Hend Attia1, Mona El Nagdy2 and Radwa M Abdel Halim2.
1 Clinical and Chemical Pathology-Haematology, School of Medicine, Newgiza University, Giza, Egypt.
2 Clinical and Chemical Pathology, Kasr Alainy, Cairo University, Cairo, Egypt.
Correspondence to:
Dr. Hend Attia, MD. Lecturer of Clinical and Chemical pathology, School
of Medicine, Newgiza University, Giza, Egypt. Kilo 22 Cairo -
Alexandria Desert Road, Giza, Egypt. Tel: +2 01060533327. Email:
Hend.mokhtar@ngu.edu.eg ORCID: 0000-0002-9948-6068
Published: September 1, 2023
Received: April 23, 2023
Accepted: August 8, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023046 DOI
10.4084/MJHID.2023.046
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objectives:
Research supports the role of monocyte/macrophage activation in
COVID-19 immunopathology. This study aimed to evaluate sCD14 and sCD163
- the monocyte activation markers - and to investigate their relation
to hematological parameters and blood morphology in COVID-19 infection.
Methods:
This is a case-control study that included 70 COVID-19 patients.
Patients were subdivided into two groups: 23 severely diseased
ICU-admitted patients and another group of 47 non-ICU-admitted
patients. sCD163 and sCD14 levels were determined using ELISA.
Results:
sCD163 and sCD14 showed significantly higher levels in sera of patients
compared to the control group, with significantly higher levels of
sCD163 in ICU-admitted patients than non-ICU admitted patients.
Receiver operating characteristic curve analysis demonstrated the
usefulness of sCD163 with a cut-off value of 734 ng/mL as a potential
marker to discriminate between ICU and non-ICU admitted COVID-19
patients (sensitivity of 81.16%; specificity of 96.67% and positive
predictive value of 98% with area under the curve of 0.930). sCD163
levels showed a positive correlation with total white blood cells,
absolute neutrophilic count, Neutrophil/Lymphocyte ratio, and a
negative correlation with platelet count. sCD14 levels positively
correlated with D-dimer values associated with a shift to the left and
neutrophilic toxic granulations in blood morphology.
Conclusion:
High sCD163 and sCD14 levels, hematological parameters, and blood
morphology reflect monocyte activation in COVID-19 infection. sCD163 is
a potential marker of disease severity. These findings support further
study of therapeutics targeting macrophage activity in COVID-19
patients with high sCD163 levels.
|
Introduction
The coronavirus disease of 2019 (COVID-19) infection has resulted in 4.8 million cases and 323,000 deaths worldwide[1-3] until May 2020. At present (August 2023), WHO shows 768.983.095 confirmed cases and 6.953.743 deaths (https://covid19.who.int/)
Monocytes
and macrophages have been implicated in the pathogenesis of COVID-19
infection, as evidenced by the detection of infiltration of
monocyte-derived macrophages in affected lungs of severely diseased
COVID-19 patients.[4,5]
CD14 and CD163 are
myeloid differentiation markers found primarily on monocytes and
macrophages. These markers have been reported as reliable biomarkers of
monocyte-macrophage activation, which can be measured as soluble CD14
(sCD14) and CD163 (sCD163) released in plasma and serum.[4]
Monocyte
and macrophage activation and inflammatory immune response with the
production of a cytokine storm have been described in severe COVID-19
disease.[4] M2- Like anti-inflammatory Macrophage
activity in the lungs of COVID-19 patients has been reported in severe
lung affection and pulmonary fibrosis.[5] Level of
sCD163, the marker for M2-like macrophages detected in the serum of
COVID-19 patients, maybe a possible indicator of high M2-like
macrophages activity associated with lung damage and pulmonary fibrosis
in COVID-19 infection that requires intensive care unit (ICU)
admission.[5,6]
sCD14 is a biomarker of
monocyte/macrophage activation in COVID-19 infection. In addition, its
role in the activation of endothelial cells increases adhesion molecule
expression and procoagulant activity, which causes thrombosis in
COVID-19 pneumonia.[7] Evaluation of sCD14 in COVID-19
infection reflects an overriding act of monocyte and macrophage immune
response in COVID-19 infection.[7]
The
pathogenesis of COVID-19 is associated with immune dysregulation and
changes in cellular compartments, particularly macrophage M1 and M2
subtypes, which affect the level of released sCD163 and sCD14. These
significant immune system changes are also manifested by hematological
blood count changes in the form of lymphopenia and increased
neutrophil/lymphocyte (N/L) ratio, especially in patients with severe
COVID-19 illness.[8,9] The hematopoietic system, blood
count, and various hematological parameters are all affected by
COVID-19 infection, and these effects may have diagnostic and
prognostic significance.[10] Interestingly, emerging
evidence links COVID-19 progression and the appearance of variable
morphological changes in circulating blood cells and abnormal findings
on blood smears.[11,12]
The study aimed to
evaluate sCD14 and sCD163 - the monocyte activation markers - as
predictors of disease severity and ICU admission in COVID-19 and to
investigate their relation to hematological parameters, peripheral
blood morphological changes, and inflammatory biomarkers.
Material and Methods
Research design.
This case-control study was carried out for two months and was
conducted on 100 subjects. The study included 70 PCR-positive COVID-19
patients admitted to Kasr Al-Ainy Hospital, Cairo University. The
sample size was calculated using sample power 3. The power was set at
80% and the alpha level at 0.05. The study complied with the Research
Ethics Committee, Faculty of Medicine, Cairo University (N13-2023) and
adhered to the guidelines of the Declaration of Helsinki. Written
consent was obtained from all participants.
Subjects and material used.
The study included 100 subjects. The studied subjects were divided into
two groups: Group A included 70 PCR-positive COVID-19 patients with a
mean age of 59.93 years, 29 female patients (41.4%) and 41 male
patients (58.6%) with a female ratio of 1.4:1. Group B included 30
healthy volunteers with a mean age of 49.7. COVID-19 Patients (group A)
were subdivided into two groups: A group of 23 severely diseased
patients who required ICU admission and another group of 47 non-ICU
admitted patients. Patients were admitted to ICU according to the
published World Health Organization Clinical Management of COVID-19
management.[13]
Sample preparation.
Seven milliliters of venous blood were collected from each participant
and divided as follows: 3 mL of blood in a plain dry sterile
vacutainer, samples were allowed to clot at room temperature, and then
centrifuged at 3000 g for 10 min. The serum was separated into three
aliquots; the first was used to analyze liver and kidney function
tests, and the second was used to assay C-reactive protein (CRP),
ferritin, procalcitonin, and interleukin-6. The third aliquots were
immediately frozen at -200C for assay
of sCD163 and sCD14. Two milliliters of blood in sterile K2-EDTA
vacutainers were used for complete blood picture and peripheral blood
film preparation. Two milliliters of blood were collected on sodium
citrate vacutainers, and plasma was separated for Prothrombin time
(PT), Partial thromboplastin time (PTT), and D-dimer assays.
Laboratory analysis.
The studied subjects confirmed to have COVID-19 by PCR (Cobas 6800 PCR
system) were subjected to laboratory investigations: (a) routine tests,
including complete blood picture, carried out using a Beckman Coulter
LH 750 hematology analyzer, and liver function tests (ALT and AST) and
kidney function tests. (Urea and creatinine) carried out using a
Beckman Coulter AU680 automated chemistry analyzer (Beckman Coulter,
Inc., Brea, CA, USA); (b) immunological tests, including CRP and
D-dimer carried out on Cobas c501 while ferritin, interleukin-6 and
procalcitonin carried out on Cobas e601 (Roche Diagnostics GmbH,
Indianapolis, IN, USA).
sCD163 levels were determined using
Elabscience Enzyme-linked immunosorbent assay (ELISA) (Elabscience,
Biotechnology Inc. China, catalog number: E-EL-H0036), and levels of
sCD14 were determined using SinoGeneClon ELISA (SinoGeneClon, Biotech
Co., Ltd, China, catalog number: SG-10117), following the
manufacturer’s instruction of both kits.
Statistics.
Version 23 of the Statistical Package for Social Science (IBM SPSS) was
used to gather and analyze the data. The interquartile range and median
were used to present quantitative data. Pearson's correlation
coefficients were used to study the relationship between sCD14 and
sCD163 levels in ICU and non-ICU patients. Values under 0.05 were
regarded as significant. Numbers and percentages were used to depict
the qualitative data, and the Chi-square test or Fisher exact test was
used to compare the groups. When comparing two independent groups with
quantitative data and a parametric distribution, the independent t-test
was used, whereas the Mann-Whitney test was used for non-parametric
distributions. The correlation between two numerical parameters within
the same group was evaluated using Spearman correlation coefficients.
Results
Descriptive data of the studied patients.
In this study, the patient group included 41 males and 29 females.
Their mean age was 59.93 years. Of 70 COVID-19 patients, 23 (32.9%) had
progressive disease requiring ICU admission. Demographics, laboratory
parameters, and peripheral blood smear findings of ICU and non-ICU
admitted patients are summarized in (Table 1).
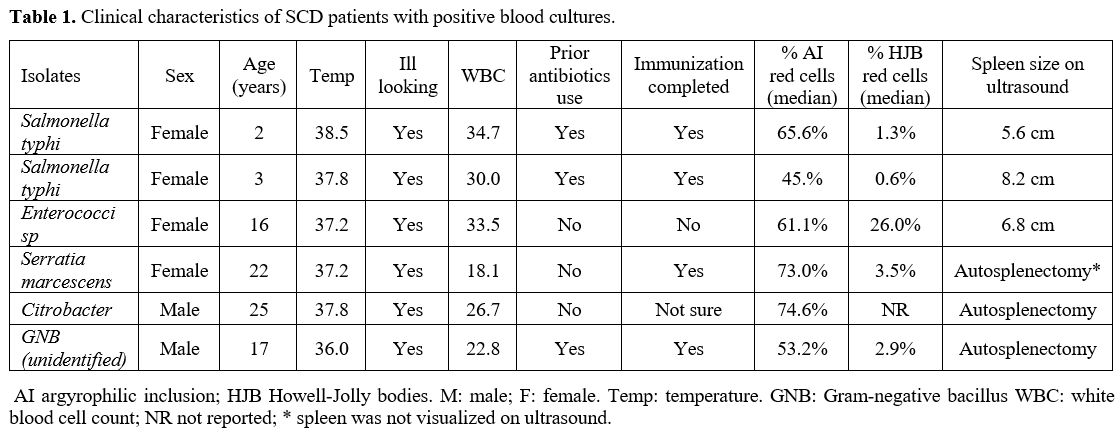 |
- Table
1. A summary of demographics, laboratory parameters and peripheral
blood smear findings of ICU and non-ICU admitted patients.
|
Serum sCD163 and sCD14 levels in the studied groups.
COVID-19 patients had significantly higher levels of sCD163 and sCD14
than the control group (sCD163 median 1198 ng/mL in COVID-19 patients
vs. 245 ng/mL in Control group; sCD14 median 9.3 ng/mL in COVID-19
patients vs. 8.35 ng/mL in the control group) (P<0.0001 and P=0.001,
respectively). ICU patients had significantly higher levels of sCD163
than non-ICU patients (1135 ng/mL vs. 1064 ng/mL, respectively)
(p=0.034). However, there was no statistically significant difference
in sCD14 levels between ICU and non-ICU patients (Table 1).
The performance of sCD163 as a marker of severe disease and ICU admission.
Receiver operating characteristic (ROC) curve analysis was performed to
evaluate the usefulness of CD163 as a potential marker to discriminate
between ICU and non-ICU admitted COVID-19 patients. A chosen cut-off
value of 734 ng/mL demonstrated a sensitivity of 81.16%, specificity of
96.67%, and positive predictive value of 98% with area under the curve
of 0.930 (Figure 1).
 |
- Figure 1. The Receiver operating characteristic (ROC) curve of CD 163 and CD 14 regarding ICU versus non ICU admitted patients.
|
Comparison of hematological findings among studied groups: changes in total leukocytic count (TLC) and morphology.
COVID-19 patients had significantly higher TLC, absolute neutrophilic
count (ANC), and N/L ratio than the control group (P<0.001,
P<0.001, and P<0.001, respectively) and significantly lower
absolute lymphocytic, monocytic, and eosinophilic counts (P<0.001,
P<0.001, and P<0.001, respectively). Patients admitted to ICU had
a significantly higher N/L ratio (P=0.009) and ANC (P=0.029), as well
as a considerably lower absolute lymphocytic (P=0.023) and monocytic
count (P=0.024) than non-ICU admitted patients.
The morphological
features of the study control, and ICU-admitted and non-ICU-admitted
groups were significantly different. COVID-19 patients had considerably
more myelocytes, metamyelocytes, shift to the left, and neutrophilic
toxic granulation (P=0.003, P=0.006, P=0.006 and P=0.003, respectively)
than the control group. The ICU-admitted patients with severe disease
had more frequent toxic granulation in the peripheral smear that was
significantly more evident compared to non-ICU admitted patients
(P=0.012) (Table 1, Figure 2A and 2B). Only Five cases out of the 70 studied COVID-19 subjects showed neutrophilic vacuolations (Figure 2C-2F)
 |
- Figure 2.
Morphological changes in peripheral blood in COVID-19 infection slides
stained using Leishman stain. A and B; Neutrophil granulocyte with
marked cytoplasmic hyper-granularity (toxic granulations). C-F;
neutrophils vacuolization.
|
Changes in red blood cell indices.
The patient group had a significantly lower mean corpuscular volume
(MCV) (P=0.031) and a significantly higher red cell distribution width
(RDW) (P=0.000) than the control group. Patients requiring ICU
admission had significantly lower MCV and mean corpuscular hemoglobin
(MCH) compared to the non-ICU admitted group (P=0.012 and P=0.019,
respectively) (Table 1).
Changes in platelets and D-dimer. Abnormal platelet morphological findings were detected, such as platelet aggregation (n=6/70) (Figure 3E, 3F, and Figure 4B) and macro-platelets (n=8/70) (Figure 3A-3D),
but statistical analysis revealed no significant difference between
studied groups. Only one critically ill ICU patient had abnormal
neutrophil-platelet aggregates (Figure 4A).
COVID-19 patients had a lower platelet count and higher mean platelet
volume (MPV) than the control group (P=0.000 and P=0.000,
respectively). Compared to non-ICU admitted subjects, ICU patients had
significantly higher MPV (p=0.025).
 |
Figure 3. Platelet
morphology in COVID-19 infection slides stained using Leishman stain.
3A-3D; macro-platelets. 3E and 3F; platelet aggregates independent of
platelet count. |
 |
Figure 4. Platelet
morphology in COVID-19 infection slides stained using Leishman stain.
4A; Platelet neutrophil aggregates. 4B; Platelet aggregates. |
Patients
showed a significantly higher D-dimer than the control group
(P<0.001). D-dimer was also considerably higher in ICU patients
compared to the non-ICU group (p=0.003)
Changes in inflammatory markers.
Inflammatory markers in the form of serum ferritin and CRP levels were
significantly higher in patients compared to controls (P<0.001 and
P<0.001). Inflammatory markers, ferritin and CRP were also markedly
higher in ICU patients compared to non-ICU admitted subjects (P=0.001
and P=0.013, respectively).
Correlation between sCD163 and sCD14 levels and clinical and laboratory parameters.
In COVID-19 patients, levels of sCD163 levels showed positive
correlation with TLC (r=0.281, P=0.019), ANC (r=0.325, P=0.006), and
N/L ratio (r=0.377, P=0.001). A positive correlation between
levels of sCD163 and peripheral smear shift to left in form of
myelocytes and metamyelocytes (r=0.290 P=0.016 and r=0.261, P=0.030
respectively) was also detected. There was a negative correlation
between sCD163 levels and platelet count (r=-0.256, P=0.033), which
could be attributed to platelet activation by monocyte activation in
COVID-19 illness.
Interestingly, a positive correlation between
levels of sCD14 and D-dimer (r=0.271, P=0.030) was detected. A negative
correlation was also found between CD14 levels and INR (r= -0.674,
P=0.023) values. High levels of sCD14 were significantly associated
with peripheral smear shift to the left and neutrophilic toxic
granulations (P=0.036 and P=0.045, respectively).
Discussion
In
the present study, we measured sCD163 and sCD14 levels, the
monocyte/macrophage activation biomarkers in COVID-19 illness.
Monocytes and macrophages have been implicated in the pathogenesis of
COVID-19 infection.[4,7,14]
The first main group of results in this study were the significantly
higher levels of sCD163 and sCD14 levels in sera of COVID-19 patients
compared to the control subjects (P<0.0001 and P=0.001,
respectively). These findings are consistent with a recent study by
Gómez-Rial et al., 2020, who found that COVID-19 pneumonia patients had
higher levels of sCD163 and sCD14 than the control group.[4]
In
the current study, ICU-admitted patients with severe disease had
significantly higher sCD163 levels than non-ICU admitted patients
(P=0.034). Zingaropoli et al., 2021 published similar findings,
reporting higher sCD163 in patients with COVID-19 progression to acute
respiratory distress syndrome (ARDS) (p=0.002).[7] Our
results, on the other hand, contradict those of Gómez-Rial et al., 2020
which reported no significant difference in sCD163 levels between ICU
and non-ICU admitted patients.[4]
Along with the results of Zingaropoli et al. study in 2021,[7]
our findings highlight the clinical utility of sCD163 in determining
the severity of COVID-19 pneumonia and support previously reported data
of higher sCD163 levels in COVID-19 patients with poor outcome.[7,12,14,15]
Furthermore, we discovered a cut-off value of 734 ng/mL for sCD163
serum level, which was related to disease severity and ICU admission
(sensitivity 81.16% and specificity 96.67%). Our data highlight the
usefulness of sCD163 as a potential marker of predicting severity and
may shed light on the early use of monocyte immune-modulating therapy.
CD163
is a scavenger receptor that serves as a marker for M2-like
macrophages. The anti-inflammatory and immunosuppressive properties of
M2 macrophages aid in tissue repair and wound healing.[4,5] TGF-β and other anti-inflammatory cytokines are secreted by M2- macrophages.[15-18]
Kaku et al. 2014 study described a positive correlation between the
expression of the M2- macrophage CD163 marker on alveolar cells and
disease severity in chronic obstructive pulmonary disease.[6] sCD163 is shed into the serum via a shedding mechanism by the surface membranes of these activated macrophages,[19,20] which explains the significantly higher sCD163 levels in our ICU patients.
Nouno
et al., 2019 found M2-alveolar macrophages co-localized with high CD163
expression in interstitial pulmonary fibrosis patients' lungs in serial
sections.[21] The findings by Nouno et al., 2019
study explain the significantly higher level of sCD163 in our ICU
patients. Our results and previous research highlight the significance
of sCD163 levels as a possible indicator of M2-like macrophage activity
in the lungs of COVID-19 patients and support the therapeutic targets
of macrophage (M2) activation suppression.[11,18-21]
In
contrast, we found no statistically significant difference in sCD14
levels between ICU and non-ICU patients. Our findings are supported by
a previously reported transient increase in CD14-positive monocytes in
mild COVID-19 and its absence in severe COVID-19 infection, which was
explained by severe myeloid cell dysregulation.[22,23]
Blood counts are an important tool for estimating disease severity and mortality risk.[24]
The second group of results in this study was the significant
differences in blood count and peripheral morphology between our study
groups, implying that blood picture and peripheral morphology play an
important role in determining disease severity in COVID-19 infection.
Similar
to previous research, we discovered that COVID-19 patients had
significantly higher TLC, ANC, and N/L ratio (P<0.001, P<0.001,
and P<0.001, respectively) and significantly lower absolute
lymphocytic, monocytic, and eosinophilic count (P<0.001, P<0.001,
and P<0.001, respectively) compared to the control group.[25,26]
Patients admitted to ICU had a significantly higher N/L ratio and ANC
(P=0.009 and P=0.029) but a considerably lower absolute lymphocytic and
monocytic count (p=0.023 and P=0.024). These findings are consistent
with the results of previous studies.[26-28]
Regarding
the morphological examination of peripheral smears, we identified that
COVID-19 patients had significantly more frequent myelocytes,
metamyelocytes, shift to the left, and neutrophilic toxic granulation
than the control group (P=0.003, P=0.006, P=0.006 and P=0.003,
respectively). Similar results were obtained by previously published
studies.[29,30] When compared to non-ICU patients,
ICU-admitted patients had a significantly higher frequency of toxic
granulations in their peripheral smear (P=0.012) (Figure 2A and 2B),
which may be explained by a secondary bacterial infection as an
underlying cause of disease severity in our ICU admitted patients.
Comparing
our morphological findings to previous research, a study published in
2022 by Jain et al., which included 80 COVID-19-positive patients (41
ICU and 39 non-ICU) and 32 COVID-19-negative ICU patients, found
similar results. According to Jain et al. study, the overall mean TLC
count and ANC were higher in ICU patients compared to non-ICU patients
(WBC, 12.43 ± 1.5 vs. 10.8 ± 1.5 ×103/μl, p = 0.25 and mean ANC, 10.60 ± 1.3 vs. 5.32 ± 1.4 ×103/μl, p = 0.24, respectively) with higher frequency of left myeloid shift (p = 0.021).[31]
We
also reported on other morphological findings, such as neutrophilic
vacuolization, identified in five of our 70 COVID-19 patients (Figure 2C-2F). COVID-19 illness has been associated with neutrophilic vacuolization in peripheral blood.[28,32]
A study by Pozdnyakova et al. 2021 described different morphologic
alterations in 100% of patients with COVID-19 (90 patients).[28]
The most frequent morphologic finding was cytoplasmic vacuolization,
present in multiple cell types with varying frequency, including
neutrophils.[28]
To the best of our knowledge,
this is the first study to investigate the relationship between sCD14
and sCD163 levels, peripheral blood morphological findings, and other
blood count parameters. In our research, soluble CD163 levels
correlated positively with the N/L ratio, which has been described as
an independent prognostic biomarker in determining COVID-19 prognosis
and treatment efficacy.[33-37] In addition to the N/L
ratio, a positive correlation was found between sCD163 levels and ANC.
The morphological study of COVID-19 patients’ blood smears showed a
significant association with high CD 163 levels in the form of shift to
left and hyper-granular neutrophils with toxic granulations (p=0.003) (Figure 2A and 2B).
Patients
with COVID-19 infection had significantly lower platelet count, which
correlated negatively with higher sCD163 levels (r=-0.256, p=0.033),
indicating that monocytic activation is strongly linked to platelet
activation and consumption. Our findings are consistent with a previous
meta-analysis on a cohort of 7,613 COVID-19 patients by Jiang et al.,
2020, which found that lower platelet count is associated with severe
disease and poor outcomes.[38]
Low platelet count is a multifactorial finding in COVID-19 disease.[39,40]
Platelet consumption in COVID-19 disease has been attributed to
endothelial damage, platelet aggregates in the lung, bone marrow
suppression, and immune clearance.[39,40] According
to Thachil et al. study in 2020, the formation of pulmonary thrombi is
necessary for preventing viremic spread through the bloodstream, has an
anti-infective role, and produces platelet consumption.[41]
According
to our findings, COVID-19 patients had significantly higher MPV than
control subjects. We observed substantially higher MPV values in
ICU-admitted patients than in non-ICU patients (p=0.025). Our MPV
findings are consistent with the findings of Güçlü et al. study, which
described the MPV as a supplementary test in predicting the severity
and mortality in COVID-19 patients.[42] The trend
toward higher MPV persists even in COVID-19 patients with normal
platelet count, according to a study published in 2021 by Wool et al.[43]
These
MPV findings may reflect an ongoing platelet activation in COVID-19
infection. The high platelet size has been associated with a high
number of surface receptors and increased platelet content of ATP. The
larger platelets are active, with a higher potential for protein
synthesis and hemostasis.[44] Our results revealed no correlation between MPV values and sCD163 levels.
Regarding platelet morphology, we identified blood smear macro-platelets only in 8 of our COVID-19 patients (Figure 3-A, C, and D).
Pezeshki et al. 2021 study, which included 89 hospitalized COVID-19
patients, reported macro-platelets in 42.7% of studied COVID-19
patients.[45] Other studies also reported giant platelets in COVID-19 patients.[46,47] According to prior research, the lung is another source of megakaryocytes where platelets can be derived from this tissue.[48] Given that the COVID-19 virus primarily affects the lungs, there could be an explanation for our findings.
Six
out of 70 patients showed platelets aggregates in blood smear (3 ICU
admitted and 3 non-ICU admitted patients). Neutrophil-platelet
aggregates were also detected in only one patient (Figure 4A).
In 2021, Rampotas and Pavord reported platelet aggregates and
macro-thrombocytes in blood films of 20 ICU patients with COVID-19
infection, indicating increased platelet activity.[49]
The findings of Rampotas and Pavord, combined with ours, could provide
additional evidence of platelets' role in COVID-19-related thrombotic
complications. We found no significant correlation between platelet
aggregates on peripheral blood smears and sCD163 levels.
Regarding
D-dimer and other inflammatory markers, highly significant levels of
D-dimer, serum ferritin, and CRP were found in the COVID-19-infected
group of patients compared to the control group. In line with previous
research, D-dimer, serum ferritin, and CRP levels were significantly
higher in our ICU patients with disease progression than in non-ICU
admitted patients in our study.[49-51] We found no
correlation between sCD163 and D-dimer, serum ferritin, or CRP.
Similarly, Volfovitch et al., 2022 study discovered that sCD163 levels
and ferritin values correlated with the severity of COVID-19 infection,
but there was no significant correlation between ferritin rise and
sCD163 levels.[52] Similar to our findings, Zingaropoli et al., 2021 discovered no significant relationship between D-dimer and CD163.[7]
In
terms of the relationship between sCD14 levels and other peripheral
blood findings, our study is the first to show a significant
association between high sCD14 levels and left shift and toxic
granulations in neutrophils (p=0.036 and 0.045, respectively), but not
other white cell changes.
We found a positive correlation between
sCD14 levels and D-dimer (r=0.271, P=0.030). Regarding the D-dimer, our
findings are consistent with those of Zingaropoli et al., 2021,[7]
who discovered a positive correlation between plasma levels of sCD14
and D-dimer, implying a link between monocyte activation and
hypercoagulability. Our findings support the previously published
hypothesis that sCD14 activates endothelial cells, increasing adhesion
molecule expression and procoagulant activity, the main cause of
coagulation activation in COVID-19 pneumonia.[7,53]
Other studies showed a correlation between TF expression and plasma
levels of sCD14, the lipopolysaccharide (LPS) receptor produced by
monocytes upon in vivo LPS activation.[54,55]
Conclusions
The
data of this study highlight the role of sCD163 as a biomarker of
M2-macrophage activation in severe COVID-19 disease. Our findings
emphasize the role of monocytes and sCD14 in the activation process of
hypercoagulability associated with COVID-19 infection. Furthermore, the
differences in sCD163, sCD14 levels, blood count, and peripheral
morphology between ICU and non-ICU admitted patients uncover their
importance as tools in diagnosing and predicting disease progression.
The recognized preliminary data encourage further studies on a larger
scale and future clinical trial testing for therapeutic approaches
targeting immune-modulation of macrophage/monocyte (M2) response in
COVID-19 infection.
References
- Hottz ED, Azevedo-Quintanilha IG, Palhinha L,
Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P,
Bozza FA, Bozza PT. Platelet activation and platelet-monocyte aggregate
formation trigger tissue factor expression in patients with severe
COVID-19. Blood. 2020 Sep 10;136(11):1330-1341. https://doi.org/10.1182/blood.2020007252 PMid:32678428 PMCid:PMC7483437
- Zhou
P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang
CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR,
Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang
YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new
coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270-273.
https://doi.org/10.1038/s41586-020-2012-7 PMid:32015507 PMCid:PMC7095418
- World Health Organization. Coronavirus Disease (COVID-19) Situation Report-122. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200521-COVID-19-sitrep-122.pdf?sfvrsn524f20e05_2. Accessed 22 May 2020.
- Gómez-Rial
J, Currás-Tuala MJ, Rivero-Calle I, Gómez-Carballa A, Cebey-López M,
Rodríguez-Tenreiro C, Dacosta-Urbieta A, Rivero-Velasco C,
Rodríguez-Núñez N, Trastoy-Pena R, Rodríguez-García J, Salas A,
Martinón-Torres F. Increased Serum Levels of sCD14 and sCD163 Indicate
a Preponderant Role for Monocytes in COVID-19 Immunopathology. Front
Immunol. 2020 Sep 23;11:560381. https://doi.org/10.3389/fimmu.2020.560381 PMid:33072099 PMCid:PMC7538662
- Shive
CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific
marker of monocyte activation. AIDS. 2015 Jun 19;29(10):1263-5. https://doi.org/10.1097/QAD.0000000000000735 PMid:26035325 PMCid:PMC4452959
- Kaku
Y, Imaoka H, Morimatsu Y, Komohara Y, Ohnishi K, Oda H, Takenaka S,
Matsuoka M, Kawayama T, Takeya M, Hoshino T. Overexpression of CD163,
CD204 and CD206 on alveolar macrophages in the lungs of patients with
severe chronic obstructive pulmonary disease. PLoS One. 2014 Jan
30;9(1):e87400. https://doi.org/10.1371/journal.pone.0087400 PMid:24498098 PMCid:PMC3907529
- Zingaropoli
MA, Nijhawan P, Carraro A, Pasculli P, Zuccalà P, Perri V, Marocco R,
Kertusha B, Siccardi G, Del Borgo C, Curtolo A, Ajassa C, Iannetta M,
Ciardi MR, Mastroianni CM, Lichtner M. Increased sCD163 and sCD14
Plasmatic Levels and Depletion of Peripheral Blood Pro-Inflammatory
Monocytes, Myeloid and Plasmacytoid Dendritic Cells in Patients With
Severe COVID-19 Pneumonia. Front Immunol. 2021 Feb 26;12:627548. https://doi.org/10.3389/fimmu.2021.627548 PMid:33777012 PMCid:PMC7993197
- Tay
MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19:
immunity, inflammation and intervention. Nat Rev Immunol. 2020
Jun;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8 PMid:32346093 PMCid:PMC7187672
- Gracia-Hernandez
M, Sotomayor EM and Villagra A (2020) Targeting Macrophages as a
Therapeutic Option in Coronavirus Disease 2019. Front. Pharmacol.
11:577571. https://doi.org/10.3389/fphar.2020.577571 PMid:33324210 PMCid:PMC7723423
- Terpos
E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN,
Politou M, Psaltopoulou T, Gerotziafas G, Dimopoulos MA. Hematological
findings and complications of COVID-19. Am J Hematol. 2020
Jul;95(7):834-847. https://doi.org/10.1002/ajh.25829 PMid:32282949 PMCid:PMC7262337
- Berber
I., Cagasar O., Sarici A., Berber K.N., Aydogdu I., Ulutas O., AsliY.,
Bag H.G.G., Delen L.A.Peripheral blood smear findings of COVID-19
patients provide information about the severity of the disease and the
duration of hospital stay. Mediterr J Hematol Infect Dis 2021, 13(1). https://doi.org/10.4084/mjhid.2021.009 PMid:33489048 PMCid:PMC7813282
- Tan
L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, Wang Q, Miao H.
Lymphopenia predicts disease severity of COVID-19: a descriptive and
predictive study. Signal Transduct Target Ther. 2020 Mar 27;5(1):33. https://doi.org/10.1038/s41392-020-0148-4 PMid:32296069 PMCid:PMC7100419
- World Health Organization Clinical management of COVID-19: interim guidance. World Health Organization 2020, https://www.who.int/publications/i/item/clinical-management-of-covid-19 (2020, accessed 27 May 2020). https://doi.org/10.15557/PiMR.2020.0004
- Christensen
JE, Thomsen AR. Co-ordinating innate and adaptive immunity to viral
infection: mobility is the key. APMIS. 2009: 117:338-55. https://doi.org/10.1111/j.1600-0463.2009.02451.x PMid:19400861
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008 Dec;8(12):958-69. https://doi.org/10.1038/nri2448 PMid:19029990 PMCid:PMC2724991
- La
Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner
N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, Kumar A, van
Laar JAM, Lachmann G, Nichols KE, Ramanan AV, Wang Y, Wang Z, Janka G,
Henter JI. Recommendations for the management of hemophagocytic
lymphohistiocytosis in adults. Blood. 2019 Jun 6;133(23):2465-2477. https://doi.org/10.1182/blood.2018894618 PMid:30992265
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010 May 28;32(5):593-604. https://doi.org/10.1016/j.immuni.2010.05.007 PMid:20510870
- Komohara
Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2
antiinflammatory macrophage phenotype in growth of human gliomas. J
Pathol. 2008 Sep;216(1):15-24. https://doi.org/10.1002/path.2370 PMid:18553315
- Weaver
LK, Hintz-Goldstein KA, Pioli PA, Wardwell K, Qureshi N, Vogel SN,
Guyre PM. Pivotal advance: activation of cell surface Toll-like
receptors causes shedding of the hemoglobin scavenger receptor CD163. J
Leukoc Biol. 2006 Jul;80(1):26-35. https://doi.org/10.1189/jlb.1205756 PMid:16799153
- Philippidis
P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC.
Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and
heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage
responses in vitro, in resolving skin blisters in vivo, and after
cardiopulmonary bypass surgery. Circ Res. 2004 Jan 9;94(1):119-26. https://doi.org/10.1161/01.RES.0000109414.78907.F9 PMid:14656926
- Nouno
T, Okamoto M, Ohnishi K, Kaieda S, Tominaga M, Zaizen Y, Ichiki M,
Momosaki S, Nakamura M, Fujimoto K, Fukuoka J, Shimizu S, Komohara Y,
Hoshino T. Elevation of pulmonary CD163+ and CD204+ macrophages is
associated with the clinical course of idiopathic pulmonary fibrosis
patients. J Thorac Dis. 2019 Sep;11(9):4005-4017. https://doi.org/10.21037/jtd.2019.09.03 PMid:31656675 PMCid:PMC6790423
- Schulte-Schrepping
J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, Krämer B,
Krammer T, Brumhard S, Bonaguro L, De Domenico E, Wendisch D, Grasshoff
M, Kapellos TS, Beckstette M, Pecht T, Saglam A, Dietrich O, Mei HE,
Schulz AR, Conrad C, Kunkel D, Vafadarnejad E, Xu CJ, Horne A, Herbert
M, Drews A, Thibeault C, Pfeiffer M, Hippenstiel S, Hocke A,
Müller-Redetzky H, Heim KM, Machleidt F, Uhrig A, Bosquillon de Jarcy
L, Jürgens L, Stegemann M, Glösenkamp CR, Volk HD, Goffinet C,
Landthaler M, Wyler E, Georg P, Schneider M, Dang-Heine C, Neuwinger N,
Kappert K, Tauber R, Corman V, Raabe J, Kaiser KM, Vinh MT, Rieke G,
Meisel C, Ulas T, Becker M, Geffers R, Witzenrath M, Drosten C, Suttorp
N, von Kalle C, Kurth F, Händler K, Schultze JL, Aschenbrenner AC, Li
Y, Nattermann J, Sawitzki B, Saliba AE, Sander LE; Deutsche COVID-19
OMICS Initiative (DeCOI). Severe COVID-19 Is Marked by a Dysregulated
Myeloid Cell Compartment. Cell. 2020 Sep 17;182(6):1419-1440.e23.
- Qin
C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W,
Tian DS. Dysregulation of Immune Response in Patients With Coronavirus
2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020 Jul
28;71(15):762-768. https://doi.org/10.1093/cid/ciaa248 PMid:32161940 PMCid:PMC7108125
- Wang
C, Deng R, Gou L, Fu Z, Zhang X, Shao F, Wang G, Fu W, Xiao J, Ding X,
Li T, Xiao X, Li C. Preliminary study to identify severe from moderate
cases of COVID-19 using combined hematology parameters. Ann Transl Med.
2020 May;8(9):593. https://doi.org/10.21037/atm-20-3391 PMid:32566620 PMCid:PMC7290538
- Guan
WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui
DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY,
Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng
P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu
SY, Zhong NS; China Medical Treatment Expert Group for Covid-19.
Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J
Med. 2020 Apr 30;382(18):1708-1720. https://doi.org/10.1056/NEJMoa2002032 PMid:32109013 PMCid:PMC7092819
- Kilercik
M, Demirelce Ö, Serdar MA, Mikailova P, Serteser M. A new
haematocytometric index: Predicting severity and mortality risk value
in COVID-19 patients. PLoS One. 2021 Aug 5;16(8):e0254073. https://doi.org/10.1371/journal.pone.0254073 PMid:34351940 PMCid:PMC8341498
- Liu
YP, Li GM, He J, Liu Y, Li M, Zhang R, Li YL, Wu YZ, Diao B. Combined
use of the neutrophil-to-lymphocyte ratio and CRP to predict 7-day
disease severity in 84 hospitalized patients with COVID-19 pneumonia: a
retrospective cohort study. Ann Transl Med. 2020 May;8(10):635. https://doi.org/10.21037/atm-20-2372 PMid:32566572 PMCid:PMC7290615
- Pozdnyakova
O, Connell NT, Battinelli EM, Connors JM, Fell G, Kim AS. Clinical
Significance of CBC and WBC Morphology in the Diagnosis and Clinical
Course of COVID-19 Infection. Am J Clin Pathol. 2021 Feb
11;155(3):364-375. https://doi.org/10.1093/ajcp/aqaa231 PMid:33269374 PMCid:PMC7799218
- Fan
BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, Mucheli SS, Kuperan
P, Ong KH. Hematologic parameters in patients with COVID-19 infection.
Am J Hematol. 2020 Jun;95(6):E131-E134. doi: 10.1002/ajh.25774. Epub
2020 Mar 19. Erratum in: Am J Hematol. 2020 Nov;95(11):1442. https://doi.org/10.1002/ajh.25774
- Singh
A, Sood N, Narang V, Goyal A. Morphology of COVID-19-affected cells in
peripheral blood film. BMJ Case Rep. 2020 May 27;13(5):e236117. https://doi.org/10.1136/bcr-2020-236117 PMid:32467125 PMCid:PMC7276239
- Jain
S, Meena R, Kumar V, Kaur R, Tiwari U. Comparison of hematologic
abnormalities between hospitalized coronavirus disease 2019 positive
and negative patients with correlation to disease severity and outcome.
J Med Virol. 2022 Aug;94(8):3757-3767. https://doi.org/10.1002/jmv.27793 PMid:35467029 PMCid:PMC9088404
- Tummidi
S, Shankaralingappa A. Peripheral smear in COVID 19: a case report.
Hematol Transfus Cell Ther. 2021 Oct-Dec;43(4):545-547. https://doi.org/10.1016/j.htct.2021.02.011 PMid:33969271 PMCid:PMC8084623
- Han
Q, Wen X, Wang L, Han X, Shen Y, Cao J, Peng Q, Xu J, Zhao L, He J,
Yuan H. Role of hematological parameters in the diagnosis of influenza
virus infection in patients with respiratory tract infection symptoms.
J Clin Lab Anal. 2020 May;34(5):e23191. https://doi.org/10.1002/jcla.23191
- Liu
J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R,
Song M, Wang L, Zhang W, Han B, Yang L, Wang X, Zhou G, Zhang T, Li B,
Wang Y, Chen Z, Wang X. Neutrophil-to-lymphocyte ratio predicts
critical illness patients with 2019 coronavirus disease in the early
stage. J Transl Med. 2020 May 20;18(1):206. https://doi.org/10.1186/s12967-020-02374-0 PMid:32434518 PMCid:PMC7237880
- Xia
X, Wen M, Zhan S, He J, Chen W. [An increased neutrophil/lymphocyte
ratio is an early warning signal of severe COVID-19]. Nan Fang Yi Ke Da
Xue Xue Bao. 2020 Mar 30;40(3):333-336. Chinese.
- Long
L, Zeng X, Zhang X, Xiao W, Guo E, Zhan W, Yang X, Li C, Wu C, Xu T,
Zhan C, Chen Y, Jiang M, Zhong N, Lai K. Short-term outcomes of
COVID-19 and risk factors for progression. Eur Respir J. 2020 May
27;55(5):2000990. https://doi.org/10.1183/13993003.00990-2020 PMid:32312863 PMCid:PMC7173674
- Nazarullah
A, Liang C, Villarreal A, Higgins RA, Mais DD. Peripheral Blood
Examination Findings in SARS-CoV-2 Infection. Am J Clin Pathol. 2020
Aug 5;154(3):319-329. https://doi.org/10.1093/ajcp/aqaa108 PMid:32756872 PMCid:PMC7454310
- Jiang
SQ, Huang QF, Xie WM, Lv C, Quan XQ. The association between severe
COVID-19 and low platelet count: evidence from 31 observational studies
involving 7613 participants. Br J Haematol. 2020 Jul;190(1):e29-e33. https://doi.org/10.1111/bjh.16817
- Zhang
Y, Zeng X, Jiao Y, Li Z, Liu Q, Ye J, Yang M. Mechanisms involved in
the development of thrombocytopenia in patients with COVID-19. Thromb
Res. 2020 Sep;193:110-115. https://doi.org/10.1016/j.thromres.2020.06.008 PMid:32535232 PMCid:PMC7274097
- Vanderschueren
S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A,
Bobbaers H. Thrombocytopenia and prognosis in intensive care. Crit Care
Med. 2000 Jun;28(6):1871-6. https://doi.org/10.1097/00003246-200006000-00031 PMid:10890635
- Thachil J. What do monitoring platelet counts in COVID-19 teach us? J Thromb Haemost. 2020 Aug;18(8):2071-2072. https://doi.org/10.1111/jth.14879 PMid:32344467 PMCid:PMC7267313
- Güçlü
E, Kocayiğit H, Okan HD, Erkorkmaz U, Yürümez Y, Yaylacı S, Koroglu M,
Uzun C, Karabay O. Effect of COVID-19 on platelet count and its
indices. Rev Assoc Med Bras (1992). 2020 Aug;66(8):1122-1127. https://doi.org/10.1590/1806-9282.66.8.1122 PMid:32935808
- Wool GD, Miller JL. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology. 2021;88(1):15-27. https://doi.org/10.1159/000512007 PMid:33049751 PMCid:PMC7649697
- Handtke S, Thiele T. Large and small plate- lets-(When) do they differ? J Thromb Hae- most. 2020 Jun;18(6):1256-67. https://doi.org/10.1111/jth.14788 PMid:32108994
- Pezeshki,
A., Vaezi, A. & Nematollahi, P. Blood cell morphology and COVID-19
clinical course, severity, and outcome. J Hematopathol 14, 221-228
(2021). https://doi.org/10.1007/s12308-021-00459-3 PMid:34249171 PMCid:PMC8255335
- Mitra
A, Dwyre DM, Schivo M, Thompson GR 3rd, Cohen SH, Ku N, Graff JP.
Leukoerythroblastic reaction in a patient with COVID-19 infection. Am J
Hematol. 2020 Aug;95(8):999-1000. https://doi.org/10.1002/ajh.25793 PMid:32212392 PMCid:PMC7228283
- Sadigh
S, Massoth LR, Christensen BB, Stefely JA, Keefe J, Sohani AR.
Peripheral blood morphologic findings in patients with COVID-19. Int J
Lab Hematol. 2020 Dec;42(6):e248-e251. https://doi.org/10.1111/ijlh.13300 PMid:32730694
- Lefrançais
E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton
EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegué
E, Looney MR. The lung is a site of platelet biogenesis and a reservoir
for haematopoietic progenitors. Nature. 2017 Apr 6;544(7648):105-109. https://doi.org/10.1038/nature21706 PMid:28329764 PMCid:PMC5663284
- Rampotas A, Pavord S. Platelet aggregates, a marker of severe COVID-19 disease. Journal of Clinical Pathology. 2021; 74 (11). https://doi.org/10.1136/jclinpath-2020-206933 PMid:33067181
- Al-Samkari
H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi
K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP.
COVID-19 and coagulation: bleeding and thrombotic manifestations of
SARS-CoV-2 infection. Blood. 2020 Jul 23;136(4):489-500. https://doi.org/10.1182/blood.2020006520 PMid:32492712 PMCid:PMC7378457
- Gómez-Pastora
J, Weigand M, Kim J, Wu X, Strayer J, Palmer AF, Zborowski M, Yazer M,
Chalmers JJ. Hyperferritinemia in critically ill COVID-19 patients - Is
ferritin the product of inflammation or a pathogenic mediator? Clin
Chim Acta. 2020 Oct;509:249-251. https://doi.org/10.1016/j.cca.2020.06.033 PMid:32579952 PMCid:PMC7306200
- Pérez-García
N, García-González J, Requena-Mullor M, Rodríguez-Maresca MÁ,
Alarcón-Rodríguez R. Comparison of Analytical Values D-Dimer, Glucose,
Ferritin and C-Reactive Protein of Symptomatic and Asymptomatic
COVID-19 Patients. Int J Environ Res Public Health. 2022 Apr
28;19(9):5354. https://doi.org/10.3390/ijerph19095354 PMid:35564749 PMCid:PMC9102188
- Volfovitch
Y, Tsur AM, Gurevitch M, Novick D, Rabinowitz R, Mandel M, Achiron A,
Rubinstein M, Shoenfeld Y, Amital H. The intercorrelations between
blood levels of ferritin, sCD163, and IL-18 in COVID-19 patients and
their association to prognosis. Immunol Res. 2022 Dec;70(6):817-828. https://doi.org/10.1007/s12026-022-09312-w PMid:36222965 PMCid:PMC9555272
- Chakravortty
D, Kato Y, Koide N, Sugiyama T, Kawai M, Fukada M, Yoshida T, Yokochi
T. Production of tissue factor in CD14-expressing human umbilical vein
endothelial cells by lipopolysaccharide. FEMS Microbiol Lett. 1999 Sep
15;178(2):235-9. https://doi.org/10.1111/j.1574-6968.1999.tb08682.x PMid:10499273
- Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11(4):225-9. https://doi.org/10.1177/09680519050110040701




