Gianmarco Bagnato1,2, Vittorio Stefoni1,2, Alessandro Broccoli1,2, Lisa Argnani2, Cinzia Pellegrini1, Beatrice Casadei1, Francesca Bonifazi3 and Pier Luigi Zinzani1,2.
1 IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli”, Bologna, Italy.
2 Dipartimento di Scienze Mediche e Chirurgiche, Università di Bologna, Bologna, Italy.
3 IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Correspondence to:
Pier Luigi Zinzani, MD, PhD and Professor. IRCCS Azienda
Ospedaliero-Universitaria di Bologna, Istituto di Ematologia
“Seràgnoli” and Dipartimento di Scienze Mediche e Chirurgiche,
Università di Bologna. Via Massarenti, 9 – 40138 Bologna, Italy. Tel
+39.051.2143680 – Fax +39.051.6364037. E-mail:
pierluigi.zinzani@unibo.it
Published: January 01, 2024
Received: September 13, 2023
Accepted: December 10, 2023
Mediterr J Hematol Infect Dis 2024, 16(1): e2024004 DOI
10.4084/MJHID.2024.004
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
We
report the case of 2 patients with relapsed/refractory peripheral
T-cell lymphoma treated with valemetostat tosylate, a selective dual
inhibitor of histone-lysine N-methyltransferases enhancer of zest
homolog 1 and 2, and subsequently bridged to allogeneic stem cell
transplantation. Valemetostat led to a quick response and was well
tolerated, offering a promising bridge therapy to transplantation for
patients with relapsed/refractory peripheral T-cell lymphoma, which is
still an unmet medical need.
|
Introduction
Relapsed/refractory
peripheral T-cell lymphoma (R/R PTCL) has a poor prognosis, with a
median overall survival (OS) of approximately 6 months.[1-3]
The achievement of a meaningful response and subsequent allogeneic stem
cell transplantation (allo-SCT) remains the only approach that can
overcome the dismal prognosis of the disease, reaching a 5-year OS and
progression-free survival (PFS) of approximately 50% and 40%,
respectively.[4]
New emerging drugs, which may
lead to a complete response (CR), have a key role as a bridge therapy
to allo-SCT, given that the presence of active disease before allo-SCT
significantly correlates with a higher relapse rate.[5]
We
report the case of 2 patients with R/R PTCL treated with valemetostat
tosylate, a selective dual inhibitor of histone-lysine
N-methyltransferases enhancer of zest homolog 1 and 2 (EZH1/2) as a
bridge therapy to allo-SCT. Patients gave consent to publish their data.
Case Presentation
Case #1.
A 63-year-old Caucasian man was diagnosed with PTCL with T follicular
helper (TFH) phenotype, not otherwise specified, EBV negative, stage
IIIA in November 2019. He was treated with CHOEP (cyclophosphamide,
doxorubicin, vincristine, etoposide, and prednisone) for 6 cycles from
December to April 2019, achieving a partial response. Then he received
brentuximab vedotin for 9 cycles from June to December 2019, obtaining
a stable disease. Therefore, the patient was treated with 8 cycles of
gemcitabine plus cisplatin from June to December 2020, resulting in
disease progression (PD).
The patient was then referred to our
center, where he received ASTX-660, a novel non-peptidomimetic
antagonist of apoptosis proteins (cIAP1/2 and XIAP), 90 mg BID for 2
cycles from April to June 2021, resulting in PD. The treatment was
complicated by a grade 3 serum amylase and lipase increase and a grade
1 dysphagia.
Then, the patient was candidated for treatment with
valemetostat. He was in stage IIIEA (tonsil) at the beginning of the
treatment (Figure 1a). He
received valemetostat 200 mg/day on a continuous 28-day cycle from
January to September 2022 for 10 cycles. After 2 cycles, he reached a
metabolic CR (Figure 1b).
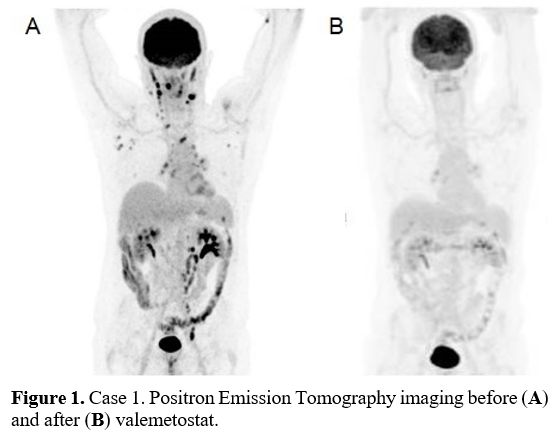 |
- Figure
1. Case 1. Positron Emission Tomography imaging before (A) and after (B) valemetostat.
|
A
grade 1 COVID-19 infection complicated the treatment. The first
negative swab occurred 1 month after the infection. The patient
experienced two episodes of grade 2 E. coli-related urinary tract
infection.
In October 2022, the patient underwent allo-SCT,
still in CR disease status from a matched unrelated donor (HLA 9/10,
for mismatch in A). The washout period between valemetostat and
allo-SCT was 14 days. The conditioning regimen consisted of thiotepa,
fludarabine, and cyclophosphamide. Acute graft versus host disease
(GVHD) prophylaxis consisted of anti-thymocyte globulin, cyclosporine A
(CSA), and methotrexate. The neutrophil engraftment occurred at day
+12.
The treatment was complicated by Streptococcus
mitis-related sepsis. At day +4, veno-occlusive disease was diagnosed,
and the patient received defibrotide 6.25 mg/kg/6h for 21 days. At day
+20, the patient was diagnosed with engraftment syndrome that required
methylprednisolone 2 mg/kg/bid, with the improvement of symptoms. After
CSA interruption, the patient experienced a progressive rash that was
consistent with GVHD skin 3, gut 0, liver 0, and global 2 that
required steroid therapy.
At the last follow-up, the patient was
in good clinical condition, and the rash was vanishing. The last
restaging, performed 3 months after the allo-SCT, confirmed that the
patient is in continuous metabolic CR.
Case #2.
In August 2020, a 50-year-old Caucasian woman was diagnosed with PTCL,
not otherwise specified, EBV positive, stage IVB for bone marrow and
tonsil involvement.
The patient received CHOEP for six cycles
from August to November 2020 and 3 lumbar punctures with intrathecal
triple therapy (methotrexate, cytarabine, and dexamethasone) as
prophylaxis for central nervous system relapse. The patient reached a
metabolic CR, but a bone marrow biopsy showed the persistence of
lymphoma involvement. Therefore, she received a high dose of cytarabine
in January 2021 with hematopoietic stem cell harvesting. A new bone
marrow biopsy was still positive for lymphoma involvement.
Then,
the patient showed rapid PD with the involvement of the left adrenal
gland, rhinopharynx, tonsil, bone marrow, spleen, and soft tissue.
Therefore, she was referred to our center where she was treated with
AFM-13 for 3 cycles from April to September 2021. After the second
cycle, the restaging showed a metabolic CR, and the bone marrow biopsy
excluded lymphoma involvement. Therefore, the patient was scheduled for
consolidation through allo-SCT, but while the third cycle was ongoing,
she experienced a new rapid PD with tonsil and spleen involvement
(stage IVsA).
In the absence of another available clinical
trial, she was treated with gemcitabine plus oxaliplatin for 3 cycles
from October to December 2021, resulting in PD.
Right before valemetostat, the patient showed disease involvement in the tongue, rhinopharynx, and bone marrow (Figure 2a).
The
patient received valemetostat 200 mg/day for 2 cycles from January to
March 2022. After the second cycle, the patient achieved a metabolic CR
(Figure 2b). A new bone marrow biopsy excluded lymphoma involvement
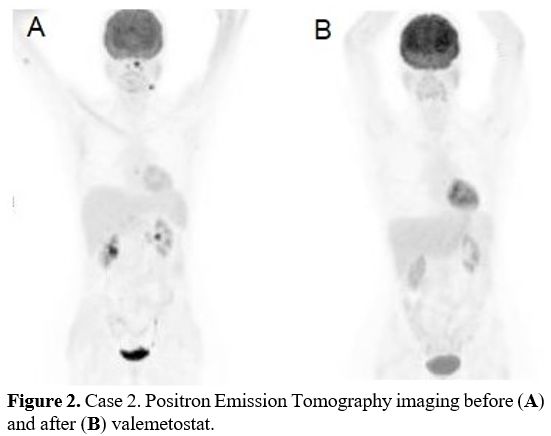 |
- Figure 2. Case 2. Positron Emission Tomography imaging before (A) and after (B) valemetostat.
|
The
treatment was well tolerated. The first cycle was complicated by the
appearance of asymptomatic purpuric skin lesions on the inferior legs
and on the right forearm. A skin biopsy showed an inflammatory process.
The skin lesions resolved after treatment with topic steroid. During
the second cycle, a grade 1 dysgeusia occurred.
The patient
received haploidentical allo-SCT using her brother as donor. The
washout period between valemetostat and allo-SCT was 14 days. The
conditioning regimen consisted of thiotepa, busulfan and fludarabine.
GVHD prophylaxis consisted of cyclophosphamide on days +3 and +4 and
CSA/ mycophenolate mofetil since day +5. The neutrophil engraftment
occurred on day 17.
The treatment was complicated by Staphylococcus coagulase negative-related sepsis and pulmonary invasive fungal infection.
At
the latest follow-up, the patient was in good clinical condition, and
ten months after allo-SCT, she is still in continuous CR.
Discussion
PTCL
still retains a poor prognosis, and its management is complicated by
the paucity of available effective drugs in the relapsed/refractory
setting.
Valemetostat tosylate is a selective dual inhibitor of
both wild-type and mutated forms of EZH1 and EZH2. EZH1 and EZH2 are
enzymatically active core subunits of polycomb repressive complex 2
(PRC2) that, by the trimethylation of the 27th
lysine of histone 3 (H3K27me3), lead to chromatin folding and
repression of genes involved in tumor suppression and cell growth.[6-10] By EZH1/2 inhibition, valemetostat leads to chromatin unfolding and unleashing transcriptional expression of these genes.[11]
Valemetostat
was recently approved in Japan for treating R/R adult T-cell
leukemia/lymphoma (ATL) based on the results of a multicenter phase 2
Japanese trial.[7,12] In this study,
25 patients with R/R ATL received valemetostat 200 mg/die orally until
the progression of disease or drug intolerance. The overall response
rate (ORR) was 48% (CR rate 20%). The median time to respond was 1.4
months. The most common grade ≥3 adverse events (AEs) were
hematological toxicities: thrombocytopenia (32%) and anemia (32%).[7]
Valemetostat also showed promising efficacy in a subset of 14 patients
with R/R ATL and 45 patients with R/R PTCL in the US and Japanese phase
1 trial in R/R NHL where the ORR was 55.6% (CR rate 24.4%) and 50% (CR
rate 21.4%), respectively. The median response time was 8.14 weeks in
both subsets.[8]
Valemetostat is currently under clinical evaluation for patients with R/R PTCL in the VALENTINE-PTCL01 trial (NCT04703192).
To
our knowledge, no published data about allo-SCT following valemetostat
treatment exists. Indeed, no patient in the phase 2 Japanese trial
underwent allo-SCT as consolidation after response with valemetostat..
Conclusions
In
our patients, valemetostat showed great rapid activity and was well
tolerated without significant AEs, leading to consolidation therapy
through allo-SCT. Valemetostat is a promising option as bridge therapy
to allo-SCT for patients with R/R PTCL, which is still an unmet medical
need. Further data are required to understand how valemetostat would
affect outcomes of subsequent allo-SCT.
Acknowledgments
We thank Massimo Agostini for data entry and AIL Bologna OdV for funding his activity (prot 2CSAIL21 Argnani).
References
- Chihara D, Fanale MA, Miranda RN, Noorani M, Westin
JR, Nastoupil LJ, Hagemeister FB, Fayad LE, Romaguera JE, Samaniego F,
Turturro F, Lee HJ, Neelapu SS, Rodriguez MA, Wang M, Fowler NH, Davis
RE, Medeiros LJ, Hosing C, Nieto YL, Oki Y. The survival outcome of
patients with relapsed/refractory peripheral T-cell lymphoma-not
otherwise specified and angioimmunoblastic T-cell lymphoma. Br J
Haematol. 2017;176(5):750-758. https://doi.org/10.1111/bjh.14477 PMid:27983760 PMCid:PMC5836501
- Mak
V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, Villa D,
Gascoyne RD, Connors JM, Savage KJ. Survival of patients with
peripheral T-cell lymphoma after first relapse or progression: spectrum
of disease and rare long-term survivors. J Clin Oncol.
2013;31(16):1970-1976. https://doi.org/10.1200/JCO.2012.44.7524 PMid:23610113
- Ellin
F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic
factors and treatment in peripheral T-cell lymphomas: a study from the
Swedish Lymphoma Registry. Blood. 2014;124(10):1570-1577. https://doi.org/10.1182/blood-2014-04-573089 PMid:25006130
- Dodero
A, Spina F, Narni F, Patriarca F, Cavattoni I, Benedetti F, Ciceri F,
Baronciani D, Scimè R, Pogliani E, Rambaldi A, Bonifazi F, Dalto S,
Bruno B, Corradini P. Allogeneic transplantation following a
reduced-intensity conditioning regimen in relapsed/refractory
peripheral T-cell lymphomas: long-term remissions and response to donor
lymphocyte infusions support the role of a graft-versus-lymphoma
effect. Leukemia. 2012;26(3):520-526. https://doi.org/10.1038/leu.2011.240 PMid:21904377
- Freytes
CO, Loberiza FR, Rizzo JD, Bashey A, Bredeson CN, Cairo MS, Gale RP,
Horowitz MM, Klumpp TR, Martino R, McCarthy PL, Molina A, Pavlovsky S,
Pecora AL, Serna DS, Tsai T, Zhang MJ, Vose JM, Lazarus HM, van Besien
K; Lymphoma Working Committee of the International Bone Marrow
Transplant Registry. Myeloablative allogeneic hematopoietic stem cell
transplantation in patients who experience relapse after autologous
stem cell transplantation for lymphoma: a report of the International
Bone Marrow Transplant Registry. Blood. 2004;104(12):3797-3803. https://doi.org/10.1182/blood-2004-01-0231 PMid:15280203
- Foss
FM, Porcu P, Horwitz SM, Izutsu K, Ishitsuka K, Kato K, Jin J, Du Y,
Inoue A. A Global Phase 2 Study of Valemetostat Tosylate (Valemetostat)
in Patients with Relapsed or Refractory (R/R) Peripheral T-Cell
Lymphoma (PTCL), Including R/R Adult T-Cell Leukemia/Lymphoma (ATL) -
Valentine-PTCL01. Blood. 2021;138(suppl 1):2533. https://doi.org/10.1182/blood-2021-144676
- Izutsu
K, Makita S, Nosaka K, Yoshimitsu M, Utsunomiya A, Kusumoto S,
Morishima S, Tsukasaki K, Kawamata T, Ono T, Rai S, Katsuya H, Ishikawa
J, Yamada H, Kato K, Tachibana M, Kakurai Y, Adachi N, Tobinai K,
Yonekura K, Ishitsuka K. An open-label, single-arm phase 2 trial of
valemetostat for relapsed or refractory adult T-cell leukemia/lymphoma.
Blood. 2023;141(10):1159-1168. https://doi.org/10.1182/blood.2022016862 PMid:36150143 PMCid:PMC10651775
- Ishitsuka
K, Izutsu K, Maruyama D, Makita S, Jacobsen ED, Horwitz S, Kusumoto S,
Allen P, Porcu P, Imaizumi Y, Yamauchi N, Morishima S, Kawamata T, Foss
FM, Utsunomiya A, Nosaka K, Serbest G, Kato K, Adachi N, Tsukasaki K,
Tobinai K. First in human study of the EZH1 EZH2 dual inhibitor
valemetostat tosylate (DS‐3201B) in patients with relapsed or
refractory non-Hodgkin lymphomas. Hematol Oncol. 2021;39(S2):014. https://doi.org/10.1002/hon.14_2879
- Yamagishi
M, Hori M, Fujikawa D, Ohsugi T, Honma D, Adachi N, Katano H, Hishima
T, Kobayashi S, Nakano K, Nakashima M, Iwanaga M, Utsunomiya A, Tanaka
Y, Okada S, Tsukasaki K, Tobinai K, Araki K, Watanabe T, Uchimaru K.
Targeting Excessive EZH1 and EZH2 Activities for Abnormal Histone
Methylation and Transcription Network in Malignant Lymphomas. Cell Rep.
2019;29(8):2321-2337.e7. https://doi.org/10.1016/j.celrep.2019.10.083 PMid:31747604
- Nakagawa
M, Kitabayashi I. Oncogenic roles of enhancer of zeste homolog 1/2 in
hematological malignancies. Cancer Sci. 2018;109(8):2342-2348. https://doi.org/10.1111/cas.13655 PMid:29845708 PMCid:PMC6113435
- Honma
D, Adachi N, Kanno O, Watanabe J, Hirasawa M, Nosaka E, Shiroishi M,
Takizawa T, Yasumatsu I, Horiuchi T, Nakao A, Suzuki K, Yamasaki T,
Nakajima K, Hayakawa M, Yamazaki T, Araki K, Fujiwara K. Blood.
2017;130(suppl1):2073.
- Daiichi
Sankyo Company Ltd. EZHARMIA® approved in Japan as first dual EZH1 and
EZH2 inhibitor therapy for patients with adult T-cell leukemia/lymphoma
[media release]. 26 Sep 2022. https://www.daiichisankyo.com/.

