Mehmet Hilmi Dogu1, Ali Irfan Emre Tekgunduz2, Burak Deveci3, Gulten Korkmaz4, Melda Comert5, Omur Gokmen Sevindik6, Osman Yokus7 and Istemi Serin7.
1 Department of Hematology, Istinye University, Faculty of Medicine, Liv Hospital Ulus, Istanbul, Turkey.
2 Department of Hematology, Istanbul Memorial Bahcelievler, Istanbul, Turkey.
3 Department of Hematology, Medstar Antalya Hospital, Antalya, Turkey.
4 Department of Hematology, Ankara City Hospital, Turkey.
5 Department of Hematology, University of Health Sciences, Gulhane Training and Research Hospital, Istanbul, Turkey.
6 Department of Hematology, Istanbul Medipol University, Faculty of Medicine, Istanbul, Turkey.
7 Department of Hematology, University of Health Sciences, Istanbul Training and Research Hospital, Istanbul, Turkey.
Correspondence to:
Istemi Serin, M.D. University of Health Sciences, Istanbul Training and
Research Hospital, Department of Hematology, Org. Nafiz GURMAN Cad.
34098, Fatih, Istanbul. Tel: +90 532 317 2393/+90 212 459 6330. E-mail:
serinistemi@hotmail.com
Published: May 1, 2023
Received: February 27, 2023
Accepted: April 18, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023031 DOI
10.4084/MJHID.2023.031
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objectives:
Gilteritinib (XOSPATA ®, Astellas) is a type I oral FLT3 inhibitor, a
tyrosine kinase A.X.L. inhibitor, involved in both c-Kit and FMS-like
tyrosine kinase 3 (FLT3) resistance. In the phase 3 ADMIRAL trial,
gilteritinib was compared with the standard of care in (R/R) acute
myeloid leukemia (A.M.L.) patients who harbored any FLT3 mutation and
showed superior efficacy with regard to response and survival.
Objectives:
This research aimed to investigate the real-life efficacy and safety of
gilteritinib in FLT3-positive R/R AML patients who were treated as a
part of an early access program held in Turkey in April 2020
(NCT03409081).
Results:
The research included 17 R/R AML patients who had received gilteritinib
from seven centers. The overall response rate was 100%. The most common
adverse events were anemia and hypokalemia (7 patients, 41.2%). Grade 4
thrombocytopenia was observed in one patient only (5.9%), leading to
permanent treatment discontinuation. Patients with peripheral edema had
a 10.47 (95% CI: 1.64-66.82) times higher risk of death than those
without peripheral edema (p<0.05).
Conclusion:
This research showed that patients with febrile neutropenia and
peripheral edema were at a high risk of death when compared to patients
without febrile neutropenia and peripheral edema.
|
Introduction
Prognosis
is relatively poor in relapsed or refractory (R/R) acute myeloid
leukemia (AML) that does not respond to standard induction
chemotherapy.[1-3] FMS-like tyrosine kinase 3 (FLT3)
is expressed in hematopoietic stem cells as a cytokine receptor
tyrosine kinase and plays a role in proliferation and differentiation.[4,5]
Approximately 25% of adult AML patients have FLT3-internal tandem
duplication (FLT3-ITD) mutations, while 10% have point mutations or
deletions of the FLT3 tyrosine kinase domain (FLT3-TKD).[4-6] FLT3-mutated AML patients are more susceptible to relapse than other AML patients.[4-6]
FLT3 tyrosine kinase inhibitors are used in the treatment of AML with different clinical activities.[7-9] As a first-generation FLT3 inhibitor, midostaurin was given combined with standard anthracycline-cytarabine therapy.[10]
In contrast to midostaurin and lestaurtinib, which are ineffective as
single agents, quizartinib and gilteritinib have proved to exert overt
clinical activity as monotherapies.[11,12]
Gilteritinib
(XOSPATA®, Astellas) is a type I oral FLT3 inhibitor, a tyrosine kinase
AXL inhibitor involved in both c-Kit and FLT3 resistance, as well as
FLT3-ITD and T.K.D. mutations.[13,14] The phase 1/2
study of gilteritinib monotherapy confirmed potent inhibition of
FLT3-provided receptor autophosphorylation at an 80 mg/day dose with an
overall response rate (ORR) of 52%.[14] In the phase 3 ADMIRAL trial,[11]
gilteritinib was compared with the standard of care in (R/R) AML
patients who harbored any FLT3 mutation and showed superior efficacy
with regard to ORR and survival.
This research aimed to
investigate the real-life efficacy and safety of gilteritinib in
FLT3-positive R/R AML patients who were treated as a part of an early
access program held in Turkey in April 2020 (NCT03409081).
Materials and Methods
The
research included 17 R/R AML patients who had received gilteritinib as
a part of the early access program from seven centers, allowing the use
of their data across Turkey between April 2020 and October 2021. In
addition to patient demographic and clinical characteristics, their
genetic risk classifications for AML, diagnostic information, response
to induction therapy, salvage therapy, exposure to FLT3 inhibitors
before gilteritinib (midostaurin or sorafenib), clinical course
following gilteritinib, adverse event profiles, and survival data
(overall survival (OS) and progression-free survival (PFS)) were
recorded.
The genetic classification was based on the 2022 ELN risk classification by genetics at initial diagnosis.[4]
The patients were required to have either FLT3-ITD or TKD (D835/I836)
mutations and all were ITD mutants. Responses to gilteritinib were
assessed on day 1 of cycle 2. The ORR was defined as complete remission
(CR), CR with incomplete platelet counts (CRp), CR with incomplete
hematologic recovery (CRi), and partial remission (PR). The preferred
dose of gilteritinib was the same in all patients, with a daily dose of
120 mg, which remained consistent throughout the follow-up period
without any necessary adjustments. Any drug-related adverse events of
any grade associated with gilteritinib were also noted.
While the
primary endpoints of the research were PFS and OS, other endpoints
included the factors affecting the adverse event profile and survival.
The research was reviewed and approved by the Ethics Committee of
Istanbul Training and Research Hospital (Date: 23.12.2022; approval
number: 402). It was conducted according to the principles of the
Declaration of Helsinki.
Statistical Methods. The central limit theorem is parametric without using a normality test based on fitness tests.[15]
However, nonparametric tests were used because the number of previous
treatment steps was an ordinal variable, and the deviation from the
mean of the time to progression and exitus was too high. The mean,
standard deviation, and minimum and maximum values were used in the
data analysis to generate the statistics in the continuous structure.
The frequency and percentage values were used to define categorical
variables. Student’s t-test and Mann-Whitney U test were used to
comparing the means of two independent groups. Chi-square/exact test
statistics were used to evaluate the relationship between categorical
variables. The Kaplan-Meier method was used to estimate overall
survival curves. The log-rank test was used to determine differences
according to risk factors, and the hazard ratio was given with a 95%
confidence interval. The statistical significance of the data was taken
as p<0.05. Data analysis was performed using IBM SPSS version 25 and
the MedCalc statistical software.
Results
Patients. The initial clinical characteristics, follow-up, and survival times of the patients are shown in Table 1.
The research included 17 patients who were diagnosed with AML The mean
age of the patients at diagnosis was 49.8±13.8 years, and the median
age was 55 years (range: 27-73). At the time of diagnosis, four
patients (23.5%) harbored any cytogenetic feature that placed them in
the adverse risk category, according to ELN 2022. The number of
patients who received allogeneic transplantation before gilteritinib
use was 2 (11.8%). Six patients were exposed to midostaurin before
gilteritinib, and only one had received both gilteritinib and sorafenib
simultaneously.
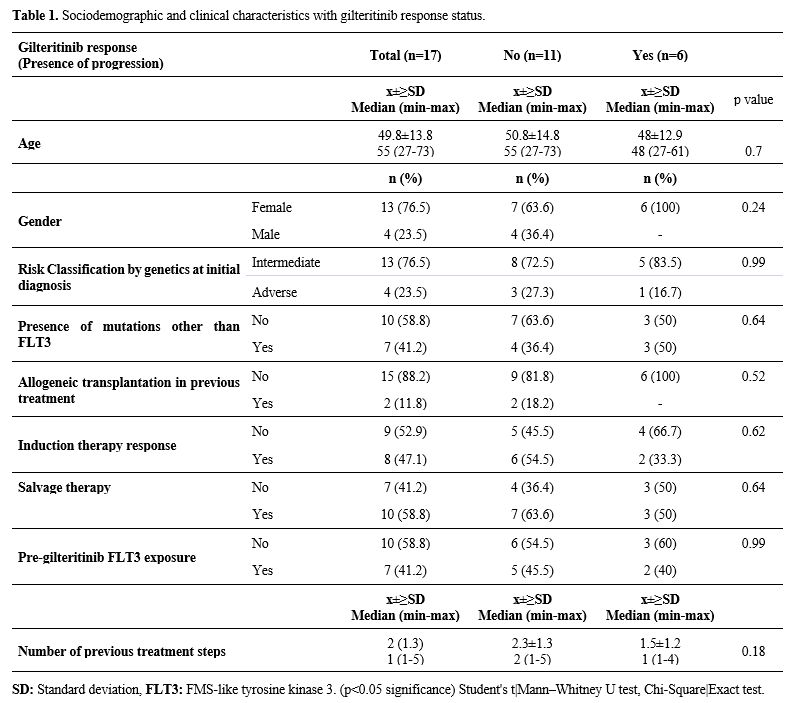 |
- Table
1. Sociodemographic and clinical characteristics with gilteritinib response status.
|
Efficacy.
The median duration of gilteritinib use was 8.5 (range: 1-21) months.
After using gilteritinib, seven patients (41.2%) received allogeneic
transplantation and two patients (11.8%) received maintenance therapy
after transplantation. All patients had at least PR with gilteritinib.
The ORR was 100% (nCR=11, 64.7%; nCRp=3, 17.6%; nCRi=1, 5.9%; nPR=2,
11.8%) (Table 2).
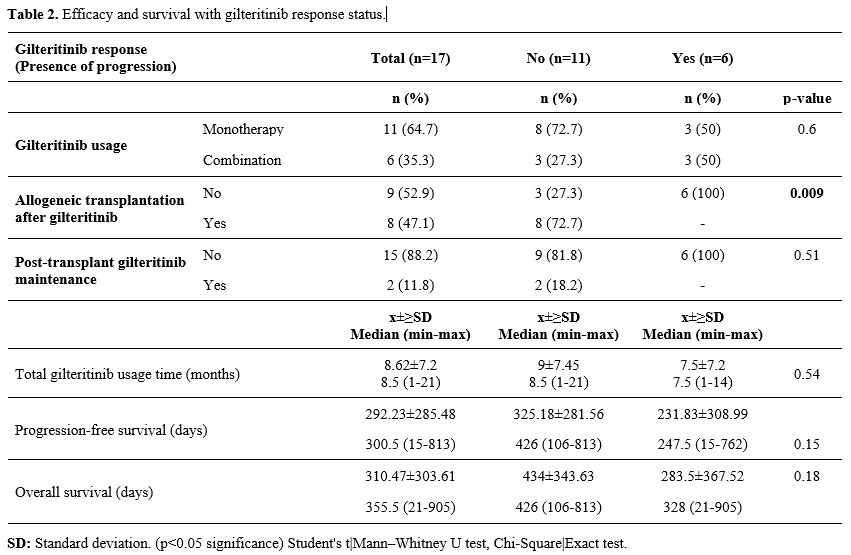 |
- Table 2. Efficacy and survival with gilteritinib response status.
|
Survival Analyses.
The median PFS was 300.5 (15-813) days, and the OS was 355.5 (21-905)
days with gilteritinib. The statistical analyses were conducted
according to the progression status during treatment and there was no
significant difference in the mean ages of the two groups (p>0.05).
Similarly, there was no significant difference between them in terms of
time to relapse, duration of gilteritinib use (months), time to
progression (days), or overall survival (days) (p>0.05) (Table 2, Figures 1 and 2).
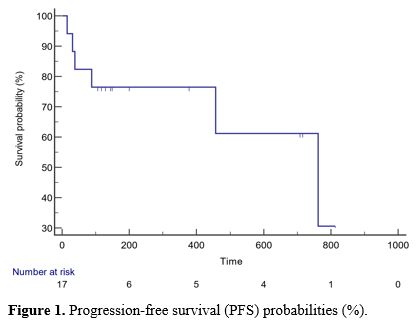 |
Figure 1. Progression-free survival (PFS) probabilities (%).
|
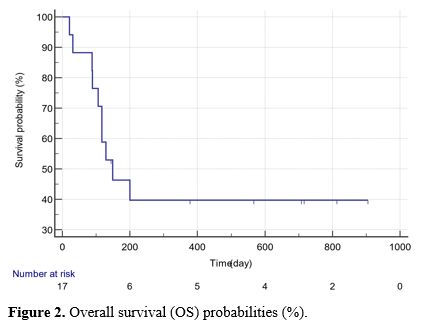 |
Figure 2. Overall survival (OS) probabilities (%).
|
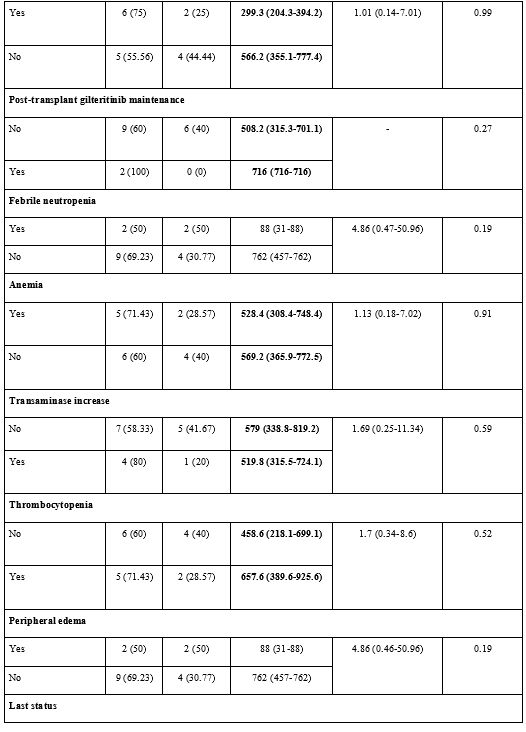
Adverse Events and Possible Effects on Survival.
The most common adverse events were anemia and hypokalemia (n=7,
41.2%). Grade 4 thrombocytopenia was observed in only one patient
(5.9%), which led to permanent treatment discontinuation; no other
patient experienced any dose reduction or curtailment (Supp. Table 1, Figure 3).
The median PFS of the patients with febrile neutropenia was 88 (95% CI:
31-88) days, while the median PFS of the patients without febrile
neutropenia was 762 (95% CI: 457-762) days (p=0.19). The median PFS of
patients with peripheral edema during gilteritinib treatment was 88
(95% CI: 31-88) days, while it was 762 (95% CI: 457-762) days in
patients without peripheral edema, with no significant difference
(p=0.19). Neither was there a significant PFS difference in terms of
adverse events (p>0.05) (Supp. Table 2, Figure 3).
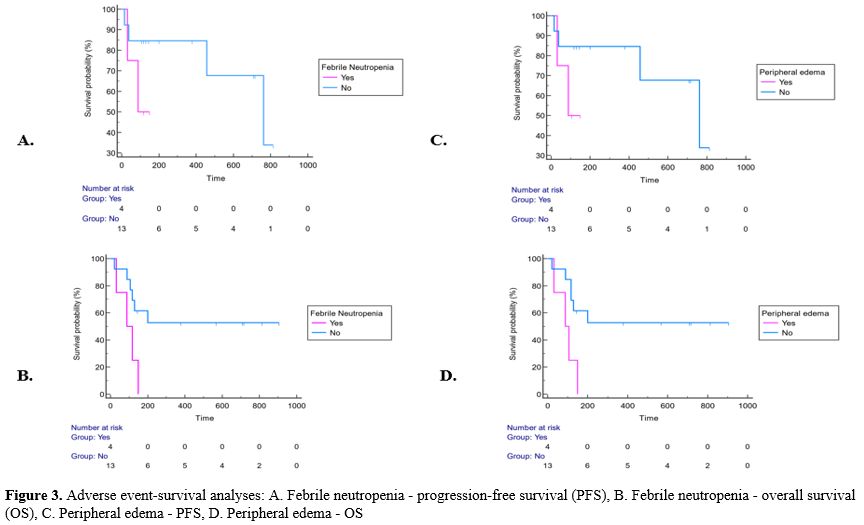 |
- Figure 3. Adverse
event-survival analyses: A. Febrile neutropenia - progression-free
survival (PFS), B. Febrile neutropenia - overall survival (OS), C.
Peripheral edema - PFS, D. Peripheral edema - OS
|
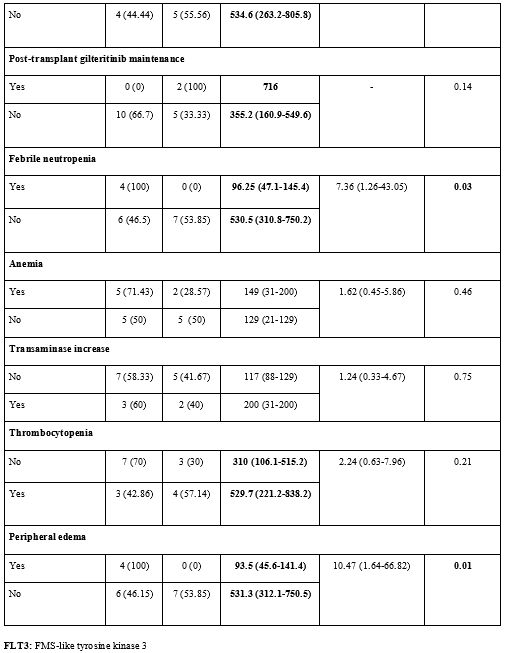
The
mean OS of patients with febrile neutropenia was 96.25 (95% CI:
47.1-145.4) days, while it was longer in patients without febrile
neutropenia and 530.5 (95% CI: 310.8-750.2) days (p=0.03). Febrile
neutropenia was found to shorten OS significantly. The risk of death in
patients with febrile neutropenia was 7.36 (95% CI: 1.26-43.05) times
higher than that in patients without febrile neutropenia (p<0.05).
While the mean OS was 93.5 (95% CI: 45.6-141.4) days in patients with
peripheral edema during gilteritinib treatment, it was longer with
531.3 (95% CI: 312.1-750.5) days in those without peripheral edema
(p=0.01). Patients with peripheral edema had a 10.47 (95% CI:
1.64-66.82) times higher risk of death than those without peripheral
edema (p<0.05) (Supp. Table 3, Figure 3).
Mortality.
Six patients experienced progression during the follow-up period
(35.3%), and 10 (58.8%) died eventually due to disease progression. No
fatal adverse event that could be possibly related to gilteritinib was
observed. Table 3 shows the
mortality rates of the patients according to their time intervals. The
30- and 60-day, 6-month, and 1-year mortality rates were 5.9% (n=1),
11.8% (n=2), 58.8% (n=10), and 64.7% (n=11), respectively.
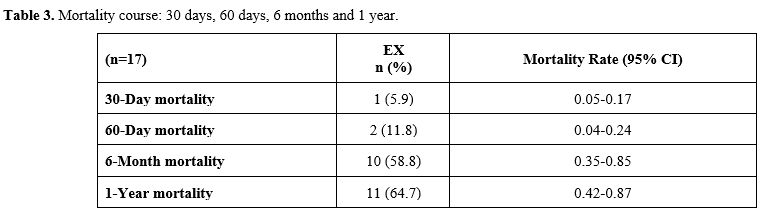 |
- Table 3. Mortality course: 30 days, 60 days, 6 months and 1 year.
|
Discussion
In
R/R AML patients, gilteritinib is considered a differentiating agent
and highly effective molecule. The present study, including Turkey’s
early access patient group, with similar features, superiorities and
differences, demonstrates the efficacy and treatment success of
gilteritinib in R/R AML patients with FLT3.
In the phase 1/2 trial of gilteritinib,[14]
the ORR in FLT3-mutated patient group was 49% at all doses (CR=9%,
CRp=5%, CRi=22%, PR=12%; n=191), while it was 52% (CR=11%, CRp=6%,
CRi=24%, PR=11%) in patients receiving ≥80 mg/day. In the ADMIRAL
trial,[11] the ORR was reported as 67.6%. The ORR was
100% (nCR=11, 64.7%; nCRp=3, 17.6%; nCRi=1, 5.9%; nPR=2, 11.8%) in
our research group, which was quite higher compared to the literature
counterparts.
In the phase 1/2 trial, the median PFS was 20 (95% CI: 14-33) and the median OS was 30 weeks (23-33).[14]
In the phase 3 ADMIRAL trial, the median PFS was 2.8 (1.4-3.7), and the
median OS was 9.3 (7.7-10.7) months in the gilteritinib group.[11]
In our research group, the median PFS was 300.5 (range: 15-813), and
the OS was 355.5 (range: 21-905) days under the treatment of
gilteritinib. Compared to phase 1/2 and phase 3 ADMIRAL studies, the
survival times in our research group were significantly longer, with
more prominent PFS.
In the phase 1/2 trial, the most common grade
3/4 adverse events were reported to be febrile neutropenia (n=97/252,
38%), anemia (n=61/252, 24%), thrombocytopenia (n=33/252, 13%), sepsis
(n=28/252, 11%) and pneumonia (n=27/252, 11%). Serious adverse events
in ≥5% of patients were febrile neutropenia (n=78/252, 31%),
progressive disease (n=43/252, 17%), sepsis (n=36/252, 14%), pneumonia
(n=27/252, 11%), acute renal failure (n=25/252, 10%), pyrexia
(n=21/252, 8%), bacteremia (n=14/252, 6%) and respiratory failure
(n=14/252, 6%). The most common reasons for treatment discontinuation
were disease progression (n=15/252, 6%) and sepsis (n=7/252, 3%). The
most common adverse event that led to treatment discontinuation was
elevated blood creatinine kinase (n=3/252, 1.2%).[14]
In the ADMIRAL trial, the most common grade 3 or higher adverse events
were febrile neutropenia (n=113, 45.9%), anemia (n=100, 40.7%), and
thrombocytopenia (n=56, 22.8%). The most common reasons for the
discontinuation of treatment with gilteritinib were relapse,
progression, lack of efficacy (50.2%), death (14.6%), and adverse
events (11.3%). Drug-related adverse events leading to the
discontinuation of gilteritinib occurred in 27 patients (11.0%). The
most common events were elevated aspartate aminotransferase levels
(n=4, 1.6%), elevated alanine aminotransferase levels (n=3, 1.2%), and
pneumonia (n=3, 1.2%).[11] In our patient group, the
most common adverse events were anemia and hypokalemia (n=7, 41.2%),
and the only adverse event with grade 3 or higher was thrombocytopenia
in one patient (5.9%). The only adverse event that led to treatment
discontinuation was thrombocytopenia (n=1, 5.9%).
Another
important finding of the research was the effect of adverse events on
survival. Patients with febrile neutropenia had a 7.36 (95% CI:
1.26-43.05) times higher risk of death than those without febrile
neutropenia. Patients with peripheral edema were found to have a 10.47
(95% CI: 1.64-66.82) times higher risk of death than patients without
peripheral edema. The OS of patients with febrile neutropenia is
expected to be significantly shorter. Considering that sepsis and
infections are some of the most common adverse events associated with
gilteritinib, the shortening of OS may be considered a natural outcome.
Peripheral edema has been associated with differentiation syndrome.[16-18]
Although there is no clear evidence, the literature has associated
patients without gilteritinib-related differentiation syndrome and
findings with improved OS and complete morphologic remission.[18]
However, not all FLT3 inhibitors are the same, and quizartinib without
differentiation is characterized by hypocellular bone marrow and
incomplete peripheral blood cell recovery.[19]
Peripheral edema and fluid retention may be a subclinical manifestation
of the differentiation syndrome that occurs independently of the
differentiation syndrome and may be associated with longer overall
survival. Gilteritinib-related cardiotoxicity has rarely been reported.
Although the definite effect is unknown,[20] gilteritinib-related cardiotoxicity was not observed in our cases, so peripheral edema was not associated with cardiotoxicity.
In
the phase 1/2 trial, a total of seven deaths were observed, which were
considered to be possibly related to treatment. Mortality was
attributed to pulmonary embolism, respiratory failure, hemoptysis,
intracranial hemorrhage, ventricular fibrillation, septic shock, and
neutropenia.[14] In the ADMIRAL trial, there were 170
deaths among 246 patients (69.1%). In the intention-to-treat
population, mortality at 30 and 60 days in the gilteritinib group was
2.0% and 7.7%, respectively. Common fatal adverse events in the
gilteritinib group were disease progression (n=30, 12.2%) and
infections (n=28, 11.4%). The most common fatal adverse events
considered possibly related to gilteritinib were pneumonia (n=3, 1.2%),
large intestine perforation (n=2, 0.8%), and septic shock (n=2, 0.8%).[11]
In our research group, the 30- and 60-day mortality rates were 5.9%
(n=1) and 11.8% (n=2), with no fatal adverse event possibly related to
gilteritinib.
The main limitation of the research was the
limited number of patients, as it consisted of patients in the early
access program only.
Conclusions
The
ORR was 100%, the median PFS under gilteritinib was 300.5 (range:
15-813), and the OS was 355.5 (range: 21-905) days. Compared to phase
1/2 and phase 3 studies, the survival times were significantly longer,
with more prominent PFS. The most common adverse events were anemia and
hypokalemia. Grade 4 thrombocytopenia was observed in only one patient,
which led to permanent treatment discontinuation, and no other patient
experienced any dose reduction or curtailment. Patients with febrile
neutropenia and peripheral edema were at a high risk of death when
compared to patients without febrile neutropenia and peripheral edema.
No fatal adverse event possibly related to gilteritinib was observed.
Availability of Data and Materials
Ethics Approval and Consent to Participate
Ethical
committee approval was received from the Istanbul Training and Research
Hospital Ethics Committee (Date: 23.12.2022; approval number: 402). The
experimental procedures were based on the Declaration of Helsinki and
relevant institutional regulations.
Authors’ Contributions
M.H.D.:
Conceptualization, Methodology, Software. I.S.: Data curation, Writing-
Original draft preparation. B.D., G.K., M.C., O.G.S., O.Y., and M.H.D.:
Software, Validation, Writing- Reviewing and Editing.
Acknowledgments
We are grateful to Ekin EKICI for her great support in the writing and editing process of the manuscript.
References
- Roboz GJ, Rosenblat T, Arellano M, Gobbi M, Altman
JK, Montesinos P, O'Connell C, Solomon SR, Pigneux A, Vey N, Hills R,
Jacobsen TF, Gianella-Borradori A, Foss Ø, Vetrhusand S, Giles FJ.
International randomized phase III study of elacytarabine versus
investigator choice in patients with relapsed/refractory acute myeloid
leukemia. J Clin Oncol. 2014; 32: 1919-1926. https://doi.org/10.1200/JCO.2013.52.8562 PMid:24841975
- Megías-Vericat
JE, Martínez-Cuadrón D, Sanz MÁ, Montesinos P. Salvage regimens using
conventional chemotherapy agents for relapsed/refractory adult AML
patients: a systematic literature review. Ann Hematol. 2018; 97:
1115-1153. https://doi.org/10.1007/s00277-018-3304-y PMid:29680875
- Ravandi
F, Ritchie EK, Sayar H, Lancet JE, Craig MD, Vey N, Strickland SA,
Schiller GJ, Jabbour E, Erba HP, Pigneux A, Horst HA, Recher C, Klimek
VM, Cortes J, Roboz GJ, Odenike O, Thomas X, Havelange V, Maertens J,
Derigs HG, Heuser M, Damon L, Powell BL, Gaidano G, Carella AM, Wei A,
Hogge D, Craig AR, Fox JA, Ward R, Smith JA, Acton G, Mehta C, Stuart
RK, Kantarjian HM. Vosaroxin plus cytarabine versus placebo plus
cytarabine in patients with first relapsed or refractory acute myeloid
leukaemia (VALOR): a randomised, controlled, double-blind,
multinational, phase 3 study. Lancet Oncol. 2015; 16:1025-1036. https://doi.org/10.1016/S1470-2045(15)00201-6 PMid:26234174
- Döhner
H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, Ebert BL,
Fenaux P, Godley LA, Hasserjian RP, Larson RA, Levine RL, Miyazaki Y,
Niederwieser D, Ossenkoppele G, Röllig C, Sierra J, Stein EM, Tallman
MS, Tien HF, Wang J, Wierzbowska A, Löwenberg B. Diagnosis and
management of AML in adults: 2022 recommendations from an international
expert panel on behalf of the ELN. Blood. 2022; 140: 1345-1377. https://doi.org/10.1182/blood.2022016867 PMid:35797463
- Kelly
LM, Yu JC, Boulton CL, Apatira M, Li J, Sullivan CM, Williams I, Amaral
SM, Curley DP, Duclos N, Neuberg D, Scarborough RM, Pandey A,
Hollenbach S, Abe K, Lokker NA, Gilliland DG, Giese NA. CT53518, a
novel selective FLT3 antagonist for the treatment of acute myelogenous
leukemia (AML). Cancer Cell. 2002; 1: 421-432. https://doi.org/10.1016/S1535-6108(02)00070-3 PMid:12124172
- Hassanein
M, Almahayni MH, Ahmed SO, Gaballa S, El Fakih R. FLT3 Inhibitors for
Treating Acute Myeloid Leukemia. Clin Lymphoma Myeloma Leuk. 2016; 16:
543-549. https://doi.org/10.1016/j.clml.2016.06.002 PMid:27450971
- Fischer
T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, Fox E, Ehninger
G, Feldman EJ, Schiller GJ, Klimek VM, Nimer SD, Gilliland DG, Dutreix
C, Huntsman-Labed A, Virkus J, Giles FJ. Phase IIB trial of oral
Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3)
and multi-targeted kinase inhibitor, in patients with acute myeloid
leukemia and high-risk myelodysplastic syndrome with either wild-type
or mutated FLT3. J Clin Oncol. 2010; 28: 4339-4345. https://doi.org/10.1200/JCO.2010.28.9678 PMid:20733134 PMCid:PMC4135183
- Cortes
J, Perl AE, Döhner H, Kantarjian H, Martinelli G, Kovacsovics T,
Rousselot P, Steffen B, Dombret H, Estey E, Strickland S, Altman JK,
Baldus CD, Burnett A, Krämer A, Russell N, Shah NP, Smith CC, Wang ES,
Ifrah N, Gammon G, Trone D, Lazzaretto D, Levis M. Quizartinib, an FLT3
inhibitor, as monotherapy in patients with relapsed or refractory acute
myeloid leukaemia: an open-label, multicentre, single-arm, phase 2
trial. Lancet Oncol. 2018; 19: 889-903. https://doi.org/10.1016/S1470-2045(18)30240-7 PMid:29859851
- Stone
RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede
C, Prior TW, Döhner K, Marcucci G, Lo-Coco F, Klisovic RB, Wei A,
Sierra J, Sanz MA, Brandwein JM, de Witte T, Niederwieser D, Appelbaum
FR, Medeiros BC, Tallman MS, Krauter J, Schlenk RF, Ganser A, Serve H,
Ehninger G, Amadori S, Larson RA, Döhner H. Midostaurin plus
Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J
Med. 2017; 377: 454-464. https://doi.org/10.1056/NEJMoa1614359 PMid:28644114 PMCid:PMC5754190
- Cortes
JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, Krämer A,
Dombret H, Hogge D, Jonas BA, Leung AY, Mehta P, Montesinos P, Radsak
M, Sica S, Arunachalam M, Holmes M, Kobayashi K, Namuyinga R, Ge N,
Yver A, Zhang Y, Levis MJ. Quizartinib versus salvage chemotherapy in
relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a
multicentre, randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 2019; 20: 984-997. https://doi.org/10.1016/S1470-2045(19)30150-0 PMid:31175001
- Perl
AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S,
Montesinos P, Baer MR, Larson RA, Ustun C, Fabbiano F, Erba HP, Di
Stasi A, Stuart R, Olin R, Kasner M, Ciceri F, Chou WC, Podoltsev N,
Recher C, Yokoyama H, Hosono N, Yoon SS, Lee JH, Pardee T, Fathi AT,
Liu C, Hasabou N, Liu X, Bahceci E, Levis MJ. Gilteritinib or
Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med.
2019; 381: 1728-1740. https://doi.org/10.1056/NEJMoa1902688 PMid:31665578
- Lee
LY, Hernandez D, Rajkhowa T, Smith SC, Raman JR, Nguyen B, Small D,
Levis M. Preclinical studies of gilteritinib, a next-generation FLT3
inhibitor. Blood. 2017; 129: 257-260. https://doi.org/10.1182/blood-2016-10-745133 PMid:27908881 PMCid:PMC5234222
- Mori
M, Kaneko N, Ueno Y, Yamada M, Tanaka R, Saito R, Shimada I, Mori K,
Kuromitsu S. Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic
activity in mouse models of FLT3 mutated acute myeloid leukemia. Invest
New Drugs. 2017; 35: 556-565. https://doi.org/10.1007/s10637-017-0470-z PMid:28516360 PMCid:PMC5613053
- Perl
AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, Claxton D, Erba
HP, Gill S, Goldberg S, Jurcic JG, Larson RA, Liu C, Ritchie E,
Schiller G, Spira AI, Strickland SA, Tibes R, Ustun C, Wang ES, Stuart
R, Röllig C, Neubauer A, Martinelli G, Bahceci E, Levis M. Selective
inhibition of FLT3 by gilteritinib in relapsed or refractory acute
myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2
study. Lancet Oncol. 2017; 18: 1061-1075. https://doi.org/10.1016/S1470-2045(17)30416-3 PMid:28645776
- Norman
G. Likert scales, levels of measurement and the "laws" of statistics.
Adv Health Sci Educ Theory Pract. 2010; 15: 625-632. https://doi.org/10.1007/s10459-010-9222-y PMid:20146096
- McMahon
CM, Canaani J, Rea B, Sargent RL, Qualtieri JN, Watt CD, Morrissette
JJD, Carroll M, Perl AE. Gilteritinib induces differentiation in
relapsed and refractory FLT3-mutated acute myeloid leukemia. Blood Adv.
2019; 3: 1581-1585. https://doi.org/10.1182/bloodadvances.2018029496 PMid:31122910 PMCid:PMC6538870
- Kondo
T, Onozawa M, Fujisawa S, Harada S, Ogasawara R, Izumiyama K, Saito M,
Morioka M, Mori A, Teshima T. Myelomonocytic differentiation of
leukemic blasts accompanied by differentiation syndrome in a case of
FLT3-ITD-positive AML treated with gilteritinib. Hematology. 2021; 26:
256-260. https://doi.org/10.1080/16078454.2021.1889111 PMid:33631087
- Sexauer
A, Perl A, Yang X, Borowitz M, Gocke C, Rajkhowa T, Thiede C, Frattini
M, Nybakken GE, Pratz K, Karp J, Smith BD, Levis M. Terminal myeloid
differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML.
Blood. 2012; 120: 4205-4214. https://doi.org/10.1182/blood-2012-01-402545 PMid:23012328 PMCid:PMC3501718
- Nybakken
GE, Canaani J, Roy D, Morrissette JD, Watt CD, Shah NP, Smith CC, Bagg
A, Carroll M, Perl AE. Quizartinib elicits differential responses that
correlate with karyotype and genotype of the leukemic clone. Leukemia.
2016; 30: 1422-1425. https://doi.org/10.1038/leu.2015.320 PMid:26585411 PMCid:PMC4927337
- Kim
L, Fowler B, Campbell CM, Slivnick J, Nawaz H, Kaka Y, Ruz P, Vallakati
A, Baliga R, Vasu S, Addison D. Acute cardiotoxicity after initiation
of the novel tyrosine kinase inhibitor gilteritinib for acute myeloid
leukemia. Cardiooncology. 2021; 7: 36. https://doi.org/10.1186/s40959-021-00122-x PMid:34686212 PMCid:PMC8531894
Supplementary Tables
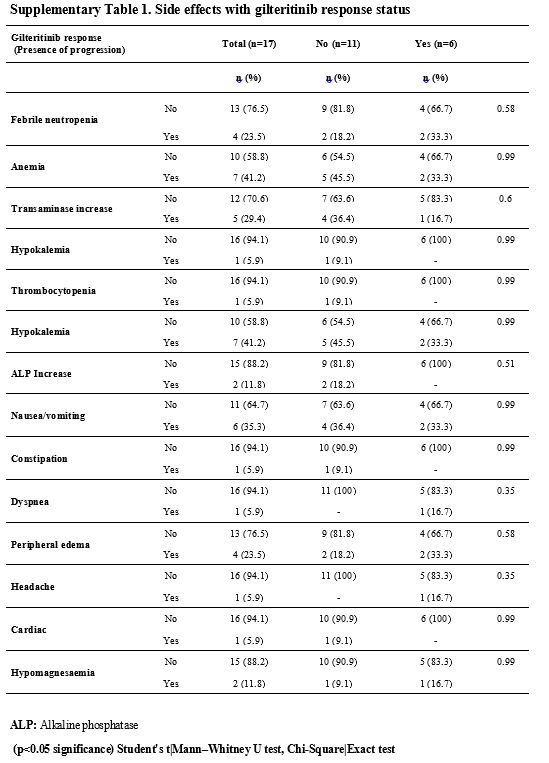 |
- Supplementary Table 1. Side effects with gilteritinib response status
|
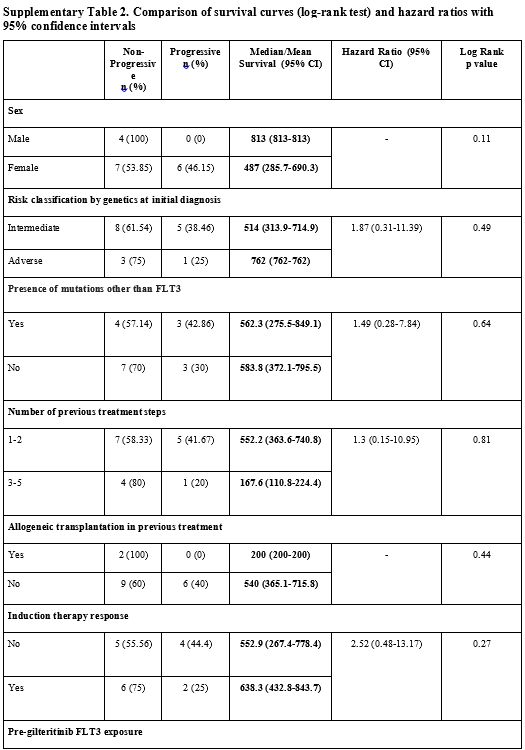 |
Supplementary Table 2. Comparison of survival curves (log-rank test) and hazard ratios with 95% confidence intervals
|
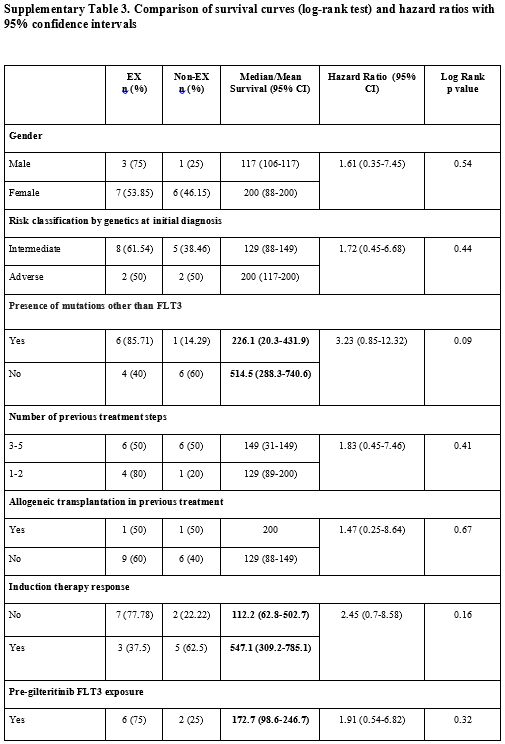 |
Supplementary Table 3. Comparison of survival curves (log-rank test) and hazard ratios with 95% confidence intervals
|