Salvatore Di Maio1, Pierluigi Marzuillo2, Shahina Daar3, Christos Kattamis4, Mehran Karimi5, Saki Forough6, Atanas Banchev7, Valeria Kaleva8, Soteroula Christou9, Carmelo Fortugno10, Polyxeni Delaporta11, Ashraf T. Soliman12, Ploutarchos Tzoulis13 and Vincenzo de Sanctis14.
1 Emeritus Director in Pediatrics, Children's Hospital "Santobono-Pausilipon", Naples, Italy.
2 Department of Woman, Child, General and Specialized Surgery, University "Luigi Vanvitelli", Naples, Italy.
3
Department of Haematology, College of Medicine and Health Sciences,
Sultan Qaboos University, Sultanate of Oman and Wallenberg Research
Centre, Stellenbosch Institute for Advanced Study, Stellenbosch
University, Stellenbosch, South Africa.
4 First Department of Paediatrics, National Kapodistrian University of Athens, Athens, Greece.
5 Department of Hematology-Oncology, American Hospital Dubai, UAE.
6 Shiraz Endocrinology and Metabolism Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
7
Expert Center for Haemophilia, Thalassemia and Other Rare Benign
Haematological Disorders, Department of Paediatric Haematology and
Oncology, University Hospital "Tzaritza Giovanna – ISUL", Medical
University, Sofia, Bulgaria.
8 Expert Center for Coagulopathies and Rare Anemias, Varna, Bulgaria.
9 Thalassemia Unit, Nicosia, Cyprus.
10
Department of Pediatric Haematoncology, Thalassaemia and Prenatal
Diagnosis Regional Center, Pugliese-Ciaccio Hospital, Catanzaro, Italy.
11 Thalassemia Unit, First Department of Pediatrics National Kapodistrian University of Athens, Athens, Greece.
12 Department of Pediatrics, Hamad General Hospital, Doha, Qatar.
13 Department of Endocrinology, Whittington Hospital, University College London, London, UK.
14 Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy.
Correspondence to:
Salvatore Di Maio, MD. Via degli Aranci, 59, Sorrento - 80067 (NA), Italy. Tel. 081 8785552. E-mail:
dimaiosalvatore@tin.it
Published: November 01, 2023
Received: May 30, 2023
Accepted: October 15, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023058 DOI
10.4084/MJHID.2023.058
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction:
To evaluate the effect of early chelation therapy (≤ 3 years) with a
variety of chelating agents on age at menarche and menstrual
characteristics in patients with transfusion-dependent thalassemia
(TDT).
Design: A
retrospective multicenter study promoted by the International Network
of Clinicians for Endocrinopathies in Thalassemia and Adolescent
Medicine (ICET-A).
Setting: Eight of 13 International Thalassemia Centers (61.5%) in the ICET-A Network participated.
Patients:
Fifty-seven female TDT patients, aged 11 to 26 years, and with early
iron chelation therapy, were eligible for the present study. They were
enrolled from one center from Iran (33 patients), 3 centers from
Bulgaria (9), 1 from Greece (8), one from Oman (4), 1 from Cyprus (2),
and 1 from Italy (1). Seven patients were excluded, four still
prepubertal (age 12-14 years) and 3 with primary amenorrhea. Therefore
50 patients were finally enrolled.
Results:
All fifty TDT patients developed spontaneous menarche at a mean age of
14.2 ± 2.24 years (range 9 – 20). A significant positive correlation
was observed between age at menarche and serum ferritin levels (r: 0.
41, p=0.005). Regular menstrual cycles were reported from 32 (64%)
patients, of whom 28 (83.3%) get menarche at age ≤ 14 years.
Complications were more frequent in patients older than 14 years at
menarche and in those with secondary amenorrhea.
Conclusions:
Age at menarche greater than 14 years was a forerunner of menstrual
irregularities and associated complications in 36% of patients despite
precocious chelation therapy. The poor adherence to treatment, to be
demonstrated in future studies, could explain the finding.
|
Introduction
Medical
advancements with regular blood transfusion therapy, iron-chelating
therapies (ICTs) and imaging methods have improved the life expectancy
of children and adolescents suffering from transfusion-dependent
thalassemia (TDT); however, as patients approach the age of puberty,
they often develop growth retardation and disorders of pubertal
development [delayed puberty, arrested puberty, late menarche and
acquired hypogonadotropic hypogonadism (AHH)], particularly if the
disease is poorly controlled with regular ICT.[1]
Up
to now, the effects of ICT on the age of menarche and subsequent
menstrual cycles have been evaluated mainly in patients who started ICT
between 5 and 10 years of age[2,3] and only in a small number of cases under the age of 5 years.[4]
Menarche
is a significant event that marks the onset of sexual and reproductive
maturation in girls and is considered a surrogate marker of general
good health in subjects affected by chronic disorders. Menarche
typically occurs within 2–3 years after thelarche (breast budding)
between the ages of 10 and 15 years, corresponding to - 2 standard
deviation (SD) and + 2 SD, respectively. The 95th percentile for menarche is 14.5 years, although many textbooks define primary amenorrhea as an absence of menses at 16 years.[5] In practice, at or before the age of 15, menarche is experienced by 98% of girls.[6]
Menstrual
regularity patterns include three main dimensions: bleeding frequency,
duration, and intensity. By the third year after menarche, 60–80% of
menstrual cycles are 21–34 days long.[7]
The
main aim of this retrospective study was to ascertain, in TDT patients
over 11 years who started iron chelation therapy early at ≤ 3 years,
the percentage of subjects with spontaneous pubertal development, the
patient's age at menarche, and the characteristics of subsequent
menstrual cycles.
Method used for the preparation and distribution of the questionnaire
A
multicenter international study using an ad hoc questionnaire in
accordance with the Declaration of Helsinki was proposed at the
beginning of September 2021 by the Coordinator (VDS) of the
International Network of Clinicians for Endocrinopathies in Thalassemia
and Adolescent Medicine (ICET-A) (first step).
After the first
draft preparation, the questionnaire was discussed by e-mail, and the
final version was validated at the beginning of October 2021 by 4
endocrinologists (De Sanctis V, Soliman AT, Tzoulis P, and Di Maio S)
and 2 hematologists (Daar S and Kattamis C) (second step).
Thirteen
centers active in the ICET-A Network were invited to participate in the
study. The questionnaire was distributed by mail to the Centers that
accepted the invitation, and the deadline for sending the requested
data was fixed to the end of December 2021 (third step).
The
questionnaire included the following information: patients’
demographics and anthropometrics data, age at first transfusion, age at
the start of chelation therapy, serum ferritin level (SF) at the start
of ICT and at menarche, associated endocrine and non-endocrine
complications, date at menarche or absence (primary amenorrhea),
menstrual pattern information collected on the basis of patients'
self-reporting, namely menstrual history during the three months
preceding the last observation [cycle regularity, cycle length (short
and long), duration of menstrual bleeding, and amount of menstrual
flow, and age at secondary amenorrhea (SA)]. Socioeconomic status,
based on parental education and occupation at the time of last
observation, behavioral patient habits (smoking, alcohol consumption),
patient's physical activity, mothers' and sisters' age at menarche, and
menses patterns were not included in the questionnaire.
The
following were excluded from the study: (a) bone marrow transplanted
patients; (b) those who were HIV positive; (c) patients with a clinical
history of isolated menarche; (d) patients who had died before the
study; (e) those with mental illness (depression, anxiety disorders,
eating disorders, and addictive behavior), and (f) patients with
chronic kidney diseases.
Material and Methods
Definitions of menarcheal age and menstrual disorders.
A menarcheal age was considered precocious before age 10 and later
above the age of 14. The age of 14 years, equal to approximately + 1.25
SD from the mean, was chosen as the threshold for defining a menarche
as "late" because an age at menarche more than 1 SD from the mean and
progressively closer to + 2 SD, represents a greater risk of pathology
and because the maternal menarche ages of individual patients were not
available.
Primary amenorrhea was defined as the absence of menses at 16 years.
The
menstrual cycle period interval was defined as the number of days from
the first day of one menstrual period to the first day of the next
menstrual period [short cycle interval: ≤ 21 days; long cycle interval
≥ 35 days and < 90 days (oligomenorrhea)]. Frequent menstrual
bleeding was defined as more than four episodes in 90 days, heavy and
prolonged menstrual bleeding was the presence of excessive menstrual
blood loss (approximated to the number of pads per day), short menses
and light menstrual bleeding (hypomenorrhea) was defined when the
menstrual cycle was 2 days or less; the absence of menstruation for
more than 3 months, at any time after menarche, in the presence of
documented AHH, was classified as SA.[4,6-9] Gynecologic age was defined as the age in years at last observation minus age at menarche.
Patients
with primary or SA were evaluated for basal pituitary–gonadal axis
(HPG) integrity and by the exclusion of other endocrine/non-endocrine
complications. The diagnosis of AHH was characterized by low levels of
estradiol (E2) in the presence of low or inappropriately normal
gonadotropin (LH and FSH) serum levels.[10]
Anthropometry and assessment of associated endocrine complications.
Height and weight were measured using a standard technique. Body mass
index (BMI) was calculated as weight in kilograms divided by the square
of height in meters. Height and weight were measured according to
international recommendations.[11] BMI was evaluated based on the World Health Organization (WHO) recommendations: underweight (<18.5 kg/m2); normal range (18.5–24.9 kg/m2); overweight (25.0– 29.9 kg/m2); obese (≥ 30 kg/m2).[12]
Associated endocrine complications. Associated endocrine complications were assessed and defined according to the I-CET position statement published in 2013.[13]
According
to the American Diabetes Association, prediabetes was defined as
follows: impaired fasting glucose (IFG) when fasting plasma glucose
(FPG) was between 5.6–6.9 mmol/L and impaired glucose tolerance (IGT)
when the 2-h plasma glucose (2-h PG) value during a 75 g oral glucose
tolerance test (OGTT) was between 7.8–11.0 mmol/L. Diabetes mellitus
was confirmed by FPG ≥7.0 mmol/L and/or a 2-h PG value during a 75 g
OGTT of ≥11.1 mmol/L.[14]
The assessment of iron overload (IOL).
The assessment of iron overload (IOL) was evaluated by SF. The
manufacturer’s normal reference range values in females were 15–150
ng/mL. Iron overload was arbitrarily classified as mild (SF < 1,000
ng/mL), moderate (SF: ≥1,000 ng/mL and < 2,000 ng/mL) or severe (SF:
≥ 2000 ng/mL).[4] Duration of chelation was defined as the age at the last observation minus the age at the start of chelation.
Statistical analysis.
Differences for continuous variables were analyzed with an
independent-sample t-test for normally distributed variables and with
the Mann-Whitney test in case of non-normality. Qualitative variables
were compared by using the chi-squared test. The Statgraphics XVII
software for Windows was used for all statistical analyses. A P value
< 0.05 was considered as significant.
Ethics.
The study was designed in accordance with the Helsinki Declaration; all
participants were informed about the nature and purpose of the study.
Each patient or their legal guardian agreed to participate in this
study and gave consent after a brief session to explain the aims.
Confidentiality, anonymity, and non-transmissibility of detailed
personal patients' data were assured. The retrospective study was
exempted from institutional Ethics Committee approval.[15] No identifiable private patient information was collected, and an anonymized dataset was analyzed.
Results
Data collected and participating Centers.
Eight of the 13 Thalassemia Centers of the ICET-A Network participated
in the study, reporting data on fifty-seven female TDT patients aged 11
to 26 years. They were from Iran (33 patients; Shiraz), Bulgaria (Sofia
n = 5, Plovdiv n = 2, Varna n = 2), Greece (8 patients; Athens), Oman
(4 patients; Muscat), Cyprus (2 patients; Nicosia), and Italy (1
patient; Catanzaro). Seven patients were excluded: 4 (2 from Cyprus, 1
from Greece and 1 from Iran) were still prepubertal (age 11-14 years),
and 3 (2 from Iran, 1 from Bulgary) had primary amenorrhea. All started
chelation therapy at or before the age of 3 years. Therefore, 50
patients were enrolled. They were born between 1995 and 2008 and aged
19.5 ± 4.2 years (range: 11 – 26). Their main clinical, therapeutic
(ICT), and laboratory characteristics are summarized in Table 1.
 |
- Table 1. Main
clinical, laboratory, and therapeutic characteristics in 50 TDT
patients with spontaneous menarche. Results are reported as mean ±
standard
- deviation (DS) and range.
|
Age
at diagnosis and age at the start of transfusion were reported in all
50 patients. SF levels were available in 44/50 patients at the start of
chelation therapy, 45/50 adolescents at menarche, and 7/8 patients at
the age of SA. BMI was reported at the age of menarche and SA.
Spontaneous menarche.
Fifty patients developed spontaneous menarche at a mean age of 14.2±
2.24 years (range 9 – 20). At the start of chelation therapy, 22
patients had received desferrioxamine mesylate (DFO), 26 patients
deferasirox (DFX), and 2 patients deferiprone (DFP).
Among the
26 patients receiving DFX, 22 had normal menstrual cycles and achieved
menarche at 13.12 ± 0.73 years when their SF level was 1834 ± 1325
ng/ml; at the start of chelation therapy, their SF was 1365 ± 985
ng/ml, not significantly different from SF levels at menarche (p =
0.27).
No correlation was found between BMI and age at menarche (n
= 45, r = -0.26; p= 0.065), while a significant inverse correlation was
found between weight percentile and age at menarche (n = 48; r = -0.39,
p = 0.0057). In 9 of 47 patients whose data were available, the
standing height at menarche was ≤ 3° percentile: 7/22 (31.8%) patients
treated with DFO; 2/23 (8.7%) patients treated with DFX (p= 0.07) and
0/2 treated with DFP. SF levels in these 9 patients were 2345 ± 1908
ng/dl (range 300 – 7000). Unfortunately, the height of the parents of
these 9 as well as the other patients was not available.
A significant correlation was observed between age at menarche and SF levels in 45 patients (r: 0. 41, p= 0.005) (Figure 1).
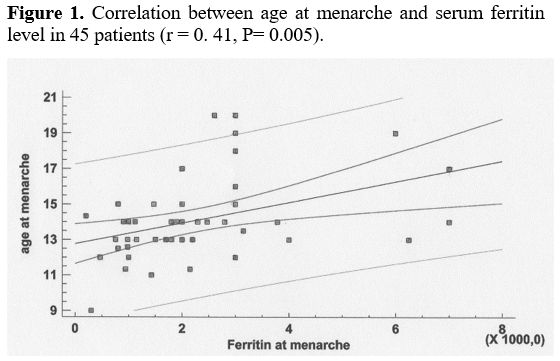 |
- Figure 1.. Correlation between age at menarche and serum ferritin level in 45 patients (r = 0. 41, P= 0.005).
|
In
particular, the mean age at menarche was 12.7 ± 1.7 (n = 10), 13.3 ±
1.1 (n = 11), and 15.0 ± 2.5 (n = 24) years in patients with mild SF
(<1,000 ng/mL), moderate SF (between 1,000 and 1,999 ng/mL), and
severe SF (from ≥ 2,000 to 7,000 ng/mL), respectively (p= 0.0067. In
the ten patients with mild iron overload, SF levels at the start of
chelation (970 ± 601 ng/dl) were not different from SF levels at
menarche (p= 0.23).
Menstrual characteristics and chelation therapy.
Thirty-two of 50 patients (64%) reported regular menstrual cycles;
their SF levels at the start of chelation (1394 ± 906 ng/ml) were not
different from SF levels at menarche (2073 ± 1522 ng/ml, P = 0.28).
Their ages at menarche were 13.14 ± 0.97 years vs 15.78 ± 2.7 years in
18 patients with irregular cycles, p = 0.0003, of whom 7 (14%) with
oligomenorrhea, 3 (6%) with short/light menses (hypomenorrhea), and 8
(16%) with SA. No patient reported heavy and prolonged menstrual
bleeding (Table 2).
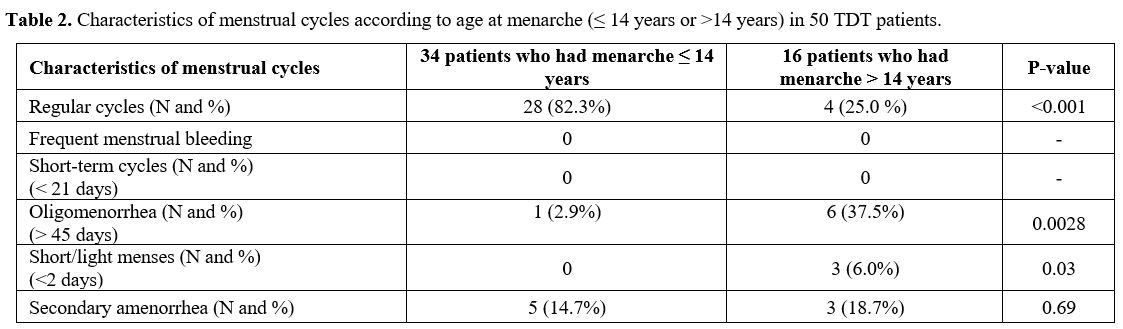 |
- Table 2. Characteristics of menstrual cycles according to age at menarche (≤ 14 years or >14 years) in 50 TDT patients.
|
Regular
menstrual cycles were more frequent in patients who experienced
menarche at age 14 years or less (82.3%) than in those who experienced
menarche at age greater than 14 years (25%; p < 0.001) (Table 2).
The group of 16 patients with menarche > 14 years of age had a
significantly higher frequency of oligomenorrhea (37.5% vs 2.9%; p=
0.0028) and hypomenorrhea (6% vs. 0%; p= 0.03) (Table 2).
The severity of IOL was not significantly associated with the characteristics of the menstrual cycles (Table 3).
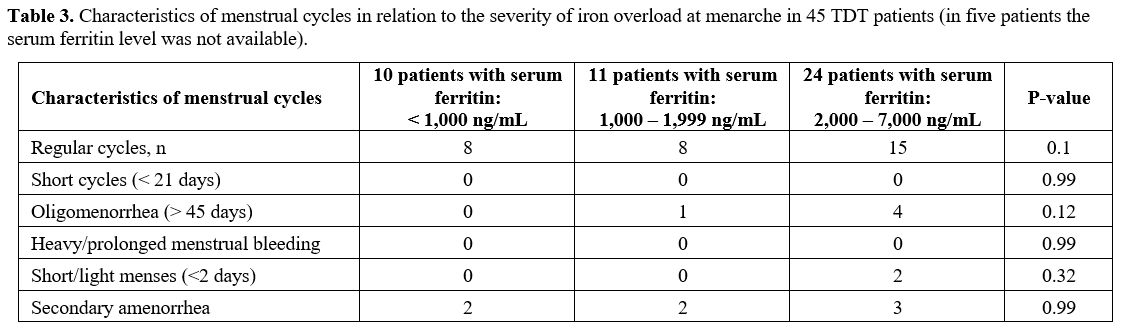 |
- Table 3.
Characteristics of menstrual cycles in relation to the severity of iron
overload at menarche in 45 TDT patients (in five patients the serum
ferritin level
- was not available).
|
Moreover,
regular menstrual cycles were more frequent in patients treated with
DFX (68.7%; P= 0.004). Their mean age at menarche was 13.12 ± 0,74
years, significantly lower than the ages at menarche of 20 patients not
on DFX = 14.9 ± 2.8 years, p = 0.0173, while their mean SF at menarche,
1840 ± 1356 (range 600 – 6250) was not different from SF levels, 2611 ±
1690 (range 300 – 7000) ng/ml of patients not on DFX, p = 0.12.
Gynecological age was 5.4 ±.3.27 years (range 1-12 years; median 4.7 years). Only one of them developed SA.
Secondary
amenorrhea was reported in 8 patients at 17.9 ± 3.5 years (range 14-23)
and preceded by oligomenorrhea. Their menarcheal age was reported as
14.2 ± 2.3 years when the registered mean SF level was 1,828 ± 1,014
ng/mL. Seven of them had received DFO and one DFX from an early age.
None had received two chelating drugs until the last observation (Table 4). At the time of SA, their gynecological age was 7.8 ± 3.2 years (range 2 – 14; median 8 years).
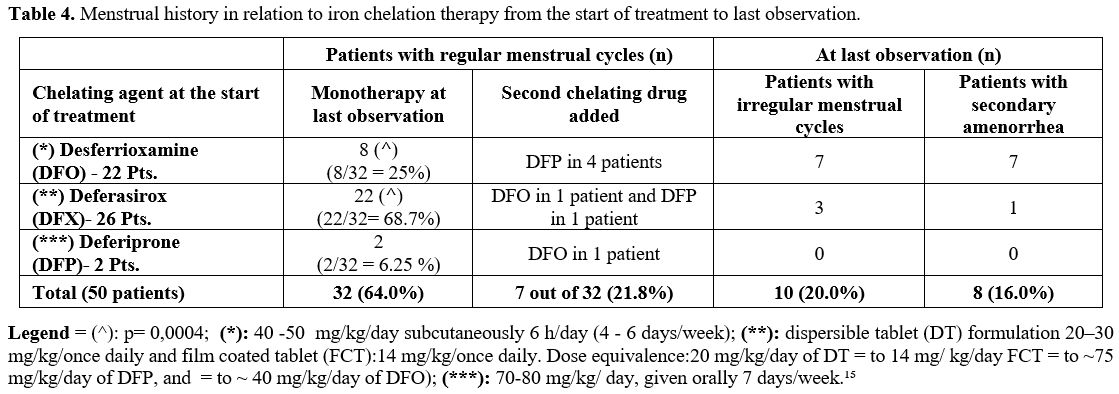 |
- Table 4. Menstrual history in relation to iron chelation therapy from the start of treatment to last observation.
|
At
the last observation, forty-three patients remained on monotherapy,
while 7 patients were receiving two ICTs (5 patients: DFO + DFP, 1
patient: DFP + DFX, and 1 patient: DFO + DFX).
Associated complications.
At last observation, the presence of associated complications was more
prevalent in patients with SA [6/8 (75%)] and in those who achieved
menarche after the age of 14 years [11/16 (68,7%)].
Glucose
dysregulation was the commonest associated complication (12/50; 24%),
followed by thyroid disorders (6/50; 12%, 2 patients with secondary
hypothyroidism and 4 with primary hypothyroidism of whom 1 had goiter),
hypoparathyroidism (1/50; 2%), and heart failure due to iron overload
associated with IGT; her SF at menarche had been 7000 ng/ml (1/50; 2%).
Discussion
In
our retrospective study on 50 adolescents and young adults with TDT who
started early ICT, the prevalence of hypogonadism due to AHH was much
lower (7.5%) than previous reports in which the prevalence reached much
higher values, even 38% (19). These data confirm the importance of
early onset of chelation therapy to attain normal sexual maturation.[4]
At the start of chelation therapy, 22 patients received DFO, 26 DFX,
and 2 patients DFP. However, their mean age at menarche (14.2 ± 2.24
years; range 9 – 20) was higher compared to data reported in the
general population in Iran (13.2 years),[16] Bulgaria (12.7 years),[17] Greece (12.2 years),[18] Oman (13.3 years),[19] and Italy (12.4 years).[20]
Although
compliance to ICT was not assessed in the present study, the efficacy
of early start of chelation, as assessed by serum ferritin levels at
menarche, showed that 10/45 of patients preserved low SF levels
<1,000 ng/mL. Their age at menarche 12.6 ± 2 years was significantly
lower compared to 14.9 ± 2.4 years of the others 35 patients, p=
0.0425. Their SF at menarche (712 ± 272 ng/ml) was not different from
SF at the start of chelation therapy (970 ± 601, p > 0.05). Eight
had regular cycles, while 2 got secondary amenorrhoea at ages 15 and 17
years after menarche at 9 and 15, respectively.
Seven of the 10
were on treatment with DFX. Thus, late menarche was reported in
patients with severe iron overload (SF: >2,000 ng/mL) compared to
those with SF < 1,000 ng/mL (12.7 years, close to the mean age at
menarche) (p= 0.0067). These data indicate that, despite the
availability of oral ICT, treatment adherence is still a serious risk
factor during pubertal age. Therefore, considerable attention should be
given to factors contributing to non-adherence to ICT during
adolescence and early adulthood, particularly those related to
patients’ education.
Notably, one patient had menarche at 9
years (BMI: 18.7 kg/m², SF: 300 ng/mL), and 9 patients had very late
menarche [ >16 years of age, mean 18.5, range 17-20 years, BMI: 20.3
kg/m² (range 16.25 - 27), mean SF: 4,000 ng/mL (range: 3,000 – 7,000
ng/mL]. Although precocious menarche is an event not reported before in
thalassemias, extremely late menarche has been reported by Psihogios et
al. (at 25 years),[21] Safarinejad et al. (at 16.8 ± 2.1years),[22] and Abd et al. (> 18 years in 2 patients).[23] Unfortunately, no data were reported on their BMI and SF level at menarche.
In
this study population, a significant correlation was found between
weight percentile and age at menarche. However, no significant
correlation was found between BMI and age at menarche, contrary to what
we observed previously on puberty and menstrual cycles in thalassemia.[4] In the early 1970s, Frisch and Revelle[24] suggested a “critical weight” theory, pointing out the relationship between weight and pubertal timing.[25]
Most
likely, the mechanism behind the association between weight status and
time of sexual maturation, at least for menarche as an endpoint, may be
more complex than a direct causal relation. As suggested by Bratke et
al.,[25] subcutaneous fat tissue like triceps (TSF)
and subscapular skinfold (SSF) could show a stronger relation with
menarche than the BMI, which measures both fat mass and lean mass.
Late
menarche in patients with TDT could be caused by other conditions, such
as functional hypogonadotrophic hypogonadism (FHH).[26]
FHH has a wide range of etiologies (stress, excessive exercise or
restrictive eating habits, chronic disease, e.g., anorexia nervosa,
inflammatory bowel disease, celiac disease, chronic renal disease, and
cystic fibrosis) that can inhibit the gonadotrophic axis by various
mechanisms. It is assumed that disorders of secretion of various
neuropeptides: neuropeptide Y (NPY), corticotropin-releasing hormone
(CRH), leptin, ghrelin, and β-endorphin may cause a disorder of pulse
GnRH secretion that results in the impairment of gonadotropin pulse
secretion.[27]
Fernandez-Fernandez et al.,[28]
who have investigated the role of ghrelin on sexual maturation, showed
that ghrelin inhibits LH secretion in vivo in prepubertal males as well
as gonadectomized male and female rats, whereas FSH remained
unaffected. Moreover, Moshtaghi-Kashanian and Razavi[29]
have hypothesized that a decreased leptin/acylated-ghrelin ratio may
constitute one additional mechanism involved in delayed puberty,
irregular menses, and amenorrhea.
In our study, irregular
periods (oligomenorrhea and hypomenorrhea; 20%) were associated with
older age of menarche (> 14 years) and other endocrine
complications. Furthermore, in 8 patients, SA was diagnosed at the age
of 14 – 23 years (17.9 ± 3.5 years; range 14-23) and was preceded by
oligomenorrhea in the absence of signs of hyperandrogenism:
ascertaining the absence of clinical (hirsutism) or biochemical
evidence of hyperandrogenism in late adolescents and young women with
oligomenorrhea is essential to rule out the frequent polycystic ovary
syndrome or other less common ovarian or adrenal disorders.
These
findings support the concept that oligomenorrhea may represent a
serious menstrual dysfunction that should be diagnosed early. However,
at the time of SA, the mean SF level was not statistically
significantly different from the mean SF registered at menarche (1,385
± 313 vs. 1,828 ± 1,014; p= 0.29), although there was a trend for
reduction. Therefore, independent of SF risk stratification into lower
or higher-risk subgroups, certain tissues, and cell types might be more
sensitive to NTBI and iron-mediated toxicity.
In a previous
study, we have documented that the duration of the menstrual history of
patients was strictly correlated to the age at the start of s.c. ICT
with DFO. In particular, the duration was 12.5 ± 8.9 years
(range: 1.4 - 28, median: 15 years) in 24 patients who started
chelation therapy < 5 years (13 with preserved menses and 11 with
SA), 7.2 ± 8.8 years (range: 0. 6 - 28.2, median: 3 years) in 54
patients who started treatment from 5 to 12.5 years, and 3.1 ± 2.3
years (range 2 - 8, median 3 years) in 8 patients who started treatment
> 12.5 years (p=< 0.01) (4). In this study, only 1 of 8 TDT
patients on treatment with DFX developed SA. However, it is important
to note that the 22 patients with regular cycles reported in this study
and treated with DFX had a relatively short gynecological age (mean:
5.7 years).
Unfortunately, only a few patients enrolled in our
study were treated with DFP in mono or combined chelation therapy.
Therefore, we are just at the beginning of a long journey that will
give us new insights over the years. Additional studies are also needed
to assess better the negative effects of IOL and the positive effects
of efficient ICTs on the reproductive system.
Glucose
dysregulation (GD) prevailed among the endocrine complications and was
more frequent in patients with SA. Although the mechanisms by which
estrogen deficiency may alter insulin action in humans are not
completely understood, animal studies have shown that estrogens
increase glucose transport and glucose utilization in muscle cells.[30-31]
These findings provide a basis for further research to explore the
effects of estrogen deficiency on GD and offer an indication for
potential therapeutic interventions.
Our work has several
limitations. The first is the lack of data on SF levels between
menarche and the last observation in young women who were still
menstruating; moreover, we have no data on parents height and regarding
ICT adherence except the low SF levels (<1,000 ng/mL) on menarche.
Other inconsistencies may be attributable to our retrospective study's
relatively small sample size, which may affect the statistical power of
our observations. Moreover, the study did not cover some information,
such as the severity of genotype and hematological phenotype that
varies among populations, girls' education, family history, and
lifestyle habits.
From another perspective, this study has
several strengths: (a) it is the first investigation focusing on a
multicenter study on menarche and menstrual cycles in patients with TDT
who started early chelation therapy with different ICT regimes; (b) it
encompasses patients with TDT treated and followed at the reference
centers for hemoglobinopathies in their own countries; (c) Third, the
preliminary results offer several ideas and reflections for future
studies.
Conclusions
Early
chelation alone does not necessarily coincide with efficient chelation
in childhood and puberty because a non-small proportion of patients,
equal to 36%, still had menstrual irregularities despite an early start
of chelation by the age of 3 years. A key finding of this study was the
significant positive correlation between age at menarche and SF level
(r: 0. 41, p= 0.005), with a mean 2-year delay in the onset of menarche
in females with severe iron overload compared with those with SF <
2,000 ng/ml. Late menarche (>14 years), related to high SF levels,
was still frequent in most Centers and was a forerunner of irregular
menstrual cycles and associated complications.
A
possible explanation is poor adherence to therapy, evidenced by high
ferritin values; however, the study design did not include this aspect.
Finally,
an interesting finding was that a significantly higher percentage of
females on treatment with DFX (68.7%) had normal menstrual cycles
compared to DFO-treated ones (25%; p= 0.0004).
This
acquisition could raise the intriguing question of the superiority of
DFX over other chelating drugs. However, the design of our study and
the low number of subjects treated with the various chelating drugs
require a robust, specific perspective study before it can be
recommended.
Adherence
to ICTs is a key prerequisite for positive treatment outcomes and is
especially important for those patients who require treatment regimens
throughout their lifetime. A specific study that also includes
compliance indicators will be able to confirm this insight.
Families,
teenagers, and young adults must understand the importance of regular
and constant ICT and should be conscious that even short periods of
interruption or irregular adherence to ICT can have late deleterious
effects on the H-P-G axis and other organs. Neglecting its importance,
despite the innovative and expensive therapies for which the National
Health Services pay high costs annually through their public funds, may
lead to complications, hospitalization, and decreased quality of life.
Author Contributions
Conceptualization:
VDS; Preparation of questionnaire: VDS, CK, SD, ATS, PT and SDM; Data
curation and analysis: SDM and PLM; Carried out the research: SD, MK,
SF, AB, VK, SC, CF and PD; Writing original draft preparation: SDM and
PLM; Reviewed the manuscript for important intellectual content and
editing: VDS, SD, CK, ATS and PT. All authors have read and approved
the final version of the manuscript.
References
- Italian Working Group on Endocrine Complications in
Non-Endocrine Disease. Multicentre Study on Prevalence of Endocrine
Complications in Thalassemia Major. Clin Endocrinol (Oxf).1995;
42(6):581-586. doi:10.1111/j.1365-2265.1995.tb02683.x. https://doi.org/10.1111/j.1365-2265.1995.tb02683.x PMid:7634497
- Bronspiegel-Weintrob
N, Olivieri NF, Tyler B, Andrews DF, Freedman HM, Holland FJ. Effect of
age at the start of iron chelation therapy on gonadal function in
β-thalassemia major. N Engl J Med. 1990;323: 713-719. doi:10.156/
NEJM199 0091 33 231104. https://doi.org/10.1056/NEJM199009133231104 PMid:2388669
- Shalitin
S, Carmi D, Weintrob N, Miskin H, Kornreich L, Zilber R, Yaniv I,
Tamary H. Serum ferritin levels as a predictor of impaired growth and
puberty in thalassemia major patients. Eur J Haematol 2005;74: 93-100.
doi:10.1111/J.1600-0609. 2004. 00371.x. https://doi.org/10.1111/j.1600-0609.2004.00371.x PMid:15654898
- Di
Maio S, Marzuillo P, Mariannis D, Christou S, Ellinides A,
Christodoulides C, de Sanctis V. A retrospective long-term study on age
at menarche and menstrual characteristics in 85 young women with
transfusion-dependent β-thalassemia (TDT). Mediterr J Hematol Infect
Dis 2021, 13(1): e2021040. doi:10.4084/MJHID.2021.040 https://doi.org/10.4084/MJHID.2021.040 PMid:34276909 PMCid:PMC8265331
- Styne
DM. Physiology and disorders of puberty, chapter 26 in: Melmed S,
Auchus RJ, Goldfine AB, Koenig RJ, Rosen CJ. Williams Textbook of
Endocrinology, 14th edition. ELSEVIER Philadelphia 2020, page1034
- American
Academy of Pediatrics, American College of Obstetricians and
Gynecologists. Menstruation in girls and adolescents: using the
menstrual cycle as a vital sign. Pediatrics. 2006 Nov;118(5):
2245-2250. doi: 10.1542/peds.2006-2481. https://doi.org/10.1542/peds.2006-2481 PMid:17079600
- Hickey
M, Balen A. Menstrual disorders in adolescence: investigation and
management. Hum Reprod Update.2003;9: 493-504.
doi:10.1093/humupd/dmg038. https://doi.org/10.1093/humupd/dmg038 PMid:14640381
- Riaz
Y, Parekh U. Oligomenorrhea. StatPearls [Internet]. Treasure Island
(FL): StatPearls Publishing; 2023 Jan. 2022 Aug 1. Bookshelf ID:
NBK560575
- De
Sanctis V, Vullo C, Katz M, Wonke B, Hoffbrand AV, Bagni B.
Hypothalamic-pituitary-gonadal axis in thalassaemic patients with
secondary amenorrhea. Obstet Gynecol.1988;72: 643-647.
- Kyriakou
A, Skordis N. Thalassaemia and Aberrations of Growth and Puberty
Mediterr J Hematol Infect Dis. 2009 Jul 27; 1(1):e2009003. doi:
10.4084/MJHID.2009.003 PMID: 21415985. https://doi.org/10.4084/mjhid.2009.003
- World
Health Organization. (2006). WHO child growth standards:
length/height-for-age, weight-for-age, weight-for-length, weight
-for-height and body mass index-for-age: methods and development. World
Health Organization. https://apps.who.int/iris/handle/10665/43413
- WHO.
Physical status: the use and interpretation of anthropometry. Report of
a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;
854:1-452.
- De
Sanctis V, Soliman AT, Elsedfy H, Skordis N, Kattamis C, Angastiniotis
M, Karimi M, Yassin MA, El Awwa A, Stoeva I, Raiola G, Galati MC,
Bedair EM, Fiscina B, El Kholy M. Growth and endocrine disorders in
thalassemia: The international network on endocrine complications in
thalassemia (I-CET) position statement and guidelines. Indian J
Endocrinol Metab. 2013; 17(1):8-18.doi:10.4103/2230-8210.107808. https://doi.org/10.4103/2230-8210.107808 PMid:23776848 PMCid:PMC3659911
- American
Diabetes Association. 2. Classification and diagnosis of diabetes-
standards of medical care in diabetes - 2017. Diabetes Care 2017;
Jan;40 (Suppl 1):S11-S24. doi: 10.2337/dc17-S005 https://doi.org/10.2337/dc17-S005 PMid:27979889
- De
Sanctis V, Soliman AT, Daar S, Tzoulis P, Fiscina B, Kattamis C,
International Network of Clinicians for Endocrinopathies in Thalassemia
and Adolescence Medicine (ICET-A). Retrospective observational studies:
Lights and shadows for medical writers. Acta Biomed.
2022;93(5):e2022319. doi: 10.23750/abm.v93i5.13179.
- Bahrami
N, Soleimani MA, Chan YH, Ghojazadeh M, Mirmiran P. Menarche age in
Iran: A meta-analysis. Iran J Nurs Midwifery Res. 2014;19(5): 444-450.
PMID: 25400670.
- Stoev R, Mitova Z. Age at Menarche in Sofia Girls /2014-2018. Acta Morphol Anthropol. 2022 29 (3-4):183-187.doi: not available. https://doi.org/10.7546/AMA.29.3-4.2022.34
- Papadimitriou
A, Stephanou N, Papantzimas G, Glynos G, Philippidis Ph. Sexual
maturation of Greek boys. Ann Hum Biol 2002 Jan-Feb;29 (1):105-108.
doi: 10.1080/03014460110054966 https://doi.org/10.1080/03014460110054966 PMid:11822482
- Musaiger
AO. Height, weight and menarcheal age of adolescent girls in Oman. Ann
Hum Biol.1991;18 (1):71-74. doi:10.1080/03014469100001412. https://doi.org/10.1080/03014469100001412 PMid:2009007
- Rigon
F, De SanctisV, Bernasconi S, Bianchin L, Bona G, Bozzola M, Buzi F,
Radetti G, Tatò L, Tonini G, De Sanctis C, Perissinotto E. Menstrual
pattern and menstrual disorders among adolescents: an update of the
Italian data. Ital J Pediatr. 2012; 38: 38.
doi:10.1186/1824-7288-38-38. https://doi.org/10.1186/1824-7288-38-38 PMid:22892329 PMCid:PMC3462713
- Psihogios
V, Rodda C, Reid E, Clark M, Clarke C, Bowden D. Reproductive health in
individuals with homozygous β-thalassemia: knowledge, attitudes, and
behavior. Fertil Steril.2002;77(1):119-127. doi: 10.
1016/S0015-0282(01)02933-8. https://doi.org/10.1016/S0015-0282(01)02933-8 PMid:11779601
- Safarinejad
MR. Reproductive Hormones and Hypothalamic-Pituitary - Ovarian Axis in
Female Patients With Homozygous β-Thalassemia Major. J Pediatr Hematol
Oncol.2010;32 (4):259-266. doi: 10.1097/MPH.0b013e3181cf8156 https://doi.org/10.1097/MPH.0b013e3181cf8156 PMid:20445415
- Abd
FR, Jiiad SS, Hamza RA. Assessment of self care management regarding
menstrual disorders among thalassemia females. Chin J Ind Hyg Occup
Dis.2022;451-458.doi:10.5281/zenodo.6624581.
- Frisch
RE, Revelle R. Height and weight at menarche and a hypothesis of
critical body weights and adolescent events. Science.1970;169:397-9.
doi: 10.1126/science.169.3943.397 https://doi.org/10.1126/science.169.3943.397 PMid:5450378
- Bratke
H, Bruserud IS, BrannsetherB, Aßmus J, Bjerknes R 3, Roelants M,
Júlíusson PB. Timing of menarche in Norwegian girls: associations with
body mass index, waist circumference and skinfold thickness. BMC
Pediatr. 2017: 17:138. doi:10.1186/ s12887-017-0893-x. https://doi.org/10.1186/s12887-017-0893-x PMid:28587648 PMCid:PMC5461627
- Varimo
T, Miettinen PJ, Känsäkoski J, Raivio T, Hero M. Congenital
hypogonadotropic hypogonadism, functional hypogonadotropism or
constitutional delay of growth and puberty? An analysis of a large
patient series from a single tertiary center. Hum Reprod. 2017;
32:147-153. doi:10.1093/humrep/dew294. https://doi.org/10.1093/humrep/dew294 PMid:27927844
- Irena
I. Etiology of Central (Hypogonadotropic). Female Hypogonadism:
Genetic, Organic and Functional Causes. Glob J Reprod Med.2017; 2 (5):
555600. doi:10.19080/GJORM.2017.02.555600. https://doi.org/10.19080/GJORM.2017.02.555600
- Fernandez-Fernandez
R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E,
Pinilla L. Effects of ghrelin upon gonadotropin-releasing hormone and
gonadotropin secretion in adult female rats: in vivo and in vitro
studies. Neuroendocrinology. 2005; 82: 245-255. doi: 10.1159/000092753.
Epub 2006 Apr 20. https://doi.org/10.1159/000092753 PMid:16721030
- Moshtaghi-Kashanian
GR, Razavi F. Ghrelin and leptin levels in relation to puberty and
reproductive function in patients with beta-thalassemia. Hormones
(Athens). 2009;8(3): 207-13. https://doi.org/10.14310/horm.2002.1237 PMid:19671520
- Puah
JA, Bailey CJ. Effect of ovarian hormones on glucose metabolism in
mouse soleus muscle. Endocrinology. 1985 Oct;117(4):1336-1340. doi:
10.1210/endo-117-4-1336 https://doi.org/10.1210/endo-117-4-1336 PMid:3896756
- Meier
DA, Garner CW. Estradiol stimulation of glucose transport in rat
uterus. Endocrinology. 1987 Oct; 121(4):1366-1374. doi:
10.1210/endo-121-4-1366 https://doi.org/10.1210/endo-121-4-1366 PMid:3653031




