Francesca Fazio1, Gianfranco Lapietra1, Maria Zaira Limongi1, Stefania Intoppa1, Maria Laura Milani1, Alfonso Piciocchi3, Maurizio Martelli1, Anna Guarini2, Robin Foà1, Maria Stefania De Propris1 and Maria Teresa Petrucci1.
1 Hematology, Department of Translational and Precision Medicine, Sapienza University, 00161 Rome, Italy
2 Department of Molecular Medicine, Sapienza University, Rome, Italy.
3 Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) Data Center, Fondazione GIMEMA Franco Mandelli Onlus.
Correspondence to:
Francesca Fazio, MD, Hematology, Department of Translational and
Precision Medicine, Sapienza University of Rome, Via Benevento 6, 00161
Rome, Italy. Email:
fazio@bce.uniroma1.it
Published: September 1, 2023
Received: May 17, 2023
Accepted: August 8, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023047 DOI
10.4084/MJHID.2023.047
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Multiple myeloma (MM) is a
heterogeneous malignancy characterized by the proliferation of abnormal
plasma cells in the bone marrow. Multiparametric flow cytometry (MFC)
plays a role in the work-up of the disease in view of the aberrant
expression of surface antigens. Our study aimed at describing the
antigenic profile detected by MFC in a series of newly diagnosed MM
patients to correlate the level of expression with other features of
the disease. Between April 2018 and June 2022, 84 consecutive MM
patients were studied at presentation. CD56 and CD117 were commonly
detected, while CD45, CD28, CD20, CD19, CD13 and CD33 were less
recurrent. CD20 expression was associated with the type of secretory MM
(p=0.041) and with a higher disease burden (p=0.038). CD28 positivity
correlated with a lower platelet count at baseline (p=0.005) and with a
lower rate of complete response (p=0.038). Furthermore, CD28 positivity
and a lower CD138 expression tended to associate with the high-risk
chromosomal translocations t(14;16) and t(4;14). The results of this
study indicate that in the diagnostic work-up of MM, MFC may help to
identify different patient subsets and improve risk stratification.
These observations need to be validated in larger series of patients
with a longer follow-up.
|
Introduction
Multiple
myeloma (MM) is a heterogeneous disorder characterized by the expansion
of clonal plasma cells (PCs) in the bone marrow (BM), often associated
with a detectable monoclonal immunoglobulin in the serum and/or urine.
The European Myeloma Network has underlined the clinical utility of
multiparametric flow-cytometry (MFC) analysis in the diagnostic work-up
and follow-up of MM patients.[1,2] The prerequisite of MFC in MM is to discriminate within the whole PC compartment between normal and aberrant clonal PCs.
PCs
are considered end-stage B cells, lacking surface expression of the
most common markers of the B-cell lineage, such as CD22, CD20, and
surface membrane immunoglobulins. Clonal PCs show a heterogeneous
expression of CD19, CD45lo, and CD56−/lo, together with high amounts of
CD38, CD138, and c(cytoplasmic)VS38.[3,4] Their
identification is favored by the concomitant expression of other
surface antigens, such as CD28, CD20, CD33, CD13, CD117, and CD56.[5,6]
Specific panels of antibody combinations have been designed, and the
definition of clonal PCs is established due to the variable association
of these antigens with cytoplasmic immunoglobulin k or λ chain
staining. The prognostic impact of the immunophenotypic profile of
clonal PCs has been suggested based on the results of a large series of
transplant-eligible newly diagnosed MM patients treated with high-dose
chemotherapy followed by autologous stem cell transplantation (ASCT).
The expression of CD19 and CD28, as well as the absence of CD117 on
clonal PCs, have been associated with a shorter time to progression.[7]
The
aim of our study was to assess the immunophenotypic characteristics of
clonal PCs on a consecutive series of newly diagnosed MM patients
managed at our Center and to investigate the possible correlation
between the aberrant phenotype, the clinical characteristics of the
disease, and cytogenetic abnormalities.
Materials and Methods
Between
April 2018 and June 2022, we analyzed BM samples from 84 consecutive
newly diagnosed MM patients managed at the Hematology Center of the
Sapienza University of Rome by flow cytometry. Informed consent was
obtained from all individual participants, and the study was in
accordance with the ethical standard of the institutional national
research committee and the 1964 Helsinki Declaration.
Bone marrow
samples in sodium citrate were required for each patient, and MFC was
performed after erythrocyte-lysis. The samples were quickly processed,
considering that down-regulation of CD138 expression has been
demonstrated on aged PC samples. MFC immunophenotyping study was
conducted using an 8-12 color combination of the following monoclonal
antibodies (CD45/CD38/CD138/CD19/CD20/CD28/CD56/CD117/CD13/CD33/cVS38/cIgkappa/cIglambda),
using the FACSCanto II/FACSLyric flow cytometers and the
PAINT-A-GATE/FACSDIVA software. Specifically, the antigen expression
was considered positive if more than 10% of PC displayed a level of
expression. The specific antibody combinations of each staining tube
with markers and their respective fluorochromes are summarized in Table 1.
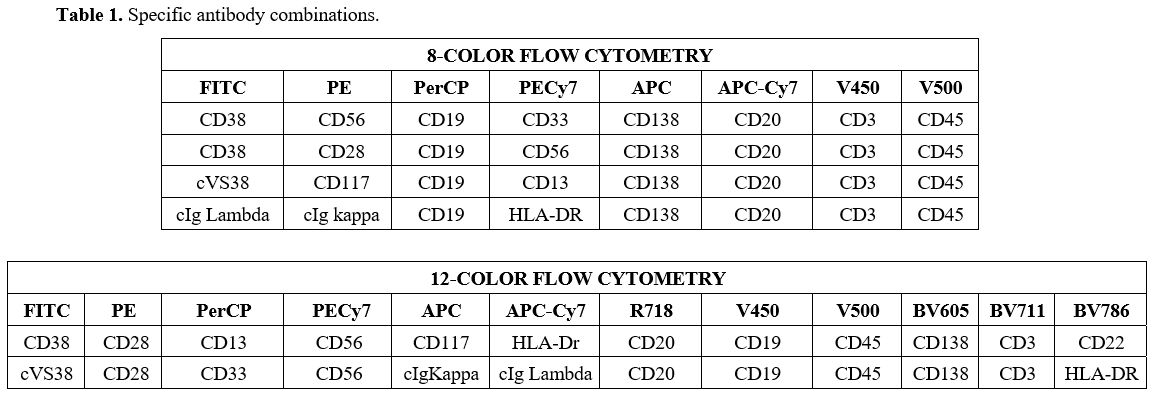 |
- Table 1. Specific antibody combinations.
|
For
the intracytoplasmic staining of cIg kappa, cIg lambda, and cVS38, a BD
fixation and permeabilization KIT was used, followed by labeling with
specific antibodies (BD Intrasure KIT).
As recommended, the
combination of CD38 and CD138 was used to identify PCs in MM. For an
optimized exclusion of other non-PC populations potentially
contaminating the CD38hi CD138+ PC gate, CD45 was simultaneously
stained, in addition to sideward (SSC) and forward (FSC) light scatter.
Within this population, the Ig light chain Kappa/Lambda ratio was used
for discriminating between clonal aberrant cells and their normal
counterparts. In rare events, at least 500,000 total cells were
acquired with a plasma cell identification cluster of at least 50 cells.
Fluorescence
in situ hybridization (FISH) was performed on purified PCs by
immune-magnetic separation using anti-CD38 microbeads.
Patients'
characteristics were summarized using cross-tabulations for categorical
variables or utilizing median and range for continuous variables.
Non-parametric tests were performed for comparisons between groups:
Chi-Squared and Fisher Exact test in case of categorical variables,
Mann-Whitney and Kruskal-Wallis test in case of continuous variables.
All analysis was performed using R software (R: A language and
environment for statistical computing. R Foundation for Statistical
Computing, Vienna, Austria).
Results
Fifty-two
newly diagnosed MM patients were males and 32 females. The median age
was 61 (28-88); 30 patients were 65. Patients’ risk stratification was
based on the International Staging System (ISS) and revised ISS
(R-ISS).[8] Thirty-eight % of patients were ISS I, and
46% were R-ISS II. Fifteen % of patients showed high-risk cytogenetic
abnormalities, according to R-ISS [del17p, t(4;14) and t(14,16)]. The
number of high-risk patients increased (43%), including those harboring
amp1q and gain1q.
Eighty-five % of patients were considered
eligible for ASCT. The median BM PCs observed by conventional
cytomorphology staining from bone marrow aspirate was 30% (10-90%). As
expected, the median of BM PCs detected by MFC was lower (8%, range
0.1-94%), probably due to a dilution effect. The main therapeutic
regimens used were bortezomib-based combinations, such as VTd
(bortezomib, thalidomide, and dexamethasone) in transplant-eligible
patients and VMP (bortezomib, melphalan, and prednisone) in
transplant-ineligible patients. The overall response rate of the entire
cohort was 85%. The baseline clinical characteristics and the frontline
induction treatments of the 84 MM patients are summarized in Tables 2 and 3, respectively.
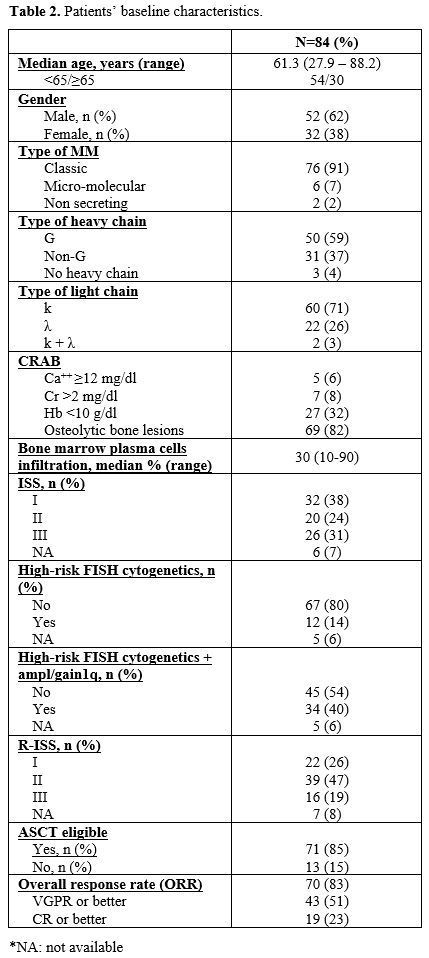 |
Table 2. Patients’ baseline characteristics. |
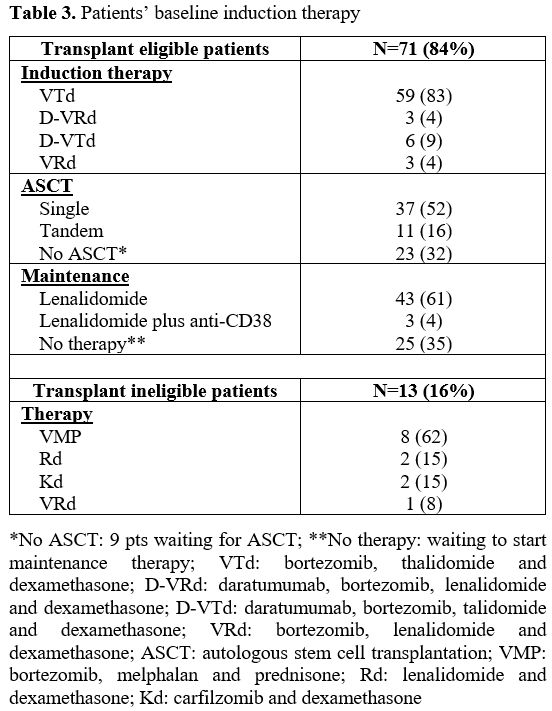 |
Table 3, Patients’ baseline induction therapy |
BM
clonal PCs from a minority of patients in our cohort presented early
B-cell maturation antigen expression, such as CD19 (2%). CD20 and CD45
were detected in 17% and 52% of the clonal PCs, respectively. In 69% of
cases, BM PCs showed a bright CD56 surface expression. Among the
remaining patients, 1% showed a reduced reactivity for CD56, while CD56
was completely negative in the other cases (30%). CD117 was detected in
42% of clonal BM PCs, while CD28 and CD33 were detected in 15% and 5%
of clonal PCs, respectively.
When considering unusual antigens
on the surface of aberrant PCs (CD28, CD20, and CD45), we observed that
the expression of CD28 was mutually exclusive compared to CD56
(p<0.001). In addition, the presence of CD20 was associated with the
absence of CD28 (p=0.048). We then investigated the correlation between
CD28, CD20, and CD45 expression on clonal PCs with the patient's
characteristics and response to treatment. Expression of CD28 on clonal
PCs was associated with a significantly lower median number of
platelets at baseline [192x103 vs. 218x103
(p=0.005)], even if this difference was not clinically relevant, and
with a significantly reduced percentage of MM patients achieving a
complete response [25% vs. 66% (p=0.038)]. Focusing on high-risk
chromosomal aberrations, t(14;16) tended to associate with CD28
expression (p=0.079), while t(4;14) tended to associate with a lower
median value of CD138 mean fluorescence intensity (MFI) [974 vs 1745
(p=0.58)]. We also observed that CD20 expression on clonal PCs (18% of
all patients) was associated with the type of secretory MM compared to
non-secretory MM (p=0.041). Furthermore, patients with CD2O expression
showed a higher median level of serum monoclonal protein at baseline
compared to patients lacking CD20 [3.86 g/dl vs 2.42 g/dl (p=0.038)] (Table 4).
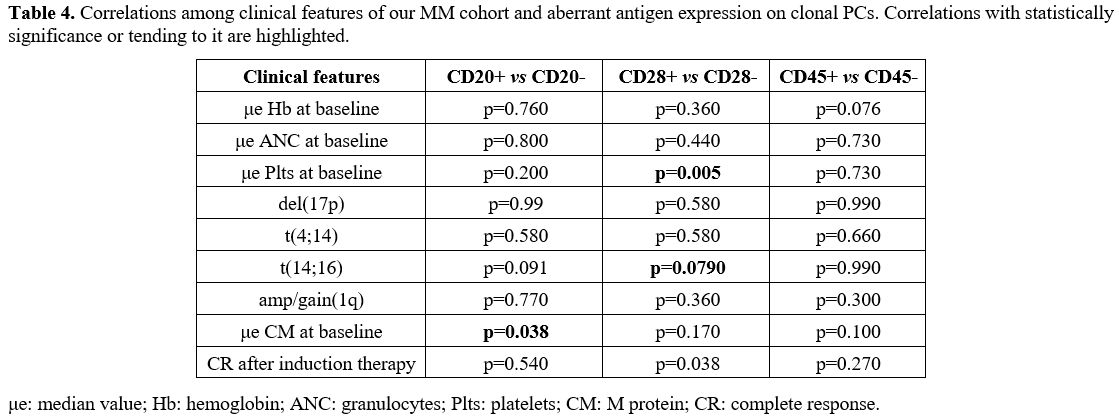 |
- Table 4. Correlations
among clinical features of our MM cohort and aberrant antigen
expression on clonal PCs. Correlations with statistically significance
or tending to it are highlighted.
|
Discussion
Despite
the relatively limited sample size, these data on a consecutive series
of newly diagnosed MM patients confirm that the antigenic surface
profile of MM PCs is highly variable, in line with the characteristic
heterogeneity of the disease. These results are in agreement with
previously published data[1,2] and confirm the
accuracy, reproducibility, and utility of flow cytometry to dissect
within clonal PCs in MM patients at presentation. In particular, CD20
and CD28 were the two surface antigens that showed the greatest
correlation with high tumor burden features in our series and could,
therefore, help identify upfront MM patients with a likely aggressive
evolution. CD28 is a T-cell costimulatory receptor, usually associated
with a rapidly evolving disease and resistance to frontline therapy.[9]
In our cohort, CD28 expression correlated with the absence of CD56
(p<0.001). The neural cell adhesion CD56 antigen is a membrane
glycoprotein, usually expressed on the surface of neoplastic PCs.[10]
Even if the role of CD56 in the evolution of MM is highly debated, a
recent study has postulated that its absence could be associated with a
lower degree of maturation of the neoplastic cells and unfavorable
prognostic parameters but not with outcome.[10] Thus,
based on the results of our study, clonal PCs with CD28 positivity and
absence of CD56 might identify a subset of patients with baseline
unfavorable disease characteristics. This observation is supported by
the evidence that CD28 expression correlates with both a low platelet
count (p=0.005) and with the high-risk t(14;16) chromosomal abnormality
(p=0.079).[11] In addition, this subgroup of patients
achieved significantly lower complete response rates (p=0.038) compared
to patients with CD28 negativity. An extended cohort of patients and a
prolonged follow-up are warranted to have more significant and relevant
data about the prognostic role of CD28 antigen expression on PC. MM
patients with CD20 positivity had higher levels of serum monoclonal
component (p=0.038), confirming that the aberrant expression of this
antigen could define a more aggressive MM subset.
Interestingly,
our analysis shows that t(4;14) correlates with a lower median CD138
MFI. CD138 is usually highly expressed on the surface of MM cells; it
mediates cell adhesion, and its loss may contribute to the
dissemination of the disease out of the BM.[12] Therefore, lower levels of expression of this protein may define a condition with high-risk aberrations.
In
conclusion, our study confirms the high heterogeneity of MM patients.
In this setting, MFC represents a simple, reproducible, and
cost-effective tool that could help to identify MM subsets at diagnosis
and improve risk stratification. A larger series of cases with a
prolonged follow-up is warranted to confirm these preliminary
observations and corroborate the clinical correlations.
Acknowledgments
This
work was supported by Associazione Italiana Ricerca sul Cancro (AIRC),
Special 5x1000 Program Metastases (21198), Milan, Italy (RF).
References
- Rawstron AC, Orfao A, Beksac M, Bezdickova L,
Brooimans RA, Bumbea H, Dalva K, Fuhler G, Gratama J, Hose D, Kovarova
L, Lioznov M, Mateo G, Morilla R, Mylin AK, Omedé P, Pellat-Deceunynck
C, Perez Andres M, Petrucci M, Ruggeri M, Rymkiewicz G, Schmitz A,
Schreder M, Seynaeve C, Spacek M, de Tute RM, Van Valckenborgh E,
Weston-Bell N, Owen RG, San Miguel JF, Sonneveld P, Johnsen HE;
European Myeloma Network. Report of the European Myeloma Network on
multiparametric flow cytometry in multiple myeloma and related
disorders. Haematologica. 2008 Mar;93(3):431-8. DOI:
10.3324/haematol.11080 https://doi.org/10.3324/haematol.11080 PMid:18268286
- Flores-Montero
J, de Tute R, Paiva B, Perez JJ, Böttcher S, Wind H, Sanoja L, Puig N,
Lecrevisse Q, Vidriales MB, van Dongen JJ, Orfao A. Immunophenotype of
normal vs. myeloma plasma cells: Toward antibody panel specifications
for MRD detection in multiple myeloma. Cytometry B Clin Cytom. 2016
Jan;90(1):61-72. DOI: 10.1002/cyto.b.21265 https://doi.org/10.1002/cyto.b.21265 PMid:26100534
- Paiva
B, Almeida J, Pérez-Andrés M, Mateo G, López A, Rasillo A, Vídriales
MB, López-Berges MC, Miguel JF, Orfao A. Utility of flow cytometry
immunophenotyping in multiple myeloma and other clonal plasma
cell-related disorders. Cytometry B Clin Cytom. 2010 Jul;78(4):239-52.
DOI: 10.1002/cyto.b.20512 https://doi.org/10.1002/cyto.b.20512 PMid:20155853
- Courville
EL, Yohe S, Shivers P, Linden MA. VS38 identifies myeloma cells with
dim CD38 expression and plasma cells following daratumumab therapy,
which interferes with CD38 detection for 4 to 6 months. Am J Clin
Pathol. 2020 Jan 2;153(2):221-228. DOI: 10.1093/ajcp/aqz153 https://doi.org/10.1093/ajcp/aqz153 PMid:31679012
- Paiva
B, Vidriales MB, Pérez JJ, Mateo G, Montalbán MA, Mateos MV, Bladé J,
Lahuerta JJ, Orfao A, San Miguel JF; GEM (Grupo Español de MM)
cooperative study group; PETHEMA (Programa para el Estudio de la
Terapéutica en Hemopatías Malignas) cooperative study group.
Multiparameter flow cytometry quantification of bone marrow plasma
cells at diagnosis provides more prognostic information than
morphological assessment in myeloma patients. Haematologica. 2009
Nov;94(11):1599-602. DOI: 10.3324/haematol.2009.009100 https://doi.org/10.3324/haematol.2009.009100 PMid:19880781 PMCid:PMC2770972
- Bataille
R, Jégo G, Robillard N, Barillé-Nion S, Harousseau JL, Moreau P, Amiot
M, Pellat-Deceunynck C. The phenotype of normal, reactive and malignant
plasma cells. Identification of "many and multiple myelomas" and of new
targets for myeloma therapy. Haematologica. 2006
Sep;91(9):1234-40. PMID:16956823
- Mateo
G, Montalbán MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutiérrez N,
Rosiñol L, Montejano L, Bladé J, Martínez R, de la Rubia J,
Diaz-Mediavilla J, Sureda A, Ribera JM, Ojanguren JM, de Arriba F,
Palomera L, Terol MJ, Orfao A, San Miguel JF; PETHEMA Study Group; GEM
Study Group. Prognostic value of immunophenotyping in multiple myeloma:
a study by the PETHEMA/GEM cooperative study groups on patients
uniformly treated with high-dose therapy. J Clin Oncol. 2008 Jun
1;26(16):2737-44. DOI: 10.1200/JCO.2007.15.4120 https://doi.org/10.1200/JCO.2007.15.4120 PMid:18443352
- Palumbo
A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L,
Richardson P, Caltagirone S, Lahuerta JJ, Facon T, Bringhen S, Gay F,
Attal M, Passera R, Spencer A, Offidani M, Kumar S, Musto P, Lonial S,
Petrucci MT, Orlowski RZ, Zamagni E, Morgan G, Dimopoulos MA, Durie BG,
Anderson KC, Sonneveld P, San Miguel J, Cavo M, Rajkumar SV, Moreau P.
Revised International Staging System for Multiple Myeloma: A Report
From International Myeloma Working Group. J Clin Oncol. 2015 Sep
10;33(26):2863-9. DOI: 10.1200/JCO.2015.61.2267 https://doi.org/10.1200/JCO.2015.61.2267 PMid:26240224 PMCid:PMC4846284
- Bahlis
NJ, King AM, Kolonias D, Carlson LM, Liu HY, Hussein MA, Terebelo HR,
Byrne GE Jr, Levine BL, Boise LH, Lee KP. CD28-mediated regulation of
multiple myeloma cell proliferation and survival. Blood. 2007 Jun
1;109(11):5002-10. DOI: 10.1182/blood-2006-03-012542 https://doi.org/10.1182/blood-2006-03-012542 PMid:17311991 PMCid:PMC1885531
- Koumpis
E, Tassi I, Malea T, Papathanasiou K, Papakonstantinou I, Serpanou A,
Tsolas E, Kapsali E, Vassilakopoulos TP, Papoudou-Bai A, Hatzimichael
E. CD56 expression in multiple myeloma: Correlation with poor
prognostic markers but no effect on outcome. Pathol Res Pract. 2021
Sep;225:153567. DOI: 10.1016/j.prp.2021.153567 https://doi.org/10.1016/j.prp.2021.153567 PMid:34352440
- Abdallah
N, Rajkumar SV, Greipp P, Kapoor P, Gertz MA, Dispenzieri A, Baughn LB,
Lacy MQ, Hayman SR, Buadi FK, Dingli D, Go RS, Hwa YL, Fonder A, Hobbs
M, Lin Y, Leung N, Kourelis T, Warsame R, Siddiqui M, Lust J, Kyle RA,
Bergsagel L, Ketterling R, Kumar SK. Cytogenetic abnormalities in
multiple myeloma: association with disease characteristics and
treatment response. Blood Cancer J. 2020 Aug 11;10(8):82. DOI:
10.1038/s41408-020-00348-5 https://doi.org/10.1038/s41408-020-00348-5 PMid:32782240 PMCid:PMC7419564
- Aref
S, Goda T, El-Sherbiny M. Syndecan-1 in multiple myeloma: relationship
to conventional prognostic factors. Hematology. 2003 Aug;8(4):221-8.
DOI: 10.1080/1024533031000153630 https://doi.org/10.1080/1024533031000153630 PMid:12911939
[TOP]



