzquiza M,
Nunes-Rodrigues R, Ammatuna E, Obr A, Herbrecht R,
Núñez-Martín-Buitrago L, Mancini V, Shwaylia H, Sciumè M, Essame J,
Nygaard M, Batinić J, Gonzaga Y, Regalado-Artamendi I, Karlsson LK,
Shapetska M, Hanakova M, El-Ashwah S, Borbényi Z, Çolak GM, Nordlander
A, Dragonetti G, Maraglino AME, Rinaldi A, De Ramón-Sánchez C, Cornely
OA; EPICOVIDEHA working group. COVID-19 infection in adult patients
with hematological malignancies: a European Hematology Association
Survey (EPICOVIDEHA). J Hematol Oncol. 2021;14(1):168. https://doi.org/10.1186/s13045-021-01177-0 PMID: 34649563
- Pinato
DJ, Zambelli A, Aguilar-Company J, Bower M, Sng C, Salazar R, Bertuzzi
A, Brunet J, Mesia R, Segui E, Biello F, Generali D, Grisanti S, Rizzo
G, Libertini M, Maconi A, Harbeck N, Vincenzi B, Bertulli R, Ottaviani
D, Carbo A, Bruna R, Benafif S, Marrari A, Wuerstlein R, Carmona-Garcia
MC, Chopra N, Tondini C, Mirallas O, Tovazzi V, Betti M, Provenzano S,
Fotia V, Cruz CA, Dalla Pria A, D'Avanzo F, Evans JS, Saoudi-Gonzalez
N, Felip E, Galazi M, Garcia-Fructuoso I, Lee AJX, Newsom-Davis T,
Patriarca A, Garcia-Illescas D, Reyes R, Dileo P, Sharkey R, Wong YNS,
Ferrante D, Marco-Hernandez J, Sureda A, Maluquer C, Ruiz-Camps I,
Gaidano G, Rimassa L, Chiudinelli L, Izuzquiza M, Cabirta A, Franchi M,
Santoro A, Prat A, Tabernero J, Gennari A. Clinical portrait of the
SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020
Jul 31;10(10):1465–74. https://doi.org/10.1158/2159-8290.CD-20-0773 PMID: 32737082
- Vijenthira
A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, Martín-Moro F,
Razanamahery J, Riches JC, Zwicker J, Patell R, Vekemans MC, Scarfò L,
Chatzikonstantinou T, Yildiz H, Lattenist R, Mantzaris I, Wood WA,
Hicks LK. Outcomes of patients with hematologic malignancies and
COVID-19: a systematic review and meta-analysis of 3377 patients.
Blood. 2020 Dec 17;136(25):2881-2892. https://doi.org/10.1182/blood.2020008824 PMID: 33113551
- García-Suárez
J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V,
Hernández-Rivas JÁ, Gil-Manso R, Kwon M, Sánchez-Godoy P,
Martínez-Barranco P, Colás-Lahuerta B, Herrera P, Benito-Parra L,
Alegre A, Velasco A, Matilla A, Aláez-Usón MC, Martos-Martínez R,
Martínez-Chamorro C, Susana-Quiroz K, Del Campo JF, de la Fuente A,
Herráez R, Pascual A, Gómez E, Pérez-Oteyza J, Ruiz E, Alonso A,
González-Medina J, Martín-Buitrago LN, Canales M, González-Gascón I,
Vicente-Ayuso MC, Valenciano S, Roa MG, Monteliu PE, López-Jiménez J,
Escobar CE, Ortiz-Martín J, Diez-Martin JL, Martinez-Lopez J;
Asociación Madrileña de Hematología y Hemoterapia (AMHH). Impact of
hematologic malignancy and type of cancer therapy on COVID-19 severity
and mortality: lessons from a large population-based registry study. J
Hematol Oncol. 2020;13(1):133. https://doi.org/10.1186/s13045-020-00970-7 PMID: 33032660
- Visco
C, Marcheselli L, Mina R, Sassone M, Guidetti A, Penna D, Cattaneo C,
Bonuomo V, Busca A, Ferreri AJM, Bruna R, Petrucci L, Cairoli R,
Salvini M, Bertù L, Ladetto M, Pilerci S, Pinto A, Ramadan S, Marchesi
F, Cavo M, Arcaini L, Coviello E, Romano A, Musto P, Massaia M,
Fracchiolla N, Marchetti M, Scattolin A, Tisi MC, Cuneo A, Della Porta
M, Trentin L, Turrini M, Gherlinzoni F, Tafuri A, Galimberti S, Bocchia
M, Cardinali V, Cilloni D, Corso A, Armiento D, Rigacci L, La Barbera
EO, Gambacorti-Passerini C, Visani G, Vallisa D, Venditti A, Selleri C,
Conconi A, Tosi P, Lanza F, Candoni A, Krampera M, Corradini P,
Passamonti F, Merli F; ITA-HEMA-COV investigators. A prognostic model
for patients with lymphoma and COVID-19: a multicentre cohort study.
Blood Adv. 2022;6(1):327-338. https://doi.org/10.1182/bloodadvances.2021005691 PMID: 34644385
- Tıglıoglu
P, Albayrak M, Tıglıoğlu M, Ozturk HBA, Aras MR, Saglam B, Maral S. The
outcome of COVID-19 in patients with hematological malignancy. Memo.
2022;15(1):83-89. https://doi.org/10.1007/s12254-021-00775-5 PMID: 34904019
- Pagnano
KB, Peralta EH, Navarro JR, David Salas LDR, Delgado N, Moiraghi B,
Toreli ACM, Perobelli LM, Fechio L, Quixada ATS, Funke V, Bendit I,
Seguro FS, Pilleux L, Bortolini J, Lourenço ALG, Sapelli J, Nucci FM,
Pavlovsky C, Oliveira LDC, Moura MS, Palma LC, Gonçalves NN, Conchon M,
Hokama POM, Almeida LL, Zulli R, de Souza CA, Boquimpani CM. COVID-19
in chronic myeloid leukemia patients in Latin America. Leuk Lymphoma.
2021;62(13):3212-3218. https://doi.org/10.1080/10428194.2021.1950709 PMID: 34254886.
- Jain
A, Nayak L, Kulkarni UP, Mehra N, Yanamandra U, Kayal S, Damodar S,
John JM, Mehta P, Singh S, Munot P, Selvarajan S, Radhakrishnan V, Lad
D, Kapoor R, Dubashi B, Bharath RS, Jain H, Jayachandran PK, Lakshmanan
J, Mani T, Thorat J, Das S, Karunamurthy O, George B, Sengar M,
Malhotra P. Outcomes of patients with hematologic malignancies and
COVID-19 from the Hematologic Cancer Registry of India. Blood Cancer J.
2022;12(1):2. https://doi.org/10.1038/s41408-021-00599-w PMID: 34987161
- Scarfò
L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C,
Garcia-Marco JA, Hernández-Rivas JÁ, Mirás F, Baile M, Marquet J,
Niemann CU, Reda G, Munir T, Gimeno E, Marchetti M, Quaglia FM,
Varettoni M, Delgado J, Iyengar S, Janssens A, Marasca R, Ferrari A,
Cuéllar-García C, Itchaki G, Špaček M, De Paoli L, Laurenti L, Levin
MD, Lista E, Mauro FR, Šimkovič M, Van Der Spek E, Vandenberghe E,
Trentin L, Wasik-Szczepanek E, Ruchlemer R, Bron D, De Paolis MR, Del
Poeta G, Farina L, Foglietta M, Gentile M, Herishanu Y, Herold T,
Jaksic O, Kater AP, Kersting S, Malerba L, Orsucci L, Popov VM,
Sportoletti P, Yassin M, Pocali B, Barna G, Chiarenza A, Dos Santos G,
Nikitin E, Andres M, Dimou M, Doubek M, Enrico A, Hakobyan Y,
Kalashnikova O, Ortiz Pareja M, Papaioannou M, Rossi D, Shah N,
Shrestha A, Stanca O, Stavroyianni N, Strugov V, Tam C, Zdrenghea M,
Coscia M, Stamatopoulos K, Rossi G, Rambaldi A, Montserrat E, Foà R,
Cuneo A, Ghia P. COVID-19 severity and mortality in patients with
chronic lymphocytic leukemia: a joint study by ERIC, the European
Research Initiative on CLL, and CLL Campus. Leukemia.
2020;34(9):2354-2363. https://doi.org/10.1038/s41375-020-0959-x PMID: 32647324
- Lee
LYW, Cazier JB, Starkey T, Briggs SEW, Arnold R, Bisht V, Booth S,
Campton NA, Cheng VWT, Collins G, Curley HM, Earwaker P, Fittall MW,
Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJX, Lee RJ, Lee
SM, Mckenzie H, Middleton CP, Murugaesu N, Newsom-Davis T, Olsson-Brown
AC, Palles C, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A,
Sivakumar S, Smith AJ, Topping O, Turnbull CD, Várnai C, Briggs ADM,
Middleton G, Kerr R; UK Coronavirus Cancer Monitoring Project Team.
COVID-19 prevalence and mortality in patients with cancer and the
effect of primary tumour subtype and patient demographics: a
prospective cohort study. Lancet Oncol. 2020;21(10):1309-1316. Erratum
in: Lancet Oncol. 2020. https://doi.org/10.1016/S1470-2045(20)30442-3 PMID: 32853557
- Gupta
A, Desai N, Sanjeev, Chauhan P, Nityanand S, Hashim Z, Gupta M.
Clinical profile and outcome of COVID-19 in haematological
malignancies: experience from tertiary care centre in India. Ann
Hematol. 2022;101(1):69-79. https://doi.org/10.1007/s00277-021-04644-3 PMID: 34559278
- Borah
P, Mirgh S, Sharma SK, Bansal S, Dixit A, Dolai TK, Lunkad S, Gupta N,
Singh G, Jain A, Bansal D, Choudhary D, Khandelwal V, Doval D, Kumar M,
Bhargava R, Chakrabarti A, Kalashetty M, Rauthan A, Kazi B, Mandal PK,
Jeyaraman P, Naithani R; AIIMS Hematology Alumni Group. Effect of age,
comorbidity and remission status on outcome of COVID-19 in patients
with hematological malignancies. Blood Cells Mol Dis. 2021;87:102525. https://doi.org/10.1016/j.bcmd.2020.102525. PMID: 33338697
- Booth
S, Curley HM, Varnai C, Arnold R, Lee LYW, Campton NA, Cook G,
Purshouse K, Aries J, Innes A, Cook LB, Tomkins O, Oram HS, Tilby M,
Kulasekararaj A, Wrench D, Dolly S, Newsom-Davies T, Pettengell R,
Gault A, Moody S, Mittal S, Altohami M, Tillet T, Illingworth J,
Mukherjee L, Apperly J, Ashcroft J, Rabin N, Carmichael J, Cazier JB,
Kerr R, Middleton G, Collins GP, Palles C; UKCCMP team. Key findings
from the UKCCMP cohort of 877 patients with haematological malignancy
and COVID-19: disease control as an important factor relative to recent
chemotherapy or anti-CD20 therapy. Br J Haematol. 2022;196(4):892-901. https://doi.org/10.1111/bjh.17937 PMID: 34761389
- World Health Organization. WHO Interim Guidance. Clinical Management of COVID-19. Geneva, WHO, 2020. https://apps.who.int/iris/handle/10665/332196
- Kitahara
CM, Flint AJ, Berrington de Gonzalez A, Bernstein L, Brotzman M,
MacInnis RJ, Moore SC, Robien K, Rosenberg PS, Singh PN, Weiderpass E,
Adami HO, Anton-Culver H, Ballard-Barbash R, Buring JE, Freedman DM,
Fraser GE, Beane Freeman LE, Gapstur SM, Gaziano JM, Giles GG,
Håkansson N, Hoppin JA, Hu FB, Koenig K, Linet MS, Park Y, Patel AV,
Purdue MP, Schairer C, Sesso HD, Visvanathan K, White E, Wolk A,
Zeleniuch-Jacquotte A, Hartge P. Association between class III obesity
(BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective
studies. PLoS Med. 2014;11(7):e1001673. https://doi.org/10.1371/journal.pmed.1001673 PMID: 25003901
- Romano
A, Cerchione C, Conticello C, Filetti S, Bulla A, Chiarenza A, Del
Fabro V, Leotta S, Markovic U, Motta G, Parisi M, Stagno F, Palumbo GA,
Di Raimondo F. Reduced Absolute Count of Monocytes in Patients Carrying
Hematological Neoplasms and SARS-CoV2 Infection. Cancers (Basel).
2022;14(5):1173. https://doi.org/10.3390/cancers14051173 PMID: 35267478
- Yigenoglu
TN, Ata N, Altuntas F, Bascı S, Dal MS, Korkmaz S, Namdaroglu S,
Basturk A, Hacıbekiroglu T, Dogu MH, Berber İ, Dal K, Erkurt MA, Turgut
B, Ulgu MM, Celik O, Imrat E, Birinci S. The outcome of COVID-19 in
patients with hematological malignancy. J Med Virol.
2021;93(2):1099-1104. https://doi.org/10.1002/jmv.26404 PMID: 32776581
- Regalado-Artamendi
I, Jiménez-Ubieto A, Hernández-Rivas JÁ, Navarro B, Núñez L, Alaez C,
Córdoba R, Peñalver FJ, Cannata J, Estival P, Quiroz-Cervantes K, Riaza
Grau R, Velasco A, Martos R, Domingo-González A, Benito-Parra L,
Gómez-Sanz E, López-Jiménez J, Matilla A, Herraez MR, Penalva MJ,
García-Suárez J, Díez-Martín JL, Bastos-Oreiro M. Risk Factors and
Mortality of COVID-19 in Patients With Lymphoma: A Multicenter Study.
Hemasphere. 2021;5(3):e538. https://doi.org/10.1097/HS9.0000000000000538 PMID: 33604516
- Wood
WA, Neuberg DS, Thompson JC, Tallman MS, Sekeres MA, Sehn LH, Anderson
KC, Goldberg AD, Pennell NA, Niemeyer CM, Tucker E, Hewitt K, Plovnick
RM, Hicks LK. Outcomes of patients with hematologic malignancies and
COVID-19: a report from the ASH Research Collaborative Data Hub. Blood
Adv. 2020;4(23):5966-5975. https://doi.org/10.1182/bloodadvances.2020003170 PMID: 33278301
- Modemann
F, Niederwieser C, Weisel K, Bokemeyer C, Fiedler W, Ghandili S.
COVID-19 and seasonal influenza: a comparative analysis in patients
with hematological malignancies. Leuk Lymphoma. 2022;63(3):664-671. https://doi.org/10.1080/10428194.2021.1992626 PMID: 34668809.
- Piñana
JL, Martino R, García-García I, Parody R, Morales MD, Benzo G,
Gómez-Catalan I, Coll R, De La Fuente I, Luna A, Merchán B, Chinea A,
de Miguel D, Serrano A, Pérez C, Diaz C, Lopez JL, Saez AJ, Bailen R,
Zudaire T, Martínez D, Jurado M, Calbacho M, Vázquez L, Garcia-Cadenas
I, Fox L, Pimentel AI, Bautista G, Nieto A, Fernandez P, Vallejo JC,
Solano C, Valero M, Espigado I, Saldaña R, Sisinni L, Ribera JM,
Jimenez MJ, Trabazo M, Gonzalez-Vicent M, Fernández N, Talarn C,
Montoya MC, Cedillo A, Sureda A; Infectious Complications Subcommittee
of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy
Group (GETH). Risk factors and outcome of COVID-19 in patients with
hematological malignancies. Exp Hematol Oncol. 2020;9:21. https://doi.org/10.1186/s40164-020-00177-z PMID: 32864192
- Azhdari
Tehrani H, Ramezaninejad S, Mardani M, Shokouhi S, Darnahal M,
Hakamifard A. Hematologic malignancies and COVID-19 infection: A
monocenter retrospective study. Health Sci Rep. 2022;5(3):e638. https://doi.org/10.1002/hsr2.638 PMID: 35620550
- Naimi
A, Yashmi I, Jebeleh R, Imani Mofrad M, Azimian Abhar S, Jannesar Y,
Mohsen Heidary M, Pakzad R Comorbidities and mortality rate in
COVID-19 patients with hematological malignancies: A systematic review
and meta-analysis. J Clin Lab Anal. 2022;36(5):e24387. https://doi.org/10.1002/jcla.24387 PMID: 35385130
- Zhang
L, Zhu F, Xie L, Wang C, Wang J, Chen R, Jia P, Guan HQ, Peng L,
Chen Y, Peng P, Zhang P, Chu Q, Shen Q, Wang Y, Xu SY, Zhao JP, Zhou M.
Affiliations expand Clinical characteristics of COVID-19-infected
cancer patients: a retrospective case study in three hospitals within
Wuhan, China. Ann Oncol. 2020;31(7):894-901. https://doi.org/10.1016/j.annonc.2020.03.296 PMID: 32224151
- Ferrara
F, Zappasodi P, Roncoroni E, Borlenghi E, Rossi G. Impact of Covid-19
on the treatment of acute myeloid leukemia. Leukemia.
2020;34(8):2254-2256. https://doi.org/10.1038/s41375-020-0925-7 PMID: 32561842
- Civriz
Bozdağ S, Cengiz Seval G, Yönal Hindilerden İ, Hindilerden F, Andıç N,
Baydar M, Aydın Kaynar L, Toprak SK, Göksoy HS, Balık Aydın B, Demirci
U, Can F, Özkocaman V, Gündüz E, Güven ZT, Özkurt ZN, Demircioğlu S,
Beksaç M, İnce İ, Yılmaz U, Eroğlu Küçükdiler H, Abishov E, Yavuz B,
Ataş Ü, Mutlu YG, Baş V, Özkalemkaş F, Üsküdar Teke H, Gürsoy V, Çelik
S, Çiftçiler R, Yağcı M, Topçuoğlu P, Çeneli Ö, Abbasov H, Selim C, Ar
MC, Yücel OK, Sadri S, Albayrak C, Demir AM, Güler N, Keklik M, Terzi
H, Doğan A, Yegin ZA, Kurt Yüksel M, Sadri S, Yavaşoğlu İ, Beköz HS,
Aksu T, Maral S, Erol V, Kaynar L, İlhan O, Bolaman AZ, Sevindik ÖG,
Akyay A, Özcan M, Gürman G, Ünal Ş, Yavuz Y, Diz Küçükkaya R, Özsan GH.
Clinical Characteristics and Outcomes of COVID-19 in Turkish Patients
with Hematological Malignancies. Turk J Haematol. 2022;39(1):43-54. https://doi.org/10.4274/tjh.galenos.2021.2021.0287 PMID: 34521187
[TOP]





