 |
|
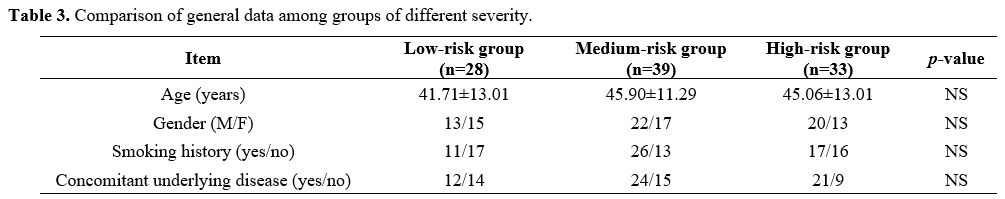
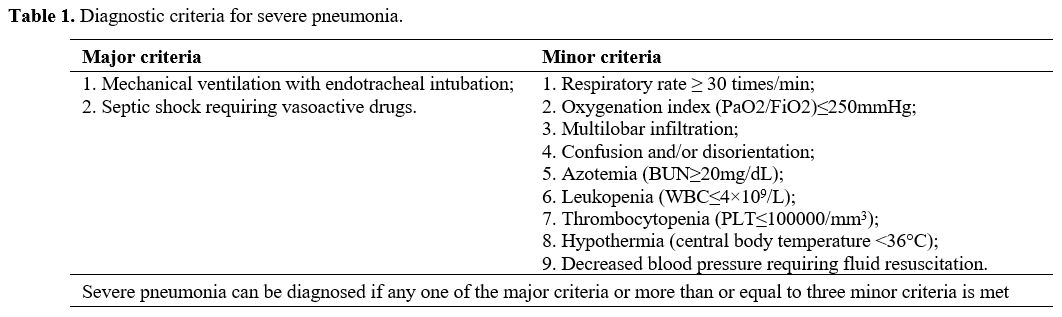
Collecting data. Electronic medical records were used to collect general information about all patients, including age, gender, smoking history, and underlying diseases. Acute Physiological and Chronic Health Score (APACHE II) was performed within 24 hours of admission to assess the severity of the disease.
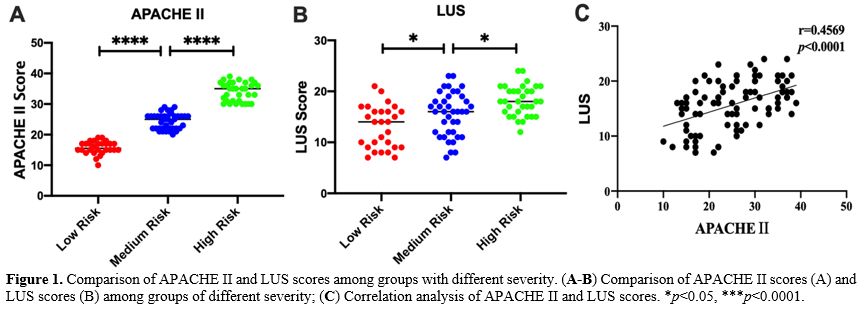
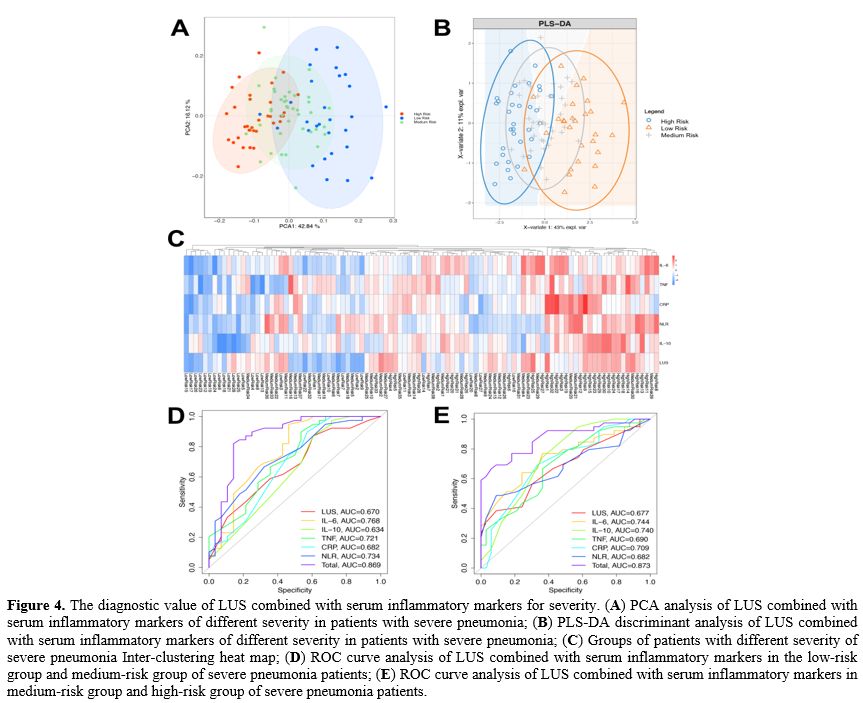
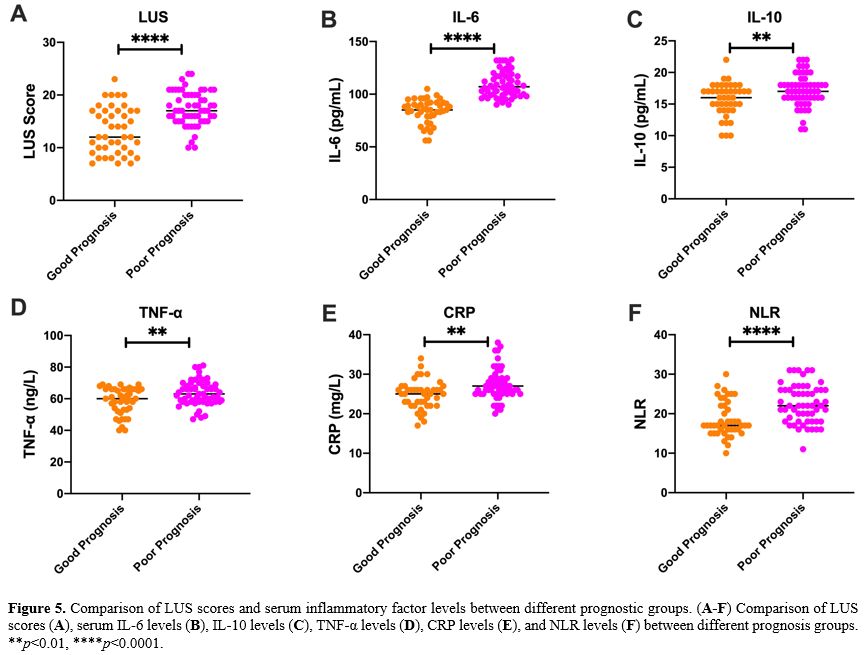
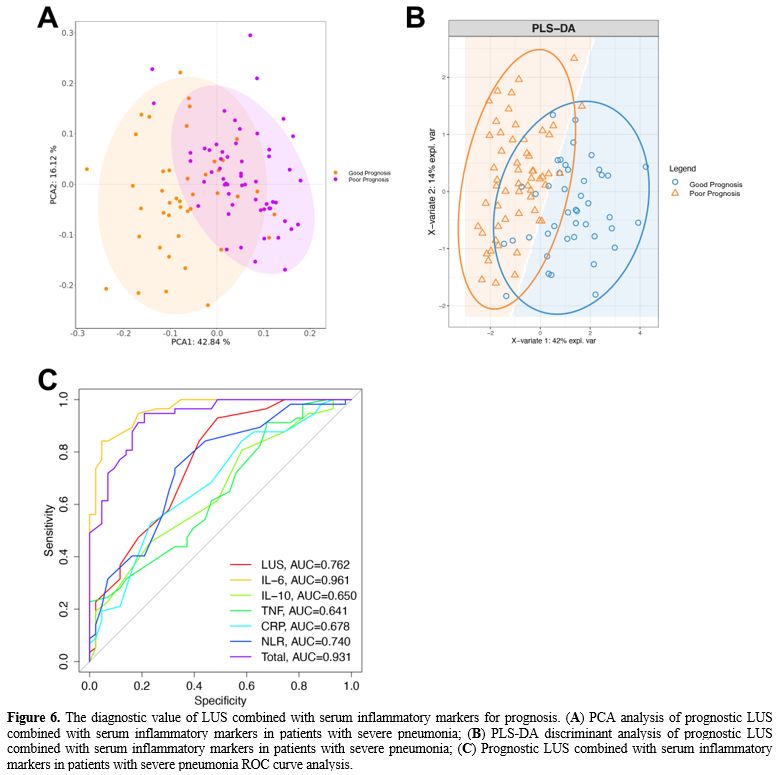
Grouping method. According to the severity of the disease, patients with severe pneumonia were divided into low-risk (LR) group (APACHE II score <20 points), medium-risk (MR) group (APACHE II score of 20-30 points) and high-risk (HR) group (APACHE II score ≥30 points). All patients were followed up for 30 days and were divided into a good prognosis (GP) group (recovered and discharged without sequelae) and a poor prognosis (PP) group (patients died or survived but had severe sequelae) according to their prognosis.
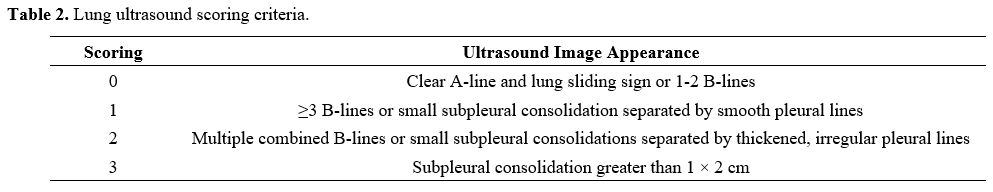
Lung Ultrasound Score (LUS). Lung ultrasonography was performed within 24 hours after admission using a SonoSound portable ultrasonograph (Sonosite, USA). The reference method was used to perform lung ultrasound division. The patient is supine and examined using the twelve-zone method. Each side is divided into three zones: anterior, lateral, and posterior, through the anterior and posterior axillary lines. Further division occurs within these zones; the three zones are divided into upper and lower parts at the horizontal position of the fourth rib space of the anterior chest (second or third rib space for women) connected by two nipples.[21,22]. Examination field: Examine each lung area in the longitudinal plane, examine each intercostal space present in this area in the transverse plane, and examine at least one complete respiratory cycle at each point. Each area was scored according to the examination results (Table 2), and all areas were aggregated to calculate the final score.[23] When there were multiple ultrasound findings in one area, the maximum value was taken. The lowest score is 0, and the highest score is 36. The higher the score, the more severe the mixed lung disease.
 |
|
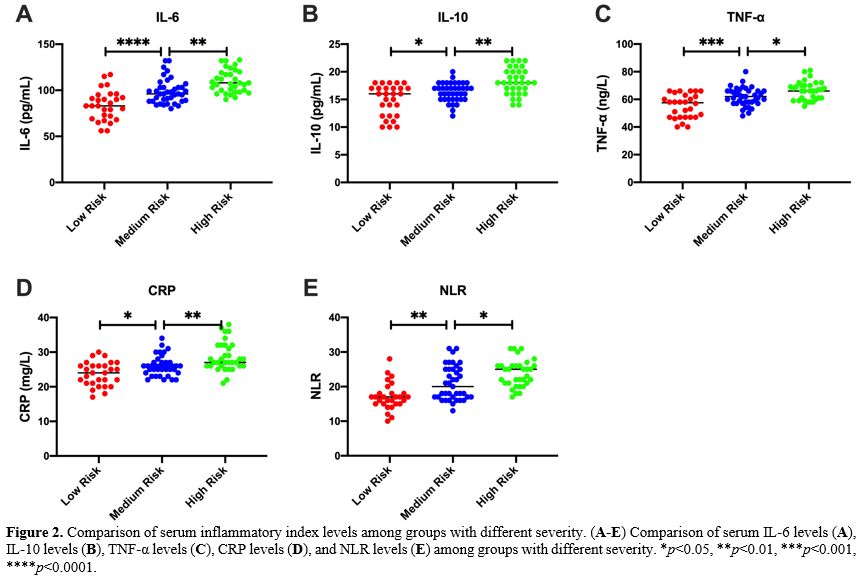
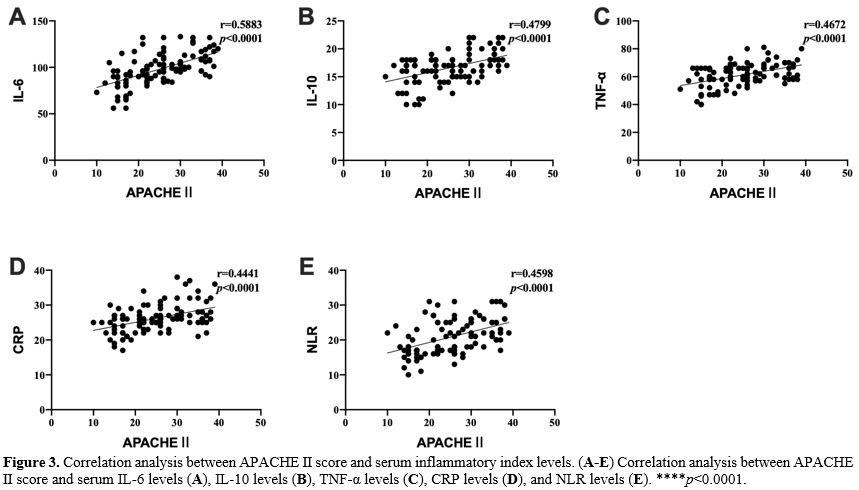
Detection of serum inflammatory markers. 5 mL of peripheral blood was drawn from the patient 24 h after admission, and serum samples were collected by centrifugation at 3000 g for 15 min. Serum IL-6, IL-10, TNF-α, CRP and NLR (neutrophil/lymphocyte) were detected using a Multiskan FC microplate reader (Thermo Company, USA) and ELISA kit (Nanjing et al., China). According to the ELISA kit instructions, add 50 μL of the diluted standards and samples to a 96-well plate and incubate at 37 °C for 30 min. After washing five times with the washing solution, add 50 μL of Reagent A (negative control tube) and 50 μL of Reagent B (assay tube) and incubated at 37 °C for 10 min, then 50 μL of stop solution was added to each well. Read the absorbance on a microplate reader and determine the sample concentration according to the standard curve.
Statistical methods. SPSS 22.0 statistical software was used to analyze and process the data, and GraphPad 8.0 software was used for drawing. T-test or chi-square test was used to compare two groups, and the one-way analysis of variance or Kruskal Wallis test was used to compare multiple groups. p < 0.05 was considered statistically significant.