R.A. Stuurman1, E. Jong2, P.C.R. Godschalk3, M.F. Corsten2 and J.E. Nagtegaal4.
1 Department of Pharmaceutical Sciences, Utrecht University, Utrecht, The Netherlands.
2 Department of Internal Medicine, Meander Medical Centre, Amersfoort, The Netherlands.
3 Department of Clinical Microbiology, Meander Medical Centre, Amersfoort, The Netherlands.
4 Department of Hospital Pharmacy, Meander Medical Centre, Amersfoort, The Netherlands.
Published: November 01, 2023
Received: June 08, 2023
Accepted: October 26, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023067 DOI
10.4084/MJHID.2023.067
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Patients
with malignant hematologic diseases or after haematopoietic stem cell
transplantation (HSCT) have weakened immune systems due to their
primary diagnosis and/or treatment.[1] Treatment can induce periods of neutropenia, defined by an absolute neutrophil count (ANC) below <500 cells/μL.[2,3] In addition to often concurrent mucositis, this predisposes to infections, which often occur in this population.[4]
In patients with chemotherapy-induced neutropenia, the prevalence of
febrile neutropenia has been suggested to rise up to 80%.[4]
Fever caused by an infection is a dangerous complication and can be
lethal; therefore, prompt antibiotic treatment is indicated.[3,4]
Meropenem is an ultra broad-spectrum antibiotic in the β-lactam class often used to treat febrile neutropenia.[5,6]
Empiric treatment with meropenem reduces mortality risk in patients
with infections caused by extended-spectrum beta-lactamase
(ESBL)-producing bacteria and other multidrug-resistant (MDR)
gram-negative bacteria.[2] However, meropenem use is
associated with an increased risk of clostridium infections and
candidemia and an increased risk of acute graft-versus-host disease in
patients undergoing allogeneic HSCT.[7-12] For these
reasons, as well as to promote antibiotic stewardship, the Dutch
Working Party on Antibiotic Policy (SWAB) has labelled carbapenems,
including meropenem, as a second-line treatment option.[13]
The
nationally recommended primary choice of antimicrobial therapy is
dependent on a pre-emptive risk stratification based on the expected
duration of neutropenia (≤7 days vs. >7 days).[13]
In high-risk neutropenic patients, antipseudomonal β-lactams such as
ceftazidime and piperacillin/tazobactam are the preferred choice of
antibiotic therapy.[13] Standard-risk neutropenic
patients with an expected short duration of neutropenia are treated
according to their Multinational Association of Supportive Care in
Cancer (MASCC) score.
Until 2023, the protocol in our hospital
advised treatment of febrile neutropenia in high-risk patients with
meropenem for at least 72 hours.[6] As no data are
available on local resistance patterns for antipseudomonal β-lactams,
making unguided changes to the treatment protocol to adhere to the
national guidelines can be challenging in this vulnerable patient
population.
This study aims to determine the frequency of bacteria
resistant to ceftazidime, piperacillin/tazobactam, and meropenem in
diagnostic cultures in haematology patients admitted with febrile
neutropenia to our hospital. Doing so can provide insight into the
appropriateness of meropenem use and possibilities for responsible
adjustments to the current empiric febrile neutropenia treatment
protocol.
Methods
Study design and outcomes.
A retrospective, observational, single-centre study was carried out at
Meander MC - a teaching hospital in the Netherlands, using a single
cohort design of adult patients admitted with hematologic disease and
febrile neutropenia between October 2018 and June 2021. The primary
outcome was the frequency of bacteria resistant to the antibiotics of
interest in diagnostic blood and urine cultures taken on admission for
febrile neutropenia. Our antibiotics of interest were ceftazidime,
piperacillin/ tazobactam, and meropenem.
Only the first diagnostic
blood and urine samples, taken after the occurrence of fever, were
included in the results. Two other relevant diagnostic cultures, namely
a line tip and wound culture, were also included in data collection as
their results were the base of treatment evaluation.
Although
meropenem has an ultra-broad-spectrum coverage, it does not treat
infections caused by some gram-positive cocci, such as Staphylococcus epidermis and Enterococcus faecalis.[14] Except for E. faecalis,
which is sensitive to piperacillin/tazobactam, the bacteria mentioned
above were resistant to both meropenem and either of the alternatives.[14]
When these gram-positive cocci are either suspected or found, they are
treated with different antibiotics, such as vancomycin.[13]
To avoid reporting results biased as a higher resistance frequency, we
displayed bacteria separately based on their resistance status to
meropenem. These are not included in the calculations of resistance
percentages to piperacillin/tazobactam and ceftazidime.
Data collection and statistical analysis.
Data collection was carried out in accordance with the Dutch Medical
Treatment Contracts Act (WGBO). The study was approved by the
scientific research committee of the hospital. Data were analysed using
SPSS (version 24). Categorical variables were reported as frequencies.
Continuous variables were defined as mean and standard deviation when
normally distributed or as a median and interquartile range when they
were not.
Results
Population demographics.
100 patients (58 male, 42 female) admitted between October 2018 and
June 2021 were enrolled in this study. The median age was 65.0
(54.0-73.8) years, the median BMI was 24.95 (22.3-29.4) kg/m2 and the median duration of hospital admission was 21.5 (9.3-31.0) days. Additional population demographics are shown in Table 1.
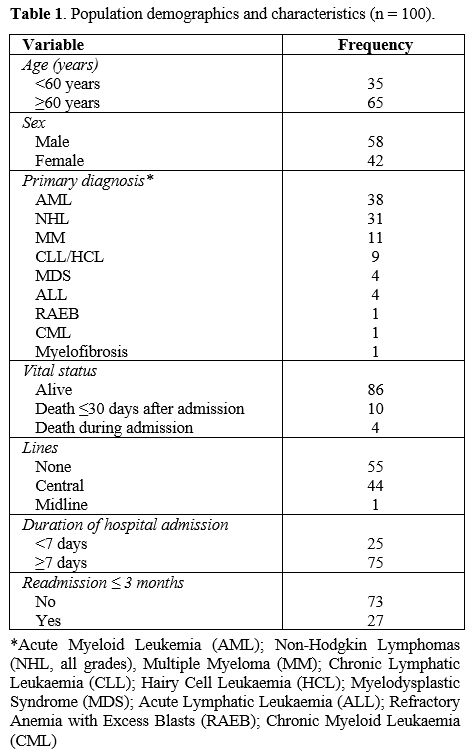 |
- Table 1. Population demographics and characteristics (n = 100).
|
Resistance frequencies.
Blood and urine cultures were taken in 100 and 62 patients,
respectively. Two other diagnostic cultures originated from a line tip
and wound. Resistance to ceftazidime was found in seven (7%) patients,
divided over seven blood cultures and one wound culture (Figure 1). Resistance to piperacillin/tazobactam was confirmed in only a single urine culture from one (1%) patient.
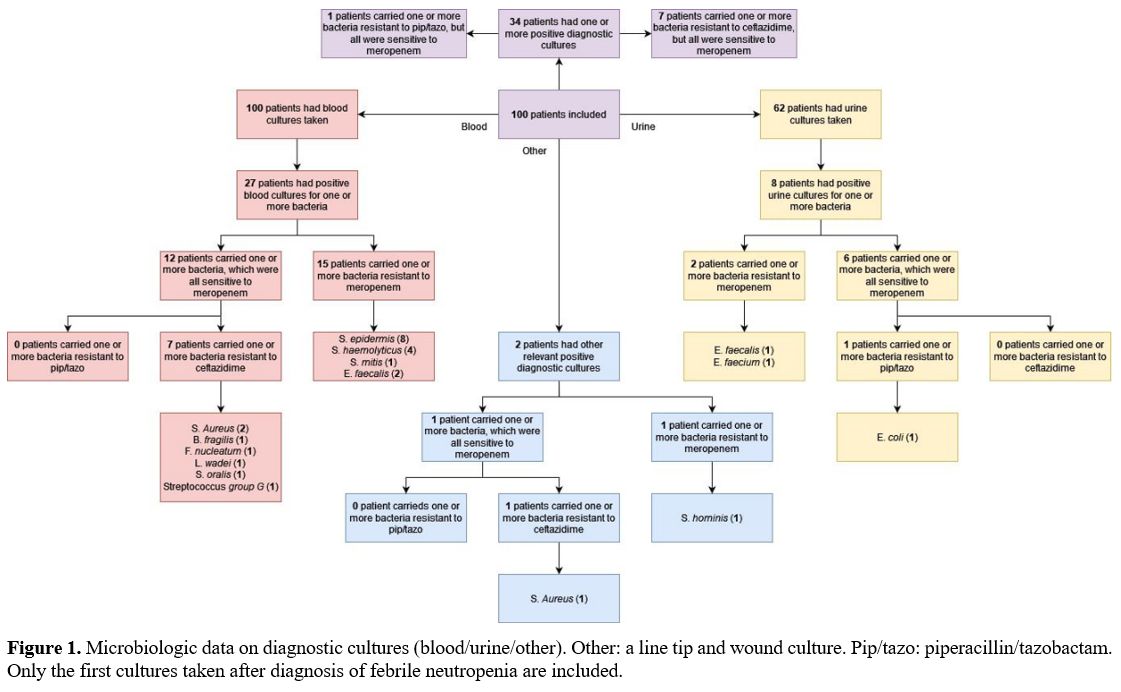 |
- Figure 1. Microbiologic
data on diagnostic cultures (blood/urine/other). Other: a line tip and
wound culture. Pip/tazo: piperacillin/tazobactam. Only the first
- cultures taken after diagnosis of febrile neutropenia are included.
|
Discussion
The
aim of this study was to determine the frequency of bacteria resistant
to ceftazidime, piperacillin/tazobactam and meropenem in diagnostic
cultures of hematologic patients admitted to our hospital with febrile
neutropenia. Retrospective analysis of diagnostic cultures showed a
resistance frequency of 7% to ceftazidime and 1% to
piperacillin/tazobactam. Furthermore, three diagnostic cultures showed E. faecalis,
which is susceptible to the latter but a poor target for meropenem.
These frequencies support that the hospital’s empiric treatment
protocol for haematology patients admitted with febrile neutropenia can
be safely adjusted from meropenem to piperacillin/tazobactam.
It is important to keep in mind that Enterobacterales with intrinsic, chromosomally encoded AmpC beta-lactamase (“AmpC producers”), such as S. marcescens, C. freundii and E. cloacae complex,
can develop resistance to penicillins and cephalosporins due to the
selection of de-repressed mutants during treatment.[15]
The risk of resistance selection is especially high during therapy with
third-generation cephalosporins such as ceftazidime, whereas
piperacillin/tazobactam is only a weak inducer of AmpC derepression.
Data from observational studies suggest that piperacillin/ tazobactam
may be a treatment option for bloodstream infections with AmpC
producers, but no clinical trials are available.[16-19]
Therefore, current guidelines for antibiotic use in our hospital do not
recommend the use of penicillins (including piperacillin/tazobactam)
and cephalosporins for the treatment of infections with AmpC producers
and susceptibility results for penicillins, and cephalosporins are not
reported to the clinicians. For critically ill patients with febrile
neutropenia admitted to the intensive care unit, the first choice of
treatment remains a carbapenem.
This study provides data from a
relatively large sample readily applicable to the hospital’s clinical
practice. However, a prospective follow-up study comparing
clinical outcomes before
and after the suggested treatment adjustments
can strengthen the recommendations made. These outcomes should involve
mortality risk and resistance patterns at a minimum to confirm the
expected benefits, including antibiotic stewardship, without impairing
clinical outcomes.
Patient characteristics available at admission,
such as age, BMI, and recent hospital admissions, hold predictive value
and allow for more precise risk stratification.[1,2]
Including prospectively validated MASCC scores or other alternatives
would allow for more accurate assessments, thus further guiding
clinicians to the most appropriate antibiotic therapy.[13,20]
Conclusions
Based
on the results of this study, we have changed our protocol of empiric
antibiotic therapy of chemotherapy-induced neutropenia from meropenem
to piperacillin/tazobactam. Making this carefully considered change
helps us promote antibiotic stewardship while preserving our patients'
safety.
Acknowledgements
We
wish to thank P.C.A.M. Buijtels for her contributions to designing the
research protocol and data collection and P.C.M. Pasker-de Jong for her
advice in the field of research design.
References
- Averbuch D, Orasch C, Cordonnier C, et al. European
guidelines for empirical antibacterial therapy for febrile neutropenic
patients in the era of growing resistance: summary of the 2011 4th
European Conference on Infections in Leukemia. Haematol.
2013;98(12):1826. https://doi.org/10.3324/haematol.2013.091025
- Khoo
AL, Zhao YJ, Teng M, et al. Evaluation of a risk-guided strategy for
empirical carbapenem use in febrile neutropenia. Ijantimicag. 2018
Sep;52(3):350-357. https://www.doi.org/10.1016/j.ijantimicag.2018.04.017
- Schmidt-Hieber
M, Teschner D, Maschmeyer G, Schalk E. Management of febrile
neutropenia in the perspective of antimicrobial de-escalation and
discontinuation. Expert review of anti-infective therapy. 2019 Dec
02;17(12):983-995. https://doi.org/10.1080/14787210.2019.1573670
- Blennow
O, Ljungman P. Infections in Hematology Patients. Concise Guide to
Hematology Cham: Springer International Publishing; 2018. p. 503-518. https://www.doi.org/10.1007/978-3-319-97873-4_38
- Wang
Y, Du Z, Chen Y, Liu Y, Yang Z. Meta-analysis: combination of meropenem
vs ceftazidime and amikacin for empirical treatment of cancer patients
with febrile neutropenia. Medicine. 2021 Feb 26;100(8):e24883. https://www.doi.org/10.1097/MD.0000000000024883
- Regelink
JC, Godschalk PCR, Russcher M. Richtlijn infectiepreventie en
antibioticabeleid hemato-oncologie Meander MC. Personal communication.
- Ballo
O, Kreisel E, Eladly F, et al. Use of carbapenems and glycopeptides
increases risk for Clostridioides difficile infections in acute myeloid
leukemia patients undergoing intensive induction chemotherapy. Ann
Hematol. 2020 Sep 24;99(11):2547-2553. https://www.doi.org/10.1007/s00277-020-04274-1
- Cornistein
W, Mora A, Orellana N, Capparelli FJ, del Castillo M. Candida:
epidemiology and risk factors for non-albicans species. Enferm Infecc
Microbiolog Clin. 2012;31(6):380-384. https://www.doi.org/10.1016/j.eimc.2012.09.011
- Ben-Ami
R, Olshtain-Pops K, Krieger M, et al. Antibiotic Exposure as a Risk
Factor for Fluconazole-Resistant Candida Bloodstream Infection.
Antimicrob Agents Chemother. 2012 May 01;56(5):2518-2523. https://www.doi.org/10.1128/AAC.05947-11
- Zaoutis
TE, Prasad PA, Localio AR, et al. Risk Factors and Predictors for
Candidemia in Pediatric Intensive Care Unit Patients: Implications for
Prevention. Clini Infect Dis. 2010 Sep 01;51(5):e38-e45. https://www.doi.org/10.1086/655698
- Paul
M, Yahav D, Bivas A, Fraser A, Leibovici L, Paul M. Anti‐pseudomonal
beta‐lactams for the initial, empirical, treatment of febrile
neutropenia: comparison of beta‐lactams. Cochrane library. 2010 Nov
10;2015(2):CD005197. https://www.doi.org/10.1002/14651858.CD005197.pub3
- Elgarten
CW, Li Y, Getz KD, et al. Broad-Spectrum Antibiotics and Risk of
Graft-versus-Host Disease in Pediatric Patients Undergoing
Transplantation for Acute Leukemia: Association of Carbapenem Use with
the Risk of Acute Graft-versus-Host Disease. Transplant Cellular Ther.
2021 Feb;27(2):177.e1-177.e8. https://www.doi.org/10.1016/j.jtct.2020.10.012
- SWAB.
The Dutch Working Party on Antibiotic Policy (SWAB) recommendations for
the diagnosis and management of febrile neutropenia in patients with
cancer Committee. [Internet]. Available at: https://swab.nl/nl/febriele-neutropenie-algemene-informatie [Accessed February 26th, 2022].
- Young P, Prisides A. Antibiotic sensitivity overview. [Internet]. Available at: https://drug.wellingtonicu.com/Appendices/5/ [Accessed February 26th, 2022].
- Macdougall
C. Beyond Susceptible and Resistant, Part I: Treatment of Infections
Due to Gram-Negative Organisms With Inducible β-Lactamases. J Pediatr
Pharmacol Ther. 2011-01-01;16(1):23. https://www.doi.org/10.5863/1551-6776-16.1.23
- Harris
PN, Wei JY, Shen AW, et al. Carbapenems versus alternative antibiotics
for the treatment of bloodstream infections caused by Enterobacter,
Citrobacter or Serratia species: a systematic review with
meta-analysis. J Antimicrob Chemother 2016;71:296-306. https://doi.org/10.1093/jac/dkv346
- Cheng
L, Nelson BC, Mehta M, et al. Piperacillin-tazobactam versus other
antibacterial agents for treatment of bloodstream infections due to
AmpC beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents
Chemother 2017;61:e00276-17. https://doi.org/10.1128/AAC.00276-17
- Tan
SH, Ng TM, Chew KL, et al. Outcomes of treating AmpC-producing
Enterobacterales bacteraemia with carbapenems vs. non-carbapenems. Int
J Antimicrob Agents 2020;55:105860. https://doi.org/10.1016/j.ijantimicag.2019.105860
- Drozdinsky
G, Neuberger A, Rakedzon S, et al. Treatment of bacteremia caused by
Enterobacter spp.: should the potential for ampC induction dictate
therapy? A retrospective study. Microb Drug Resist 2021;27:4104. https://doi.org/10.1089/mdr.2020.0234
- Choi
A, Park I, Lee HS, Chung J, Kim MJ, Park YS. Usefulness of complete
blood count parameters to predict poor outcomes in cancer patients with
febrile neutropenia presenting to the emergency department. Annals of
medicine. 2022 Dec 31;54(1):599-609. https://www.doi.org/10.1080/07853890.2022.2031271

