Ya-wei Li1, Qing Wan1, Ying Cheng2 and Hong-bo Hu1.
1 Department of Laboratory, Maternal and Child Health Hospital of Hubei Province, China.
2 Department of Pediatrics, Maternal and Child Health Hospital of Hubei Province, China.
Correspondence to:
Hong-bo Hu, Department of Laboratory, NO. 745 Wu Luo Road, Hongshan
District, Wuhan City, Hubei Province, P.R. China, 430070. E-mail:
hongbo1172@163.com
Published: September 1, 2023
Received: June 21, 2023
Accepted: August 9, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023049 DOI
10.4084/MJHID.2023.049
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Kawasaki
disease (KD) is a common childhood disease that primarily affects
small- and medium-sized arteries, particularly the coronary arteries,
causing severe cardiovascular disease. It is generally accepted that KD
is an autoimmune disorder activated by various microbial agents.[1-4]
Previous research found that roughly one-third of children with KD developed respiratory symptoms.[5,6]
Before confirming the diagnosis of KD, febrile children with
respiratory symptoms undergo laboratory testing to identify respiratory
viruses.[5,6] Human rhinoviruses (HRV), a common cause
of the common cold, can lead to various clinical manifestations beyond
the typical upper respiratory symptoms, ranging from exacerbations of
underlying lung diseases to extrapulmonary complications.[7]
There have been isolated case reports tentatively suggesting a
potential association between HRV infections and KD in children.[8,9]
However, convincing evidence from well-designed epidemiological studies
is still lacking, and the mechanisms behind this putative association
remain elusive.
This study aimed
to investigate the possible role of HRV infection in the development of
KD. Children with KD who tested negative for respiratory viruses and
those who tested positive for other respiratory viruses served as
controls. By analyzing and comparing the three groups' demographic,
clinical, and laboratory characteristics, this study attempted to gain
further insight into the potential contribution of HRV infection to the
pathogenesis of KD.
Methods
Patient selection.
According to the American Heart Association, KD is distinguished by a
5-day fever and at least four of the five primary clinical
characteristics of KD.[9] Between January 2022 and
December 2022, children hospitalized with KD who tested positive for
HRV were enrolled as the study subjects. At the same time, children
with KD who tested positive for other respiratory viruses and those
with negative respiratory virus tests were enrolled as control groups
for comparison.
Laboratory tests.
In addition to Mycoplasma pneumoniae and Chlamydia, a panel of
respiratory viruses, including Flu A (H1N1 and H3N2) and B, respiratory
syncytial virus (RSV), human parainfluenza virus (HPIV), HRV, human
metapneumovirus (HMPV), human coronavirus (HCoV: NL63, OC43, 229E, and
HKU1), human adenovirus (HAdV), and human bocavirus (HBoV)were detected
in these specimens using commercial polymerase chain reaction (PCR)
–capillary electrophoresis kits (Ningbo Haiers Gene Technology Co.,
Ltd., China).
Exclusion criteria.
The exclusion criteria included the following: (1) patients with
potential chronic diseases (e.g., congenital anomalies; genetic
disorders; immunodeficiency; and autoimmune, cardiovascular,
endocrinologic, hematological, hepatobiliary tract, or respiratory
diseases), (2) coinfection with bacteria or other pathogens, and (3)
patients with insufficient clinical data.
Statistical analyses.
The statistical analyses were performed using SPSS ver. 21.0 software
(SPSS, Inc., Chicago, IL, USA). Chi-square or Fisher's exact tests were
used to compare group frequency distributions. Normally distributed
continuous data are presented as mean ± standard deviation. The mean
values between groups were compared using the independent sample
t-test. A P value of <0.05 was considered statistically significant.
Results
Demographic information and basic clinical features of the cases.
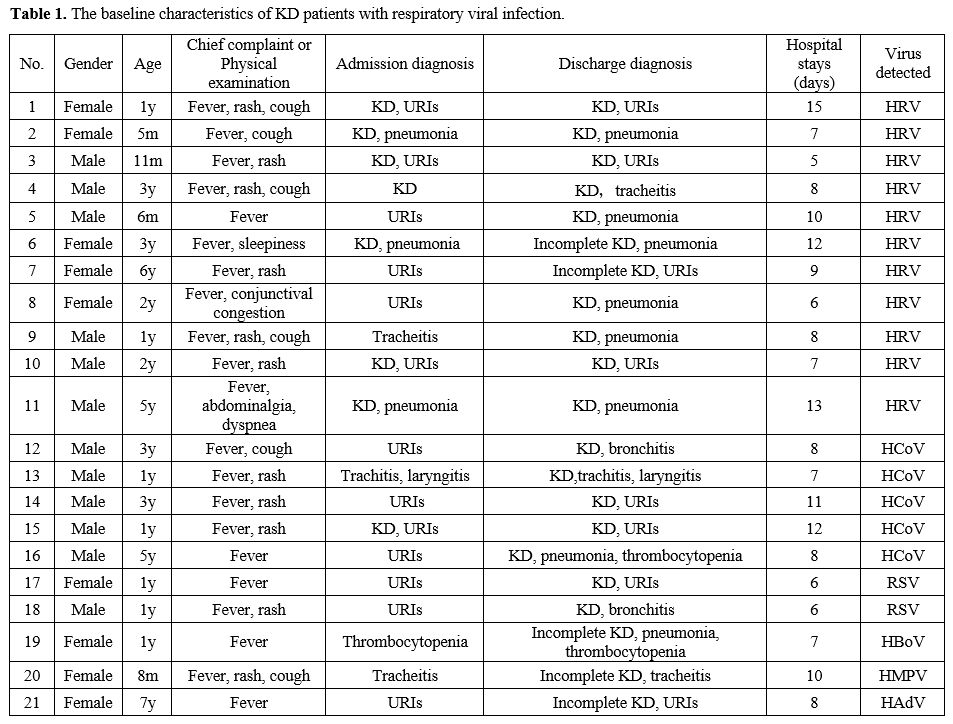
A total of 21 children with KD tested positive for respiratory viruses,
including 11 who were positive for HRV and 10 who were positive for
other respiratory viruses: 5 for HCoV, 1 for HBoV, 2 for RSV, 1 for
HAdV, and 1 for HMPV. Of the 11 KD children with HRV infection, 4
(36.4%) were admitted with respiratory tract infection, and 6 (54.5%)
were admitted with KD accompanied by respiratory tract infection. In
the discharge diagnosis, four cases (36.4%) had upper respiratory tract
infections (URIs), one (9.1%) had tracheitis, and six (54.5%) had
pneumonia. The demographic information and basic clinical features of
the cases are listed in Table 1.
 |
- Table
1. The baseline characteristics of KD patients with respiratory viral infection.
|
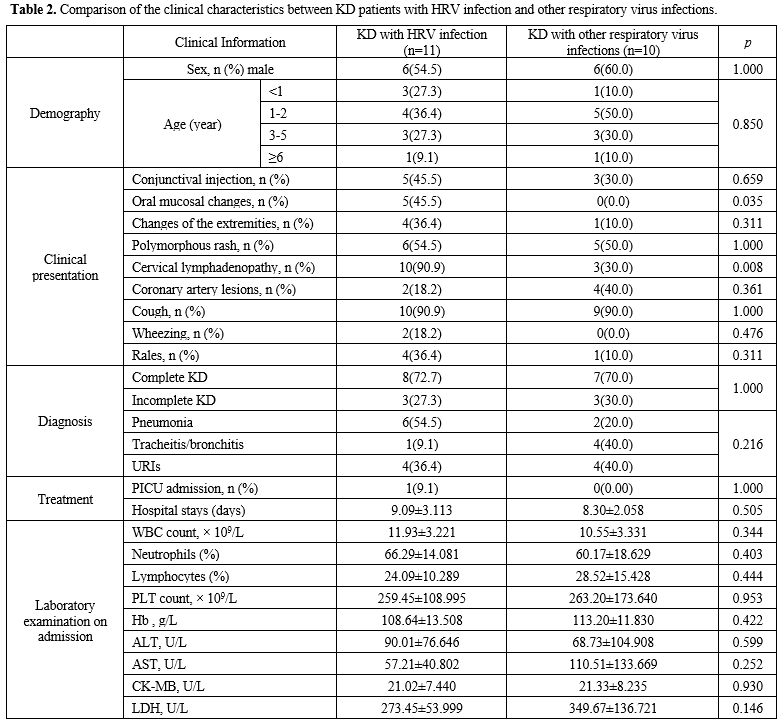
Comparison of the clinical characteristics between KD patients with HRV infection and with other respiratory viral infections. The clinical characteristics between KD patients with HRV infection and other respiratory viral infections are listed in Table 2.
Patients with HRV infection had the highest rate of clinical
presentation as oral mucosal changes (p = 0.035) and cervical
lymphadenopathy (p = 0.008).
 |
- Table 2. Comparison of
the clinical characteristics between KD patients with HRV infection and
other respiratory virus infections.
|
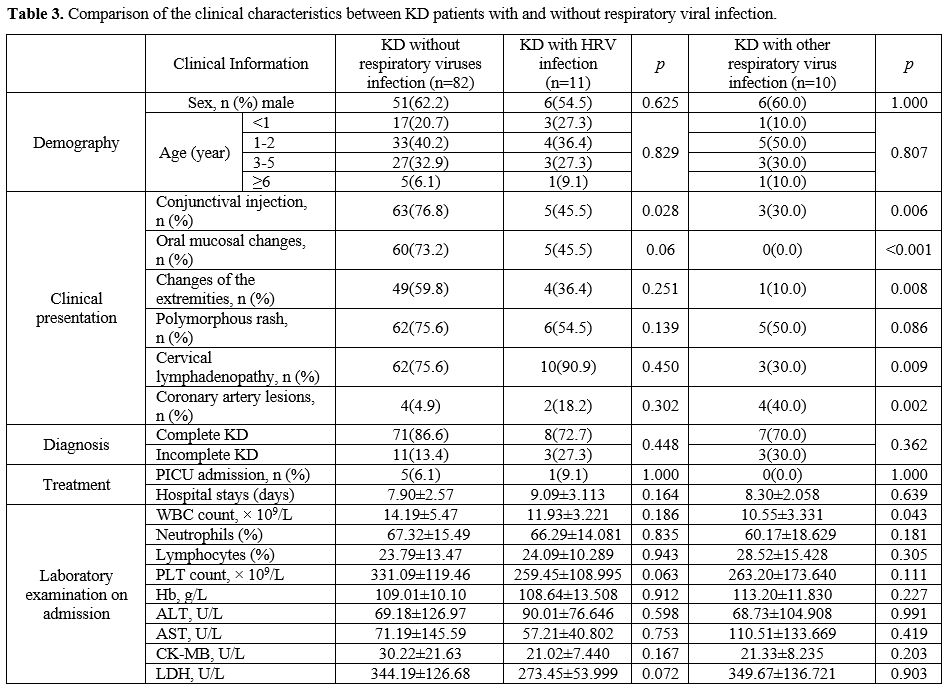
Comparison of the clinical characteristics between KD patients with and without respiratory viral infection. The results presented in Table 3
indicated that the rate of conjunctival injection was lower in the KD
group with HRV infection than in the group of KD patients who tested
negative for respiratory viruses (p = 0.028). The KD group with other
respiratory viral infections had significantly lower conjunctival
injection (p = 0.006), oral mucosal changes (p < 0.001), extremity
changes (p = 0.008), cervical lymphadenopathy (p = 0.009), and white
blood cell count (p = 0.043). In contrast, coronary artery lesions (p =
0.002) were substantially elevated.
 |
- Table 3. Comparison of the clinical characteristics between KD patients with and without respiratory viral infection.
|
Discussion
Of
the 11 KD children with HRV infection, 36.4% had a respiratory tract
infection at the time of admission, and 54.5% had KD and a respiratory
tract infection. While this study could not conclusively establish the
sequence of events leading from HRV respiratory infection to KD onset,
the association suggests that HRV infection likely contributes to the
risk of developing KD, at least in some children. HRV infection may act
as a co-factor that, in concert with other genetic and environmental
factors, plays a role in the pathogenesis of KD in susceptible
individuals. According to recent studies, one-half of patients with KD
were positive for a respiratory virus by PCR, and a large proportion of
patients with KD presented with concurrent respiratory symptoms.[5,11,12]
We
found that children with HRV-associated KD had a much higher incidence
of oral mucosal changes and cervical lymphadenopathy than children with
KD related to other viruses. Even though numerous respiratory viruses
display similar symptoms, they can have contrasted clinical
manifestations.[13,14] In other words, while various
respiratory viruses appear to trigger a generally comparable syndrome
known as KD with overlapping symptoms and signs, differences remain in
the precise clinical manifestations between instances linked to
separate viruses. The observed clinical differences could stem from
intrinsic HRV tropism for upper respiratory tissues or divergent immune
responses provoked by HRV during KD pathogenesis compared to mechanisms
of other respiratory viruses triggering KD.
In this study,
children with Kawasaki disease linked to respiratory virus infection
(virus-positive KD group) exhibited significantly lower rates of
conjunctival injection, oral mucosal changes, extremity changes, and
swollen lymph nodes than children without detectable respiratory virus
(virus-negative KD group). However, coronary artery lesions were
significantly more common in the virus-positive KD group, particularly
in the subset of children with HCoV (3 cases) or HMPV (1 case)
detected. These results suggest that:
1. Respiratory
virus-associated KD may represent a clinically distinct subset with a
different symptom profile due to distinct disease mechanisms.
2.
HCoV and HMPV, in particular, may be linked to a very severe course of
KD with a higher propensity for coronary lesions, potentially due to
differences in viral tropism or host response. These viruses could more
readily infect tissues involved in coronary damage or elicit an immune
reaction promoting vasculitis. Genetics may also play a role in disease
severity.
3. Larger scale studies are still needed to validate
links between individual respiratory viruses and KD severity, as this
study has limited power due to small sample sizes, especially for HMPV (1 case).
4.
Longer follow-up is also needed to determine longer-term outcomes, as
the degree of initial coronary changes does not necessarily directly
correlate with the need for intervention or resolution of disease. Some
cases with less initial coronary damage could progress over time, while
others remain stable. Furthermore, studies have indicated that IVIG can
stave off coronary artery abnormalities but has limited efficacy when
treating existing coronary damage.[15] All patients
with coronary artery anomalies in our study, both in the case and
control groups, were given IVIG within two days of being diagnosed with
KD. Only one case with concurrent HRV infection showed no response to
IVIG treatment. As a result, this element has little impact on our
conclusions.
Our study also has some limitations. First, this is a
retrospective study, and not all KD patients underwent respiratory
pathogen testing, so some potential positive cases may have been
missed. Second, the number of positive cases is relatively small, which
may lead to statistical bias. Finally, pathogen testing only involved
the detection of common respiratory pathogens. In the control group,
some infected KD cases may have had potential infections from
non-respiratory viruses. Further large-scale, multi-center prospective
studies are warranted to validate and extend these findings.
Conclusions
In
this study, we reported 11 HRV-associated KD and 10 cases of other
respiratory virus-associated KD cases. We compared the parameters
between these two groups and respiratory virus-negative KD cases.
Larger sample sizes are needed to confirm these differences and
elucidate whether KD cases triggered by different infectious agents
have distinct mechanisms of pathogenesis, which will facilitate more
personalized diagnosis and treatment approaches tailored to the
specific infectious trigger.
References
- Hu HB, Shang XP, Wu JG, Cai YL. The Immunologic
Profiles of Kawasaki Disease Triggered by Mycoplasma pneumoniae
Infection. Fetal Pediatr Pathol. 2022;1-9.
doi:10.1080/15513815.2022.2154133 https://doi.org/10.1080/15513815.2022.2154133 PMid:36484731
- Ünlü
AM, Holm M, Krusenstjerna-Hafstrøm T, Glarup M, Bjerre J, Herlin T.
Changes in Kawasaki disease incidence and phenotype during the COVID-19
pandemic. Dan Med J. 2023;70(6):A10220600. Published 2023 May 15. PMID:
37341355
- Neubauer
HC, Lopez MA, Haq HA, Ouellette L, Ramirez AA, Wallace SS. Viral
Coinfections in Kawasaki Disease: A Meta-analysis [published online
ahead of print, 2023 May 12]. Hosp Pediatr. 2023;e2023007150.
doi:10.1542/hpeds.2023-007150 https://doi.org/10.1542/hpeds.2023-007150 PMid:37170763
- Rigante
D. Kawasaki Disease as the Immune-Mediated Echo of a Viral Infection.
Mediterr J Hematol Infect Dis. 2020;12(1):e2020039. Published 2020 Jul
1. doi:10.4084/MJHID.2020.039 https://doi.org/10.4084/mjhid.2020.039 PMid:32670517 PMCid:PMC7340244
- Turnier
JL, Anderson MS, Heizer HR, Jone PN, Glodé MP, Dominguez SR. Concurrent
Respiratory Viruses and Kawasaki Disease. Pediatrics. 2015;136(3):
e609-e614. doi:10.1542/peds.2015-0950 https://doi.org/10.1542/peds.2015-0950 PMid:26304824
- Baker
AL, Lu M, Minich LL, et al. Associated symptoms in the ten days before
diagnosis of Kawasaki disease. J Pediatr. 2009;154(4):592-595.e2. doi:
10.1016/j.jpeds.2008.10.006 https://doi.org/10.1016/j.jpeds.2008.10.006 PMid:19038400 PMCid:PMC2745188
- To
KK, Lau SK, Chan KH, et al. Pulmonary and extrapulmonary complications
of human rhinovirus infection in critically ill patients. J Clin Virol.
2016; 77:85-91. doi: 10.1016/j.jcv.2016.02.014 https://doi.org/10.1016/j.jcv.2016.02.014 PMid:26921740
- Tan
YRL, Chow CC, Ganesan I, Leow HME. Hydrocele in a case of atypical
Kawasaki disease: case report and review of diagnostic criteria. BMC
Pediatr. 2021;21(1):279. Published 2021 Jun 15.
doi:10.1186/s12887-021-02758-1 https://doi.org/10.1186/s12887-021-02758-1 PMid:34130639 PMCid:PMC8204479
- Ohnishi
T, Sato S, Noda A, Tanaka M, Suganuma E. A case of concurrent
rhinovirus infection and Kawasaki disease complicated with acute
encephalopathy. Pediatr Int. 2022;64(1): e15167. doi:10.1111/ped.15167 https://doi.org/10.1111/ped.15167
- Newburger
JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term
management of Kawasaki disease: a statement for health professionals
from the Committee on Rheumatic Fever, Endocarditis and Kawasaki
Disease, Council on Cardiovascular Disease in the Young, American Heart
Association. Circulation. 2004;110(17):2747-2771. doi:
10.1161/01.CIR.0000145143.19711.78 https://doi.org/10.1161/01.CIR.0000145143.19711.78 PMid:15505111
- Kim
JH, Yu JJ, Lee J, et al. Detection rate and clinical impact of
respiratory viruses in children with Kawasaki disease. Korean J
Pediatr. 2012;55(12):470-473. doi:10.3345/kjp.2012.55.12.470 https://doi.org/10.3345/kjp.2012.55.12.470 PMid:23300502 PMCid:PMC3534160
- Chang
LY, Lu CY, Shao PL, et al. Viral infections associated with Kawasaki
disease. J Formos Med Assoc. 2014;113(3):148-154. doi:
10.1016/j.jfma.2013.12.008 https://doi.org/10.1016/j.jfma.2013.12.008 PMid:24495555 PMCid:PMC7125523
- Awad
S, Khader Y, Mansi M, et al. Viral Surveillance of Children with Acute
Respiratory Infection in Two Main Hospitals in Northern Jordan, Irbid,
during Winter of 2016. J Pediatr Infect Dis. 2020;15(1):1-10.
doi:10.1055/s-0039-1692972 https://doi.org/10.1055/s-0039-1692972 PMid:32300275 PMCid:PMC7117070
- Shokrollahi
MR, Noorbakhsh S, Monavari HR, Ghavidel Darestani S, Vosoughi Motlagh
A, Javadi Nia S. Acute nonbacterial gastroenteritis in hospitalized
children: a cross sectional study. Jundishapur J Microbiol. 2014;7(12):
e11840. Published 2014 Dec 1. doi:10.5812/jjm.11840 https://doi.org/10.5812/jjm.11840
- Rigante
D, Andreozzi L, Fastiggi M, Bracci B, Natale MF, Esposito S. Critical
Overview of the Risk Scoring Systems to Predict Non-Responsiveness to
Intravenous Immunoglobulin in Kawasaki Syndrome. Int J Mol Sci.
2016;17(3):278. Published 2016 Feb 24. doi:10.3390/ijms17030278 https://doi.org/10.3390/ijms17030278 PMid:26927060 PMCid:PMC4813142
[TOP]


