Daniele
Avenoso1, Fabio Serpenti1,
Liron Barnea Slonim2, Styliani Bouziana1,
Francesco Dazzi1, Guy Hannah1,
Michelle Kenyon1, Varun Mehra1,
Austin Kulasekararaj1, Pramila Krishamurthy1,
Mili Naresh Shah1, Sharon Lionel1,
Antonio Pagliuca1 and Victoria Potter1.
1
King’s College Hospital NHS Foundation Trust, Department of
haematological medicine, Denmark Hill, London.
2 King’s College Hospital NHS Foundation Trust,
Department of Histopathology.
Correspondence to:
Dr
Daniele Avenoso, King's College Hospital NHS Foundation Trust,
Department of Haematological Medicine, Denmark Hill, London. Tel: +4420
3299 9000. E-mail:
d.avenoso@nhs.net
Published: January 01, 2024
Received: September 02, 2023
Accepted: December 06, 2023
Mediterr J Hematol Infect Dis 2024, 16(1): e2024002 DOI
10.4084/MJHID.2024.002
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Allogeneic haematopoietic stem-cell transplant is an option,
potentially curative, for high-risk acute myeloid leukaemia (AML) and
myelodysplastic syndrome (MDS) patients. Post-transplant
cyclophosphamide administration allows for the selection of
haploidentical donors in patients who are eligible for the procedure
but do not have a fully matched donor since it can overcome the HLA
barrier. There is still an active debate on whether intensifying the
conditioning regimen is necessary with haploidentical donors when
peripheral blood stem cells are used as the graft source.
Herein,
we report our decennial experience of haploidentical stem-cell
transplant using peripheral blood stem cells (haplo-PBSC) at King’s
College Hospital.
Objectives:
The primary objective was to evaluate overall survival (OS) following
haplo-PBSC. Secondary objectives were total OS for patients with less
than two previous lines of therapy, OS according to cytomegalovirus
(CMV) reactivation, incidence of transplant-related mortality (TRM),
graft-versus-host disease (GVHD) and GVHD-relapse-free survival (GRFS).
Results:
One-year and three-year total OS were 62% and 43%, respectively, with a
median OS of 22 months. One-year and three-year OS for patients with ≤2
and those with >2 previous lines of therapy were 72% and 55%, and
60% and 22%, respectively (p-value=0.04). The median OS in patients
with >2 previous and ≤2 lines of therapy was 16 and 49 months,
respectively. Cumulative incidence (CI) of relapse was 25% with a
median time to relapse of 5 months (range 1 – 38 months).
Conclusions:
Haploidentical haematopoietic stem-cell transplant is potentially
curative in chemo-sensitive AML and MDS and offers a high rate of
prolonged remission. Our cohort further confirms the role of
consolidative haploidentical transplant in patients in complete
remission and highlights that patients with heavily pre-treated disease
may not benefit from this strategy.
|
Introduction
Curative
strategy for high-risk acute myeloid leukaemia (AML) and
myelodysplastic syndromes (MDS) still relies on the
graft-versus-leukaemia (GVL) effect following allogeneic haematopoietic
stem cell transplant (allo-HSCT).[1,2]
Despite
the improvement in the HLA tissue typing techniques and the evolution
of mismatched unrelated donor transplants, a non-negligible proportion
of allo-HSCT eligible patients lack a fully matched donor, and the
guidelines still need to be provided for optimal donor selection for
these patients.[3]
The administration of
post-transplant cyclophosphamide (PTCY) after the infusion of bone
marrow cells from haploidentical sibling donors[4] has
become a viable option to remove the HLA barrier, and nowadays,
mismatched related donors can be selected in the absence of a fully
matched donor. As with other donor types, the main reasons for
transplant failure remain disease relapse and transplant-related
mortality (TRM).
Over the past two decades, several strategies
have been implemented in an attempt to improve the anti-leukaemia
effect of haplo-HSCT, including the use of peripheral blood stem cells
(PBSC) instead of bone marrow (BM),[5-7] intensification of the conditioning regimen[8,9] or a combination of both.
A
single-centre retrospective study reported a higher rate of both grade
III/IV acute graft-versus-host disease (GVHD) and
steroid-refractory GVHD (SR-GVHD) with haploidentical PBSC, without any
difference in the relapse rate compared to those that received BM.[10]
A multicentric study suggested lower relapse rates with the use of PBSC compared to BM as a source of the graft,[7]
and despite initial concerns regarding an increased risk of GVHD with
haplo-PBSC, different groups demonstrated similar outcomes compared to
BM.[6,11]
Data from a large
retrospective haplo-HSCT study suggests that the degree of HLA mismatch
is the best predictor of success in this setting, regardless of the
type of conditioning (myeloablative conditioning (MAC) or reduced
intensity conditioning (RIC))[12,13]
Recently,
a large retrospective study showed that haplo-HSCT and matched sibling
donors (MSD) have similar 1-year and 3-year overall survival (OS)
rates.[14] The administration of PTCY following
haplo-HSCT significantly reduced the incidence of chronic GVHD compared
to MSD (26% versus 56%). Despite the decreased incidence of chronic
GVHD within the haplo-HSCT cohort, there was no survival advantage due
to late-onset lethal infections and the occurrence of secondary
malignancies.[14]
Also, an EBMT study performed
in acute leukaemia patients undergoing transplant in first complete
remission reported no differences in OS and leukaemia-free survival
between haplo-BM and PBSC from matched unrelated donors. Interestingly,
this study showed a significantly reduced incidence of chronic GVHD and
extensive GVHD in patients who underwent haplo-BM.[15]
In 2017, a CIBMTR study enriched for RIC cases found that although OS
was not different, peripheral blood compared to BM had aGVHD and cGVHD
risk greater and relapse risk less.[16]
Herein,
we report the outcome of AML and MDS patients transplanted with
haploidentical PBSC with a uniform GVHD prophylaxis regimen with PTCY,
tacrolimus and mycophenolate mofetil.
Patients and Methods
Between
August 2010 and August 2021, 72 haploidentical hematopoietic stem-cell
transplants were performed at King’s College Hospital in London, United
Kingdom.
Forty cases of AML and MDS were analysed in this single-centre retrospective cohort study (Figure 1).
AML and MDS were diagnosed according to WHO criteria and stratified
according to the disease risk index as previously described.[17,18]
 |
- Figure 1. Haplo-HSCT
population at King’s College Hospital in 10 years. *Two cases of
primary induction failure achieved first complete remission (CR1) after
two lines of therapies; one patient achieved CR1 after three lines of
therapy. +Two cases needed three lines of therapy to achieve second
complete remission (CR2).
|
Neutrophil
engraftment was defined as a neutrophil count of ≥1,000/µL for two
consecutive days without G-CSF support. Platelet engraftment was
defined as platelets count ≥20,000/µL for two consecutive days without
platelets transfusion in the two previous days.
Chimerism analysis
of peripheral blood and bone marrow was performed on days +28, +56,
+100, +180 and +365 with short tandem repeat (STR) testing by
polymerase chain reaction (PCR) followed by fragment-length analysis as
previously described.[19]
All patients signed consent forms approved by the institutional review board.
HLA tissue typing and matching.
Donors and recipients were typed using Third Generation Sequencing
(TGS) and Next Generation Sequencing (NGS) techniques for HLA-A, -B,
-C, -DRB1, -DQ with high resolution. Donors were considered
haploidentical if they shared a haplotype with the patient.
Transplant procedure.
HSCT was performed with G-CSF mobilised PBSC. Nonmyeloablative (NMA)
regimen consisted of cyclophosphamide 14.5 mg/kg on days -6 and -5,
fludarabine 30 mg/m2/day from day -6
to day -2, and low-dose total body irradiation (2 Gy) on day -1
(FCTBI). Myeloablative MAC regimen included Thiotepa 5 mg/Kg on days -6
and -5, Busulfan 3.2 mg/Kg on days -4, -3, -2 and fludarabine 50 mg/m2 from day -4 till day -2.
GVHD
prophylaxis consisted of post-transplant cyclophosphamide (50 mg/kg) on
days +3 and +4. On day +5, tacrolimus and mycophenolate mofetil (MMF)
were started. Tacrolimus (at a total dose of 1 mg) was administered as
a once-a-day infusion and switched to oral tablets at discharge. The
doses were adjusted to obtain serum levels between 10 and 15 ng/ml. MMF
was administered at 15 mg/kg p.o. three times per day until day +35.
G-CSF was started on day +5 in all patients.
NMA conditioning was
the standard of care for patients undergoing haplo-HSCT. MAC was
offered exceptionally only to patients aged <55 years and with an
HCT-CI score of <3 or with either active disease or leukaemia-free
status.
Supportive Care.
Anti-microbial prophylaxis was initiated at the time of conditioning.
It consisted of Acyclovir 400 mg twice daily, Ciprofloxacin 500 mg once
daily, and Posaconazole 300 mg once daily until neutrophil engraftment.
At the resolution of neutropenia, patients started fluconazole
prophylaxis until tacrolimus administration was discontinued.
Penicillin V 500 mg BD was started at neutrophil engraftment and
continued for life. Letermovir for cytomegalovirus (CMV) prophylaxis
was offered in November 2019. Biweekly CMV, adenovirus and Epstein-Bar
virus (EBV) monitoring by quantitative PCR was performed from the start
of conditioning until day +100 and weekly until day +180. Patients
received red blood cell and platelet transfusions according to standard
operative procedures at our institution.
Diagnosis and Treatment of Graft-Versus-Host Disease. Clinical diagnosis of acute and chronic GVHD (aGVHD and cGVHD, respectively) was made based on standard criteria.[20]
When possible, confirmation by histologic analysis of skin and/or
gastrointestinal biopsy specimens was performed. First-line and
second-line therapy for GVHD were provided according to institutional
protocols.
Statistical Analysis. Continuous variables were described as median and range, and categorical variables as frequencies.
Overall
survival (OS) was estimated using the Kaplan-Meier curves and defined
as the time from starting HSCT to death from any cause or the last
follow-up for living patients.
Progression-free survival (PFS) was
estimated with Kaplan-Meier curves and was defined as the time from
HSCT to relapse or death (whichever came first) or last follow-up.
GVHD-Relapse
free survival (GRFS) was defined as the time from HSCT to either grade
3/4 acute GVHD or moderate to severe chronic GVHD or relapse or death
from any cause.
Cumulative incidence (CI) analysis was performed
for transplant-related mortality (TRM), relapse and GVHD incidence
(either acute or chronic). TRM was defined as death due to any cause
other than progression of the underlying malignancy, with death due to
relapse as a competing event. Relapse was defined as recurrence of the
underlying hematologic malignancy, and death due to any other cause
(TRM) was a competing event for this analysis.
For cumulative
incidence analysis of GVHD, death without aGVHD in the first 100 days
was considered a competing event for the aGVHD, whereas relapse or
death in the absence of cGVHD was considered a competing event for
cGVHD.
Chi-square statistics was used to compare categorical variables, and the Mann-Whitney test was used for continuous variables.
The primary objective was to evaluate OS following transplantation with haploidentical PBSC.
Secondary
objectives were OS for patients with less than two previous lines of
therapy, OS according to CMV reactivation, and the incidence of TRM,
GVHD and GRFS. All analyses were done with SPSS software Version
29.0.1.0, and two-tailed p-values ≤ 0.05 were considered significant.
Results
Patient characteristics
Twenty/eight
patients (13 females and 15 males) and twelve MDS patients (12 males)
were transplanted with haploidentical sibling donors. Patient baseline
characteristics are summarised in Table 1. 90% of patients received NMA conditioning, and the remaining 10% received MAC.
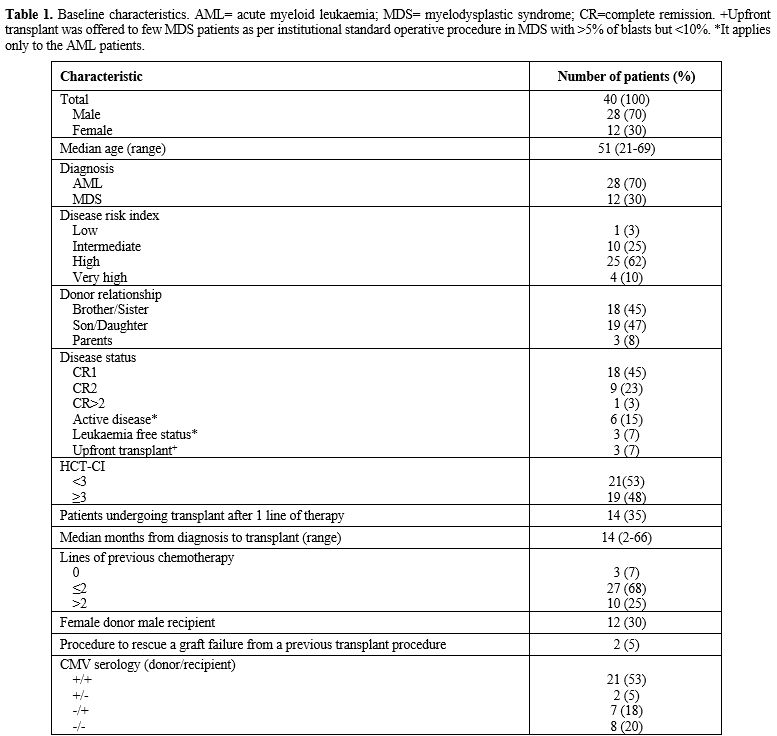 |
- Table 1. Baseline
characteristics. AML= acute myeloid leukaemia; MDS= myelodysplastic
syndrome; CR=complete remission. +Upfront transplant was offered to few
MDS patients as per institutional standard operative procedure in MDS
with >5% of blasts but <10%. *It applies only to the AML patients.
|
The median age of the cohort was 51 (range 21 - 69); 19 patients were 55 or older.
Eighteen
patients (45%) underwent allo-HSCT in first complete remission (CR1),
nine (23%) when a second complete remission (CR2) was achieved, one
patient (3%) was transplanted in CR>2, six AML patients (15%) had
active disease and three AML patients were in morphologic leukaemia
free state[2] (7%). Three (7%) MDS patients underwent upfront allo-HSCT.
88%
of the patients in CR1 and CR2 had ≤2 previous lines of therapy, and
12% had >2 lines of therapy. 53% of the remaining patients had >2
lines of therapy. All but one patient with active disease had >2
lines of therapy. Median follow-up time was 23 months (range 1 – 144
months).
Graft characteristics, engraftment and chimerism. Unmanipulated peripheral blood haematopoietic stem cells with a median of 5.9x106 CD34+cells/Kg (3.37 - 14.6) were infused. Median time to neutrophils ≥1000/µL was 18 days (13-42), and 25 days (14-44) to platelets ≥20.000/µL.
Two cases of primary graft failure occurred. Median unfractionated,
CD3+ and CD15+ chimerism at 365 days after transplant were 100%, 100%
and 100%, respectively.
Overall survival, progression-free survival, and relapse rate. One-year and three-year total OS were 62% and 43%, respectively, with a median OS of 22 months (Figure 2; range 1 -142).
One-year
and three-year OS for patients with ≤2 and those with >2 previous
lines of therapy were 72% and 55%, and 60% and 22%, respectively
(p-value=0.04 – Log Rank, Figure 3). The median OS in patients with >2 previous and ≤2 lines of therapy were 16 and 49 months, respectively.
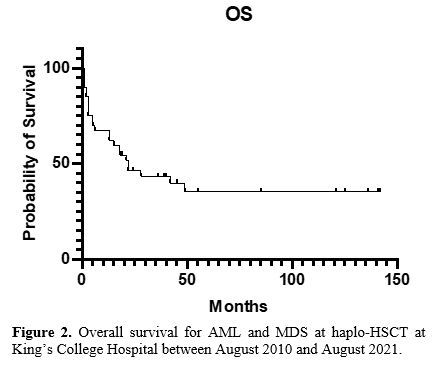 |
Figure 2. Overall survival for AML and MDS at haplo-HSCT at King’s College Hospital between August 2010 and August 2021. |
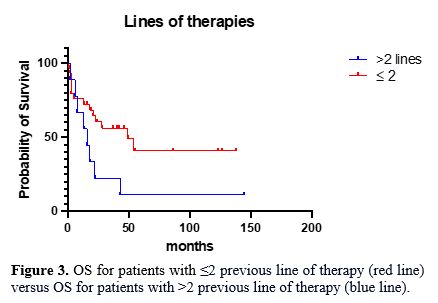 |
Figure 3. OS for
patients with ≤2 previous line of therapy (red line) versus OS for
patients with >2 previous line of therapy (blue line).
|
The presence of TP53 mutations
divides MDSs with complex karyotypes (CK-MDSs) into distinct prognostic
groups. In a cohort of 359
Twenty-eight
patients (70%) were CMV IgG positive. One-year and three-year OS for
patients with and without CMV reactivation were 65% and, 58%, and 40%
and 51%, respectively (p-value not significant).
One-year and three-year total PFS was 57% and 46%, respectively.
One-year
and three-year PFS for patients with ≤2 and those with >2 previous
lines of therapy were 62% and 40%, and 50% and 30%, respectively
(p-value not significant).
Cumulative incidence (CI) of relapse was 25% with a median time to relapse of 5 months (range 1 – 38 months).
One-year
and three-year CI of relapse for patients with ≤2 and in patients with
>2 previous lines of therapy were 13% and 49%, and 17% and 49%,
respectively (p-value 0.05)
Graft versus host disease.
Acute GVHD (aGVHD) grade I-II occurred in 50% of the patients, with no
grade III/IV observed. The median time from transplant to aGVHD onset
was 55 days (range 28 - 210).
Mild chronic GVHD (cGVHD) occurred
in 3% of the patients. The incidence of moderate and severe chronic
GVHD was 5% and 10% of all patients, respectively (Figure 4). The median time to chronic GVHD was 280 days (range 111 – 552).
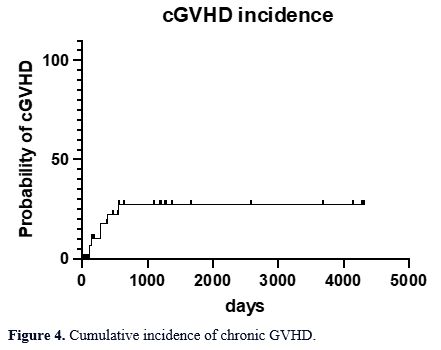 |
- Figure 4. Cumulative incidence of chronic GVHD.
|
Transplant related mortality.
One-year and three-year TRM was 21% and 35%, respectively. The median
time to TRM was 4 months (range 1 - 49). The most common cause of death
was infection (50% of total TRM), followed by GVHD (30%),
post-transplant lymphoproliferative disease (PTLD) (6%), unknown cause
(6%), graft failure (8%).
Patients who underwent allograft with
either active disease or >2 previous lines of therapy had a global
TRM of 90% (the two remaining patients in this cohort succumbed to
relapsed disease). In contrast, patients who had achieved CR or have
had ≤2 previous lines of therapy had a global TRM of 40%.
GVHD-relapse free survival.
The one-year GRFS rate was 51%, and the three-year GRFS was 41%, with a
median total GRFS of 13 months (range 1 - 142). One-year and three-year
GRFS in patients with ≤2 and >2 previous lines of therapy were 39%
and 33%, and 22% and 27%, respectively.
Viral reactivation.
CMV reactivation occurred in 50% of patients at a median time of 46
days post-transplant (range 13-415 days) without any evidence of organ
damage. Notably, CMV prophylaxis with letermovir became available only
in November 2019; therefore, only 7% of patients received the
treatment. EBV reactivation was detected in 67% of the patients at a
median time of 46 days post-transplant (range 9 – 1218). Only one
patient developed monomorphic PTLD that required multiple lines of
therapy and had a dismal outcome. Adenovirus reactivation was recorded
in 10% of patients at a median time of 45 days post-transplant (range
21 – 126); no cases of adenovirus disease were diagnosed.
Discussion
Haplo-HSCT is nowadays considered an established transplant option for AML and MDS patients lacking a fully matched donor.[21,22]
To
minimise the risk of relapse in AML and MDS patients, both
intensification of the conditioning regimen and/or substitution of BM
with PBSC as source of the graft have been implemented. While
intensification of the conditioning regimen led to ambiguous results,[4,8,9,23] the utilisation of PBSC has led to decreased relapse rate[6,7]
with a resulting increased risk of grade II/IV acute GVHD seen only
after the administration of MAC5. However, studies focused on the
source of stem cells showed contrasting results. A retrospective EBMT
study in patients with relapsed/refractory or active AML at the time of
haplo-HSCT showed a better leukaemia-free survival with BM rather than
with PBSC in patients ≥55 years of age.[24] This was
mainly a consequence of a higher TRM within the PBSC group. Our study
showed a similar finding: 50% of patients were 55 or older at the
time of transplant and had only PBSC as a source of stem cells. The use
of haplo PBSC within an older population could have contributed to a
higher TRM, especially within the group of patients undergoing
transplantation with >2 lines of previous therapy.
Despite our
cohort being heavily pre-treated, the current study showed a 25% 2-year
relapse rate, which is quite similar to one reported by Mehta et al.
for patients receiving haplo-PBSC.[10] Notably, the cumulative incidence of relapse is inferior compared to a large retrospective EBMT study.[24]
The retrospective nature of the current report is an important bias;
however, it is worth mentioning that it is difficult to compare with
one or more studies due to many differences on one or more points
(conditioning, stem cell source, haematological diseases, etc). A
possible explanation of a relatively low relapse rate could be a higher
CD3-positive T-cells and NK-cells in the graft, allowing a stronger
anti-leukaemia effect of haplo-PBSC.
In our decennial experience
of haploidentical transplantation, the 2-year OS for AML and MDS
patients was 46%. In patients with ≤2 previous lines of therapy, the
2-year OS was 60%, similar to the estimated 2-year OS of other groups.[9,25]
Additionally,
in our study, the incidence of grade I/II acute GVHD was 50%, with no
cases of grade III/IV, further confirming the safety of haplo-PBSC
after NMA conditioning.
In terms of chronic GVHD, the
administration of PTCY in combination with MMF and tacrolimus was
effective in minimising the risk of moderate to severe cases. This
result highlights the protective effect of PTCY even in the context of
haplo-PBSC.
Interestingly, all cases of severe GVHD within our cohort received a graft with a CD34+ dose >5x106/Kg, and this result suggests that defining a maximum haplo-CD34+ cell dose might be of benefit.
Our
cohort also showed a grim outcome in patients undergoing haplo-HSCT
with active disease or with >2 lines of therapy due to increased TRM
and relapse rates. This suggests that haplo-HSCT should be offered at
an early stage to patients for whom a fully matched donor cannot be
identified to avoid increased chances of disease relapse. Our results
are a further confirmation that advanced disease at the time of
transplant is a strong negative prognostic factor for both TRM and
early relapse.
This study suggests the feasibility and safety of
haplo-PBSC in MDS and AML patients and highlights the importance of
PTCY administration to ensure an acceptable risk of GVHD. Nevertheless,
our results further contribute to the debate about the optimal graft
source within the haplo-HSCT platform.
This is the first study
examining the role of the number of previous lines of therapy on
transplant outcomes. This single-centre experience in a limited number
of heavily pre-treated patients suggests that the GVL effect may not
suffice to guarantee durable remission, and this cohort of patients may
require maintenance therapy to minimise the risk of relapse.
Additionally, the elevated TRM in patients with >2 lines of therapy
suggests they may benefit from more frequent surveillance to minimise
the risk of lethal infectious events.
Conclusions
Haplo-PBSC
after NMA conditioning is potentially curative in AML and MDS patients
with chemo-sensitive disease and seems to offer a high rate of
prolonged remission with relatively low rates of severe GVHD and
relapse.
Author contribution
DA
and FS collected the data. DA performed statistical analysis. DA, LBS,
FS, and SB wrote the manuscript and were involved in patient care. VP
supervised the project, was involved in patient care, and wrote the
manuscript. The other authors were involved in patient care and
reviewed the manuscript. All the authors approved the final version.
References
- Sweeney, C. &
Vyas, P. The Graft-Versus-Leukemia Effect in AML. Front. Oncol. 9, 1217
(2019). https://doi.org/10.3389/fonc.2019.01217
PMid:31803612 PMCid:PMC6877747
- Döhner,
H. et al. Diagnosis and management of AML in adults: 2022
recommendations from an international expert panel on behalf of the
ELN. Blood 140, 1345-1377 (2022). https://doi.org/10.1182/blood.2022016867
PMid:35797463
- Nagler,
A. & Mohty, M. In 2022, which is prefered: haploidentical or
cord transplant? Hematology 2022, 64-73 (2022). https://doi.org/10.1182/hematology.2022000327
PMid:36485156 PMCid:PMC9820258
- Luznik,
L. et al. HLA-Haploidentical Bone Marrow Transplantation for
Hematologic Malignancies Using Nonmyeloablative Conditioning and
High-Dose, Posttransplantation Cyclophosphamide. Biol. Blood Marrow
Transplant. 14, 641-650 (2008). https://doi.org/10.1016/j.bbmt.2008.03.005
PMid:18489989 PMCid:PMC2633246
- Ruggeri,
A. et al. Bone marrow versus mobilized peripheral blood stem cells in
haploidentical transplants using posttransplantation cyclophosphamide.
Cancer 124, 1428-1437 (2018). https://doi.org/10.1002/cncr.31228
PMid:29360162
- Castagna,
L. et al. Bone Marrow Compared with Peripheral Blood Stem Cells for
Haploidentical Transplantation with a Nonmyeloablative Conditioning
Regimen and Post-transplantation Cyclophosphamide. (2014)
doi:10.1016/j.bbmt.2014.02.001. https://doi.org/10.1016/j.bbmt.2014.02.001
PMid:24530426
- O'Donnell,
P. V. et al. Nonmyeloablative bone marrow transplantation from
partially HLA-mismatched related donors using posttransplantation
cyclophosphamide. Biol. Blood Marrow Transplant. J. Am. Soc. Blood
Marrow Transplant. 8, 377-86 (2002). https://doi.org/10.1053/bbmt.2002.v8.pm12171484
PMid:12171484
- Raiola,
A. M. et al. Unmanipulated Haploidentical Bone Marrow Transplantation
and Posttransplantation Cyclophosphamide for Hematologic Malignancies
after Myeloablative Conditioning. Biol. Blood Marrow Transplant. 19,
117-122 (2013). https://doi.org/10.1016/j.bbmt.2012.08.014
PMid:22940057
- Solomon,
S. R. et al. Haploidentical transplantation using T cell replete
peripheral blood stem cells and myeloablative conditioning in patients
with high-risk hematologic malignancies who lack conventional donors is
well tolerated and produces excellent relapse-free survival: results of
a prospective phase II trial. Biol. Blood Marrow Transplant. J. Am.
Soc. Blood Marrow Transplant. 18, 1859-1866 (2012). https://doi.org/10.1016/j.bbmt.2012.06.019
PMid:22863841
- Mehta,
R. S. et al. Bone Marrow versus Peripheral Blood Grafts for
Haploidentical Hematopoietic Cell Transplantation with
Post-Transplantation Cyclophosphamide. Transplant. Cell. Ther. Off.
Publ. Am. Soc. Transplant. Cell. Ther. 27, 1003.e1-1003.e13 (2021). https://doi.org/10.1016/j.jtct.2021.09.003
PMid:34537419 PMCid:PMC8504778
- Sammassimo,
S. A Cellular Therapy with Haploidentical Peripheral Hematopoietic STEM
CELL Transplantation MAY be a Therapeutic Option in Patients with
Relapsed Lymphoma with Chemorefractory Disease. (2018). https://doi.org/10.1182/blood-2018-99-119752
- Ciurea,
S. O. et al. Improved early outcomes using a T cell replete graft
compared with T cell depleted haploidentical hematopoietic stem cell
transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood
Marrow Transplant. 18, 1835-1844 (2012). https://doi.org/10.1016/j.bbmt.2012.07.003
PMid:22796535 PMCid:PMC4320643
- Piemontese,
S. et al. A survey on unmanipulated haploidentical hematopoietic stem
cell transplantation in adults with acute leukemia. Leukemia 29,
1069-1075 (2015). https://doi.org/10.1038/leu.2014.336
PMid:25434302
- Rashidi,
A. et al. Outcomes of haploidentical vs matched sibling transplantation
for acute myeloid leukemia in first complete remission. Blood Adv. 3,
1826-1836 (2019).
- Nagler,
A. et al. Comparison of Haploidentical Bone Marrow versus Matched
Unrelated Donor Peripheral Blood Stem Cell Transplantation with
Posttransplant Cyclophosphamide in Patients with Acute Leukemia. Clin.
Cancer Res. 27, 843-851 (2021). https://doi.org/10.1158/1078-0432.CCR-20-2809
PMid:33148668
- Bashey,
A. et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated
Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor
Transplantation Using Post-Transplant Cyclophosphamide. J. Clin. Oncol.
35, 3002-3009 (2017). https://doi.org/10.1200/JCO.2017.72.8428
PMid:28644773 PMCid:PMC5590802
- Khoury,
J. D. et al. The 5th edition of the World Health Organization
Classification of Haematolymphoid Tumours: Myeloid and
Histiocytic/Dendritic Neoplasms. Leukemia 36, 1703-1719 (2022). https://doi.org/10.1038/s41375-022-01613-1
PMid:35732831 PMCid:PMC9252913
- Armand,
P. et al. Validation and refinement of the Disease Risk Index for
allogeneic stem cell transplantation. Blood 123, 3664-3671 (2014). https://doi.org/10.1182/blood-2014-01-552984
PMid:24744269 PMCid:PMC4047501
- Blouin,
A. G., Ye, F., Williams, J. & Askar, M. A Practical Guide To
Chimerism Analysis: Review of The Literature and Testing Practices
Worldwide. Hum. Immunol. 82, 838-849 (2021). https://doi.org/10.1016/j.humimm.2021.07.013
PMid:34404545 PMCid:PMC9492519
- Toubai,
T., Sun, Y. & Reddy, P. GVHD pathophysiology: is acute
different
from chronic? Best Pract. Res. Clin. Haematol. 21, 101-117 (2008). https://doi.org/10.1016/j.beha.2008.02.005
PMid:18503979
- Appelbaum,
F. R. Allogeneic hematopoietic cell transplantation for acute myeloid
leukemia when a matched related donor is not available. Hematol. Am.
Soc. Hematol. Educ. Program 412-417 (2008)
doi:10.1182/asheducation-2008.1.412. https://doi.org/10.1182/asheducation-2008.1.412
PMid:19074118
- McCurdy,
S. R. & Fuchs, E. J. Selecting the best haploidentical donor.
Semin. Hematol. 53, 246-251 (2016). https://doi.org/10.1053/j.seminhematol.2016.08.001
PMid:27788762
- Devillier,
R. et al. Reduced intensity versus non-myeloablative conditioning
regimen for haploidentical transplantation and post-transplantation
cyclophosphamide in complete remission acute myeloid leukemia: a study
from the ALWP of the EBMT. Bone Marrow Transplant. 57, 1421-1427
(2022). https://doi.org/10.1038/s41409-022-01674-x
PMid:35752739
- Baron,
F. et al. Human leukocyte antigen-haploidentical transplantation for
relapsed/refractory acute myeloid leukemia: Better leukemia-free
survival with bone marrow than with peripheral blood stem cells in
patients ≥55 years of age. Am. J. Hematol. 97, 1065-1074 (2022). https://doi.org/10.1002/ajh.26627
PMid:35696192
- Raiola,
A. M. et al. Impact of HLA Disparity in Haploidentical Bone Marrow
Transplantation Followed by High-Dose Cyclophosphamide. Biol. Blood
Marrow Transplant. (2017) doi:10.1016/j.bbmt.2017.10.002. https://doi.org/10.1016/j.bbmt.2017.10.002
PMid:29024804


