Vasiliki
Antari1, Lemonia Skoura2,
Emmanuel Hatzipantelis1, Vasiliki-Rengina
Tsinopoulou1, Konstantina Papakonstantinou1,
Efthimia Protonotariou2, Assimina
Galli-Tsinopoulou1 and Athanasios Tragiannidis1.
1 Childhood
& Adolescent Hematology Oncology Unit, 2nd Pediatric
Department,
School of Medicine, Aristotle University of Thessaloniki, AHEPA
University Hospital, Thessaloniki, Greece
2 Department
of Microbiology, School of Medicine, Aristotle University of
Thessaloniki, AHEPA University Hospital, Thessaloniki, Greece.
Correspondence to:
Athanasios Tragiannidis, MD, PhD, Assistant Professor of Pediatrics -
Pediatric Hematology-Oncology. Hematology Oncology Unit, 2
nd
Pediatric Department, School of Medicine. Faculty of Health Sciences,
Aristotle University of Thessaloniki, AHEPA Hospital, Greece. E-mail:
atragian@auth.gr,
atragian@hotmail.com
Published: November 01, 2023
Received: September 21, 2023
Accepted: October 18, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023065 DOI
10.4084/MJHID.2023.065
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
To investigate the kinetics and prognostic value of pancreatic stone
protein (PSP) and mid-regional proadrenomedullin (MR-proADM) during
episodes of febrile neutropenia (FN) in children with hematological
malignancies.
Material and methods:
We evaluated prospectively a total of 70 FN episodes in 70 children
with acute leukemias and lymphomas. CRP, PSP, and MR-proADM levels were
measured at the onset of the febrile episode (day 1), day 3, and day 7.
The outcome and survival of children were evaluated during the study
period until day 28. The performance of each marker in identifying
sepsis or severe sepsis was assessed as an area under a receiver
operating characteristic (ROC) curve. ROC curves were used for each
biomarker to derive cut-offs for sensitivity and specificity in
distinguishing sepsis from non-sepsis.
Results:
During the 2-year study period, 70 febrile neutropenia episodes in 70
children with hematological malignancies were enrolled. Of 70 episodes
of febrile neutropenia, in 17 (24%), a bacterial/fungal infection was
documented. Criteria for sepsis were fulfilled for 31 (44%) and 7 (10%)
patients were admitted to PICU. The median values of all biomarkers
on day 1 differed significantly between patients with
and without sepsis. PSP, MR-proADM, and CRP specificity were 0.82,
0.70, and 0.57, respectively. The sensitivity of PSP, MR-proADM, and
CRP were 0.84, 0.74, and 0.88, respectively.
Conclusions:
PSP and MR-proADM are promising biomarkers for early diagnosis of
sepsis during FN episodes in children with hematological malignancies.
However, PSP has a higher sensitivity and specificity.
|
Introduction
In
the era of multi-modality and targeted therapies, survival of children
with cancer has excellent rates that exceed 80% in most European and
North American countries.[1]
Relapsed and/or refractory diseases are the main cause of treatment
failure and are related to low survival rates.[1,2]
Infections are still a severe and important complication to therapy and
represent the second cause of death and a main cause of morbidity and
mortality, especially for children with acute leukemias and those who
underwent hematopoietic stem cell transplantation (HSCT).[3-6]
Febrile neutropenia (FN) is the most common adverse event of intensive
chemotherapy and cause of treatment delay.[6]
In the last two decades, the emergence of newer antiinfectives in
combination with the use of novel biomarkers of infection in our
arsenal have significantly ameliorated the outcome of febrile episodes
and the survival of patients. The prompt initiation of antimicrobial
therapy plays a crucial role in managing these episodes. In case of
persisting fever and neutropenia, escalation of antimicrobial treatment
is advised in combination with empiric use of antifungals.[5-7]
Many prognostic models for the outcome of febrile neutropenic episodes
have been reported in the literature; however, there is still a strong
need for accurate and sensitive biomarkers of infection for
distinguishing septic from non-septic and bacteremic from
non-bacteremic patients.[8-10]
CRP is an acute
inflammatory protein produced by the liver by proinflammatory cytokines
IL-1, IL-6, and IL-17 and increases 6-10 hours after the onset of acute
infections and inflammation.[11]
Measurement of CRP is highly recommended by infectious disease
societies.[12,13]
In the pediatric setting, there have been many attempts to incorporate
predictive models, including CRP, for the initial assessment of
children with FN.[8,10]
In a recent
systematic literature review, 37 studies evaluated CRP as a biomarker
of bacterial infection in high-risk febrile immunocompromised children.
CRP was related to high sensitivity, ranging from 77.7 to 92.3%, but
poor specificity (15.5–72.2%).[9]
Contrarily, higher
cut-off values have been related to a decrease in sensitivity
(24.2–77.8%) and a slight increase in specificity (63–87.3%).[9]
Originally,
lithostatic and regenerating protein 1 (Reg 1), later renamed
pancreatic stone protein (PSP), has been studied in patients with
pancreatitis and diabetes.[14] PSP
is a 14kDa
insoluble polypeptide encoded by the reg gene secreted by the pancreas,
and its response is induced by systemic inflammation and sepsis. As an
acute-phase protein, it is involved in cell proliferation during
regenerative processes through proinflammatory cytokines and in the
inflammatory response to infection. PSP has widely been used in adults
as a biomarker of sepsis in the clinical setting and has a higher
diagnostic impact in identifying infection than other used biomarkers.[14]
Due to its unique characteristic of increasing before the onset of
signs and symptoms of infection and sepsis (pre-symptomatic diagnosis),
PSP has widely been studied in different populations and age groups and
has shown to be more accurate and with higher sensitivity and
specificity than CRP, procalcitonin (PCT) and IL-6 for diagnosis and
outcome of sepsis.[15] Data on
neonates and children
are scarce. In children, the combination of PSP with PCT and CRP has
been shown to be superior to each isolated biomarker for children with
sepsis and osteomyelitis.[16-17]
In neonates, PSP has
been evaluated as a sepsis biomarker. It has been found that, alone or
when combined with PCT, it has a high negative predictive value that
rules out sepsis and helps de-escalate antibiotic treatment.[18-19] This is the first study
evaluating PSP in the pediatric hematology oncology setting.
Adrenomedullin
is a peptide with immunomodulating effects that have been shown to have
a role in the integrity and stability of the vascular endothelium after
severe infection. It seems to downregulate many processes, including
inflammation and sepsis. Its serum levels show rapid elevations during
sepsis, followed by rapid circulation clearance, making it difficult to
detect because of its half-life of 22 min.[2]
Due to
its increased stability, the precursor mid-regional pro adrenomedullin
(MR-proADM) is measured in clinical practice. MR-proADM is predictive
for poor clinical outcomes in patients with sepsis, severe respiratory
and urinary infections, and heart and kidney failure in adult and
pediatric settings.[20-23] As a
precursor amino acid
sequence that splits from proadrenomedullin, MR-proADM is used as a
surrogate marker for adrenomedullin. In the pediatric setting,
MR-proADM has widely been used as a biomarker and prognostic marker in
children with lower respiratory (mainly community-acquired pneumonia),
urinary tract infections, and malignancy.[20-23]
To
date, no combined studies have investigated the role of the standard
biomarker CRP compared to the newer (PSP, MR-proADM) for predicting
bacteremia, sepsis, and outcome in febrile neutropenic children with
hematological malignancies. Therefore, we aimed to evaluate all the
above mentioned biomarkers in this setting.
Material and methods
Patients
and study design. At the Pediatric Hematology-Oncology
Unit of the 2nd
Pediatric Department of the Aristotle University of Thessaloniki,
Greece, 70 febrile neutropenic consecutive episodes in 70 children with
hematological malignancies were collected and analyzed prospectively
from January 2020 to December 2022. For each patient, only one FN
episode was included in the study. Inclusion criteria were age under 18
years and diagnosis of acute leukemia or lymphoma without a history of
fever and antibiotic use in the last 7 days. For each patient/episode,
we collected demographic data, underlying disease, treatment, fever
characteristics (temperature, days until defervescence), duration and
severity of neutropenia, positivity of blood cultures collected during
the onset of the FN episode, and antimicrobial treatment. Total blood
counts and biochemical exams were routinely performed in all patients
during each febrile neutropenic episode. Plasma specimens for CRP and
MR-proADM and whole blood for PSP were collected at the onset of each
episode and before administering the first antibiotic treatment. All
the abovementioned biomarkers were measured on day 1, day 3, and day 7.
Samples collected for each patient were centrifugated (3000r/min for
10min) and stored at -80 °C. CRP levels were measured by
immunoturbidimetry (mg/dl; normal values <0.8mg/dl). PSP values
were
measured with the CE-marked IVD PSP capsule on the abioSCOPE® platform
(Abionic SA, Epalinges, Switzerland). This nanofluidic immunoassay
technology measures a point-of-care PSP value within 7 min via a
fingerstick test. The abioSCOPE platform can measure PSP values of up
to 600 ng/mL.[24] Higher values
were displayed as
>600 ng/mL. Serum MR-ProADM was measured by the new sandwich
immunoassay method (Novus Biologicals, USA). The assay (normal
reference range 0.33 ± 0.7 nmol/l) has an analytical detection limit of
0.05 nmol/l. Values of MR-proADM were provided as nmol/l, and the assay
had a functional sensitivity of 0.47 nmol/L.[25]
For
each patient, data about clinical signs and symptoms, vital signs,
focal infection (pneumonia, urinary tract infection, bloodstream
infection, colitis, CNS infection), transfer to ICU, and outcome on day
28 (survival, death) were prospectively collected. The study protocol
was approved by the Ethics Committee of the Medical School of Aristotle
University of Thessaloniki, Greece (42/2022).
Definitions.
Definitions of systemic inflammatory response syndrome (SIRS),
infection, sepsis, severe sepsis, and septic shock were based on those
published by the International Consensus Conference on Pediatric
Sepsis.[26] Bacteremia was defined
by positive blood
cultures in patients with FN. In the case of coagulase-negative
Staphylococcus spp, two positive blood cultures were required.
Localized infection was defined as the presence of clinical and/or
radiological findings of infection in febrile neutropenic patients
without positive bloodstream infection (BSI). Additionally, fever was
defined as a single axillary temperature ≥38.0 °C for more than one
hour or ≥38.3 °C or greater and neutropenia as absolute neutrophil
count (ANC) of <500cells/μL or the decrease of ANC to
500cells/μL in
the next 24-48 hours according to the definitions of the American
Society of Clinical Oncology and Infectious Diseases Society of
America.[27]
Statistical
analysis.
Continuous variables were described using the mean ± SD for normally
distributed data or the median (interquartile range (IQR)) for
non-normally distributed data. Comparisons of group differences for
continuous variables were made by one-way ANOVA or Mann-Whitney test as
appropriate. Categorical data were described as the number of patients
in each category with corresponding percentages. The significance of
differences in proportions was tested by the Chi-squared test. The
performance of each marker in identifying sepsis or severe sepsis was
assessed as an area under a receiver operating characteristic (ROC)
curve. ROC curves were used for each marker to derive cut-offs for
sensitivity and specificity in distinguishing sepsis from non-infective
SIRS. Statistical analyses were performed in SPSS 17.0 (IBM Corporation
Somers, NY, USA) and Prism 5 (GraphPad Software Inc. La Jolla, CA,
USA). All P-values were two-sided, and statistical significance was set
at an a-value of 0.05.
Results
During
the 2-year study period, 70 FN episodes in 70 consecutive children with
hematological malignancies were enrolled. Seventy patients' underlying
diseases were: acute lymphoblastic leukemia (55 patients), acute
myeloid leukemia (11 patients), and non-Hodgkin lymphoma (4 patients).
The study population consisted of 41 females and 29 males, and the
median age of patients was 5 (range: 1-18 years). Patients’ demographic
data and clinical characteristics are shown in Table 1. Of 70 FN
episodes enrolled, no focus of infection was documented in 45 (64%)
cases, while in 17 (24%) episodes a BSI was detected. Gram-negative
bacteria were obtained in 13 (19%) episodes, Gram-positive in 2 (3%)
episodes, and an invasive fungal infection (candidemia) was documented
in 2 (3%) episodes. Gram-negative organisms isolated were Pseudomonas aeruginosa
(5), E. coli
(4), Klebsiella
pneumonia (2), and Citrobacter
koseri (2). A coagulase-negative Staphylococcus spp
and a Candida
spp were detected both in 2 episodes. Criteria for sepsis were
fulfilled for 31 patients (44%), and 7 (10%) were admitted to the ICU.
Overall, all-cause mortality on day 28 was 4% (3 patients), while 67
patients (96%) underwent defervescence, bacteremia eradication, and
infection resolution. The median temperature value at the onset of the
FN episode was 38.5 (range: 37.5-40.0 °C). All patients were
neutropenic at onset, and the median value of WBC and absolute
neutrophil count (ANC) were 1300 (range: 10-14000/μL) and 100 (range:
0-500/μL), respectively. The mean hemoglobin value at day 1 was 8.5
(5.9-13.1g/dl), and the mean platelets value was 44000/μL
(4000-393000/μL). All demographic characteristics, clinical data,
laboratory findings, focus of infection, and outcomes of patients are
shown in Table 1.
 |
- Table
1.. Demographics, clinical characteristics, the focus of infection, and outcome of children with haematological malignancies.
|
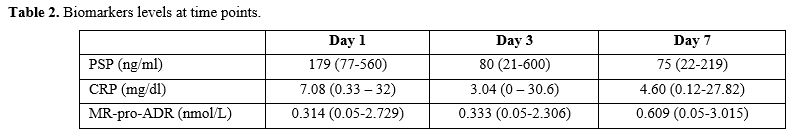
Biomarker
median levels and ranges at days 1, 3, and 7 are shown in Table 2.
CRP and PSP levels were significantly higher at the onset of the
febrile episode (day 1) and decreased on days 3 and 7. Of note, for
MR-proADM the highest median values were measured on day 7 from the
onset of the FN episode. The median length of stay in the hospital at
the onset of the FN episode for children who fulfilled the criteria for
sepsis was 22 days and differed significantly from those without (10
days) (p=0.01). Patients who fulfilled the criteria for sepsis had a
significantly lower median WBC count at onset and differed
significantly from those without (700/μL vs 1340/μL; p=0.04).
Contrarily, although the median ANC of patients with sepsis was lower
than those without, no statistical significance was found (230/μL vs
457/μL; p=0.06). Criteria for sepsis were fulfilled for 31 (44%) and 7
(10%) patients were admitted to PICU. All-cause mortality on day 28 was
4% (3 patients, two with candidemia/invasive candidiasis and one with
sepsis due to invasive aspergillosis/COVID-19 infection. All three
biomarkers were significantly higher in children with sepsis than in
those without at the onset of the FN episode. Of note, the median PSP
levels on day 1 were higher in children with sepsis compared to those
without [179ng/ml (range: 77-560ng/ml) vs. 80ng/ml (range:
21-600ng/ml)] and differed significantly (p<0.00001).
 |
- Table
2.. Biomarkers levels at time points.
|
Similarly,
CRP and MR-proADM levels on day 1 were higher in children with sepsis
and differed significantly from those without (p=0.06 and p=0.02,
respectively). Baseline characteristics, laboratory data, focus of
infection, outcome, and median values of PSP, MR-proADR, and CRP in
patients with and without sepsis during the onset of the FN episode are
shown in Table 3.
Additionally, we compared biomarker levels between patients with and
without bloodstream infections (BSI). Patients with BSI had prolonged
hospitalization compared to those without (median 20 vs 14 days) but
did not differ significantly (p=0.16). Similarly to patients with
sepsis, those with BSI had a lower median value of WBC and ANC but did
not differ significantly between the 2 groups (p=0.15 and p=0.10,
respectively). Of note, the median value of PSP on day 1 differed
significantly between patients with and without a BSI [174ng/ml (range:
81-560ng/ml) vs 89ng/ml (range: 21-600ng/ml)] (p=0.03).
 |
- Table
3.. Baseline characteristics of patients with and without sepsis.
|
Similarly,
the median value of MR-proADR on day 1 differed significantly among
patients with BSI and those without (p=0.04). Contrarily to this, the
median CRP value was higher for children with BSI but did not differ
significantly (p=0.21). Table
4
shows baseline patients’ characteristics, clinical and laboratory data,
focus on infection, outcome, median PSP, MR-pro-ADR, and CRP values in
patients with and without BSI.
In order to determine the
capability of biomarkers to detect severe infection and sepsis, ROC
curve analysis was conducted for each biomarker. The specificity of
PSP, MR-proADM, and CRP were 0.82, 0.70, and 0.57 respectively. The
sensitivity of PSP, MR-proADM, and CRP were 0.84, 0.74, and 0.88,
respectively. The positive predictive value (PPV) of PSP, MR-proADM,
and CRP were 0.81, 0.64, and 0.68, respectively. The negative
predictive value (NPV) of PSP, MR-proADM, and CRP were 0.85, 0.84, and
0.78, respectively. The AUC (area under the curve) of PSP, MR-proADR,
and CRP were 0.80 (CI 95%: 0.67 – 0.92), 0.68 (CI 95%: 0.50 – 0.86) and
0.67 (CI 95%: 0.52 – 0,82), respectively. The AUC for PSP, MR-proADR,
and CRP are shown in Figure
1-3.
 |
Table
4. Baseline line characteristics of patients with BSI. |
 |
Figure
1. AUC for PSP. |
 |
Figure
2 AUC for MR-proADR. |
 |
Figure
3. AUC for CRP.
|
Discussion
Children
and adolescents with hematological malignancies represent a particular
population at risk for developing FN after conventional antitumoral
therapies due to defects in immunity and mucosal integrity.6 Many
prognostic models for febrile neutropenic episodes have been reported
in the literature; however, it remains still challenging to predict
outcomes and distinguish septic from non-septic and bacteremic from
non-bacteremic patients.[8-10]
Our study
evaluated the role of the newer biomarkers PSP and MR-proADM compared
to CRP in febrile neutropenic children with hematological malignancies.
According to our findings, PSP is a promising biomarker for
early
diagnosis of sepsis during FN episodes in children with hematological
malignancies. PSP has the best AUC compared to MR-proADM and CRP, with
high sensitivity and specificity. For both biomarkers, sensitivity and
specificity were high, although CRP presented the highest sensitivity
(0.88) but poor specificity (0.57), as already reported in the
literature in single studies but also in the recent systematic review
by Van der Velden et al.[9]
PSP has been studied
and compared to other biomarkers in neonates and children with
infection, sepsis, osteomyelitis, and in those admitted to pediatric
ICU. To our knowledge, no data about kinetics and the role of PSP in
febrile neutropenic children with hematological malignancies are
available in the literature. In a recent review, Eggimann et al.
concluded that in 12 out of 13 studies performed in different clinical
settings in the adult population, PSP proved to be more accurate and
with a higher specificity and sensitivity in comparison to CRP, PCT,
and IL-6.[15] Apart from the adult
setting, PSP has
been evaluated as a diagnostic marker of sepsis-related organ failure
in 62 pediatric patients admitted in the ICU and has shown high
specificity and a low sensitivity value (0.92 and 0.50, respectively).
Based on their results, the authors of the abovementioned study
concluded that PSP values did not differ between patients with systemic
inflammatory response syndrome and sepsis; however, those who died had
higher PSP levels compared to survivors.[16]
Another
study in the pediatric setting evaluated and compared PSP, CRP, and PCT
as prognostic factors in children with sepsis and septic shock, and
according to their findings, the accuracy (AUC) to predict death was
for PSP=0.83, for PCT=0.76, and for CRP=0.73. These findings are
similar to ours regarding PSP and CRP, although patients were
non-neutropenic. The authors concluded that all the abovementioned
biomarkers can predict the outcome, and combining all three improves
the prediction significantly.[17]
In the setting of
the neonatal population, various studies have evaluated PSP as a
predictor of sepsis and have shown that its accuracy (AUC) for
infection and sepsis is high alone or in combination with PCT.[18,19]
To our knowledge, only a recent study by de Guadiana-Romualdo et al.
evaluated biomarkers' role in adult cancer patients. In this study,
like ours, 105 febrile neutropenic adult patients with cancer and PCT,
PSP, and sCD25 were measured at presentation to evaluate these markers'
ability to diagnose infection and outcome. All biomarkers were
significantly higher in infected patients, and PCT presented the
highest diagnostic accuracy for infection (AUC: 0.901), whereas PSP and
sCD25 had a lower and similar performance (AUC: 0.751 and 0.730,
respectively).[28] In our study,
the AUC of PSP was
superior (0.80); however, the methodology differed as we focused
particularly on the diagnostic performance of PSP for diagnosis of
early-onset sepsis in pediatric febrile neutropenic patients. Other
studies in the adult setting with sepsis-related complications have
shown that PSP levels have a high diagnostic accuracy in discriminating
the severity and outcome.[29-35]
In our study, PSP
was superior in discriminating between patients with sepsis and those
without and between patients with and without a BSI.
MR-proADR has been demonstrated to have an impact as a prognostic
marker for bacteremia, sepsis, FN, and pneumonia.[20-23,36-46]
In our study, MR-proADM has shown a sensitivity and specificity value
of 0.70 and 0.74, respectively. A recent systematic review evaluated
the diagnostic accuracy of MR-pro-ADM in identifying children with
invasive bacterial infections. In the meta-analysis, four studies were
selected that included 1404 patients aged between day one of life and
12 years. Only one study was of high quality, accounting for the
majority of patients. A single study reported the diagnostic accuracy
of MR-pro-ADM for invasive bacterial infection, reporting an AUC of
0.69.[21] Agnello et al.
investigated the roles of
presepsin and MR-proADM in 36 FN episodes of 26 children with cancer
and found that both presepsin and MR-proADRM have poor clinical
usefulness for the outcome of FN episodes.[38]
A
recent study that evaluated 36 FN episodes of 14 children with solid
tumors has shown that the first-day plasma MR-proADM levels
significantly predicted the presence of culture positivity (AUC 0.628)
and high risk patients with neutropenic fever (AUC 0.76).[42]
Demirkaya et al. compared MR-proADM with CRP and PCT in pediatric
cancer patients. Among these biomarkers, PCT demonstrated the highest
correlation with the severity of infection, and adrenomedullin levels
on day 3 were significantly higher in the microbiologically documented
infection group than those in the clinically documented infection group
and patients with fever of unknown origin.[43]
In the
adult setting, the role of MR-proADM and PCT as novel biomarkers for
predicting infections in febrile patients with hematogical malignancies
has been evaluated on 340 patients, 103 with sepsis and 159 with SIRS.
Similarly to our results, the initial pro-ADM levels were significantly
higher in neutropenic patients with BSIs than in those without
documented infections. Levels of MR-pro-ADM decreased in response to
antimicrobial therapy in patients with bacterial infection (p=0.007),
as our results also show.[41]
Our study,
although prospective, has several limitations. It is a single-center
study with a small number of patients/episodes during a short period (2
years). Despite this, our findings have demonstrated that PSP, in
particular, is a promising marker for early diagnosis of sepsis and
bacteremia in febrile neutropenic children with cancer. PSP has a
higher AUC than CRP and MR-proADM, with a higher sensitivity and
specificity, and could predict high-risk patients for sepsis-related
complications (ICU transfer, bacteremia, death). Additionally, both can
be useful, especially if combined with CRP, for the early diagnosis of
severe infection and for triaging patients based on the risk of sepsis.
PSP expected from the high sensitivity and specificity, through its
negative predictive value, seems to predict patients who develop
sepsis. Unfortunately, we had some problems timely performing PCT, so
no comparison is possible with this important marker of sepsis. Further
studies are required to understand the diagnostic accuracy of both
tests, particularly alone and in a minor grade in combination, in
predicting the outcome of high-risk febrile neutropenic children with
hematological malignancies at the onset of the episode and during its
course.
References
- Erdmann F, Frederiksen
LE, Bonaventure A, Mader L,
Hasle H, Robison LL, Winther JF. Childhood cancer: Survival, treatment
modalities, late effects, and improvements over time. Cancer Epidemiol.
2021;71(Pt B):101733. https://doi.org/10.1016/j.canep.2020.101733
PMid:32461035
- Hudson
MM, Neglia JP, Woods WG, Sandlund JT, Pui CH, Kun LE, Robison LL, Green
DM. Lessons from the past: opportunities to improve childhood cancer
survivor care through outcomes investigations of historical therapeutic
approaches for pediatric hematological malignancies. Pediatr Blood
Cancer. 2012;58(3):334-43. https://doi.org/10.1002/pbc.23385
PMid:22038641 PMCid:PMC3256299
- Loeffen
EAH, Knops RRG, Boerhof J, Feijen EAML, Merks JHM, Reedijk AMJ,
Lieverst JA, Pieters R, Boezen HM, Kremer LCM, Tissing WJE.
Treatment-related mortality in children with cancer: Prevalence and
risk factors. Eur J Cancer. 2019;121:113-122. doi:
10.1016/j.ejca.2019.08.008. https://doi.org/10.1016/j.ejca.2019.08.008
PMid:31569066
- Lund
B, Åsberg A, Heyman M, Kanerva J, Harila-Saari A, Hasle H, Söderhäll
S, Jónsson ÓG, Lydersen S, Schmiegelow K; Risk factors for treatment
related mortality in childhood acute lymphoblastic leukaemia. Pediatr
Blood Cancer. 2011;56(4):551-9. https://doi.org/10.1002/pbc.22719
PMid:21298739
- Lehrnbecher
T, Averbuch D, Castagnola E, Cesaro S, Ammann RA, Garcia-Vidal C,
Kanerva J, Lanternier F, Mesini A, Mikulska M, Pana D, Ritz N, Slavin
M, Styczynski J, Warris A, Groll AH; 8th European Conference on
Infections in Leukaemia. 8th European Conference on Infections in
Leukaemia: 2020 guidelines for the use of antibiotics in paediatric
patients with cancer or post-haematopoietic cell transplantation.
Lancet Oncol. 2021;22(6):e270-e280. doi: 10.1016/S1470-2045(20)30725-7.
https://doi.org/10.1016/S1470-2045(20)30725-7
PMid:33811814
- Lehrnbecher
T. Treatment of fever in neutropenia in pediatric oncology patients.
Curr Opin Pediatr. 2019;31(1):35-40.
https://doi.org/10.1097/MOP.0000000000000708 PMid:30461508
- Groll
AH, Pana D, Lanternier F, Mesini A, Ammann RA, Averbuch D, Castagnola
E, Cesaro S, Engelhard D, Garcia-Vidal C, Kanerva J, Ritz N, Roilides
E, Styczynski J, Warris A, Lehrnbecher T; 8th European Conference on
Infections in Leukaemia. 8th European Conference on Infections in
Leukaemia: 2020 guidelines for the diagnosis, prevention, and treatment
of invasive fungal diseases in paediatric patients with cancer or
post-haematopoietic cell transplantation. Lancet Oncol.
202;22(6):e254-e69. https://doi.org/10.1016/S1470-2045(20)30723-3
PMid:33811813
- Santolaya
ME, Alvarez AM, Avilés CL, Becker A, Venegas M, O'Ryan M, Salgado C,
Topelberg S, Tordecilla J, Varas M, Villarroel M, Viviani T, Zubieta M,
de la Maza V, Vergara A, Farfán MJ, Torres JP. Prospective validation
of a risk prediction model for severe sepsis in children with cancer
and high-risk febrile neutropenia. Pediatr Infect Dis J.
2013;32:1318-23. https://doi.org/10.1097/01.inf.0000436128.49972.16
PMid:24569305
- van
der Velden FJS, Gennery AR, Emonts M. Biomarkers for Diagnosing Febrile
Illness in Immunocompromised Children: A Systematic Review of the
Literature. Front Pediatr. 2022;10:828569. https://doi.org/10.3389/fped.2022.828569
PMid:35372147 PMCid:PMC8965604
- Ammann
RA, Bodmer N, Hirt A, Niggli FK, Nadal D, Simon A, Ozsahin H, Kontny U,
Kühne T, Popovic MB, Lüthy AR, Aebi C. Predicting adverse events in
children with fever and chemotherapy-induced neutropenia: the
prospective multicenter SPOG 2003 FN study. J Clin Oncol.
2010;28:2008-14. https://doi.org/10.1200/JCO.2009.25.8988
PMid:20231680
- https://wwwn.cdc.gov/nchs/data/nhanes/2001-2002/labmethods/l11_b_met_c_reactive_protein.pdf
- Klastersky
J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M,
Herrstedt J; ESMO Guidelines Committee. Management of febrile
neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol.
2016;27(suppl 5):v111-v118. doi: https://doi.org/10.1093/annonc/mdw325
PMid:27664247
- Kochanek
M, Schalk E, von Bergwelt-Baildon M, Beutel G, Buchheidt D, Hentrich M,
Henze L, Kiehl M, Liebregts T, von Lilienfeld-Toal M, Classen A,
Mellinghoff S, Penack O, Piepel C, Böll B. Management of sepsis in
neutropenic cancer patients: 2018 guidelines from the Infectious
Diseases Working Party (AGIHO) and Intensive Care Working Party (iCHOP)
of the German Society of Hematology and Medical Oncology (DGHO). Ann
Hematol. 2019;98:1051-69. https://doi.org/10.1007/s00277-019-03622-0
PMid:30796468 PMCid:PMC6469653
- Fidalgo
P, Nora D, Coelho L, Povoa P. Pancreatic Stone Protein: Review of a New
Biomarker in Sepsis. J Clin Med. 2022;11:1085. https://doi.org/10.3390/jcm11041085
PMid:35207355 PMCid:PMC8880320
- Eggimann
P, Que YA, Rebeaud F. Measurement of pancreatic stone protein in the
identification and management of sepsis. Biomark Med. 2019;13:135-45. https://doi.org/10.2217/bmm-2018-0194
PMid:30672312
- Jiří
Ž, Kýr M, Vavřina M, Fedora M. Pancreatic stone protein - a possible
biomarker of multiorgan failure and mortality in children sepsis.
Cytokine. 2014;66:106-11. https://doi.org/10.1016/j.cyto.2014.01.009
PMid:24594294
- Wu
Q, Nie J, Wu FX, Zou XL, Chen FY. Prognostic Value of High-Sensitivity
C-Reactive Protein, Procalcitonin and Pancreatic Stone Protein in
Pediatric Sepsis. Med Sci Monit. 2017;23:1533-9. https://doi.org/10.12659/MSM.900856
PMid:28358790 PMCid:PMC5384617
- Schlapbach
LJ, Graf R, Woerner A, Fontana M, Zimmermann-Baer U, Glauser D,
Giannoni E, Roger T, Müller C, Nelle M, Stocker M. Pancreatic stone
protein as a novel marker for neonatal sepsis. Intensive Care Med.
2013;39:754-63. https://doi.org/10.1007/s00134-012-2798-3
PMid:23296629
- Rass
AA, Talat MA, Arafa MA, El-Saadany HF, Amin EK, Abdelsalam MM, Mansour
MA, Khalifa NA, Kamel LM. The Role of Pancreatic Stone Protein in
Diagnosis of Early Onset Neonatal Sepsis. Biomed Res Int.
2016;2016:1035856. https://doi.org/10.1155/2016/1035856
PMid:27689072 PMCid:PMC5027295
- Piccioni
A, Saviano A, Cicchinelli S, Valletta F, Santoro MC, de Cunzo T, Zanza
C, Longhitano Y, Tullo G, Tilli P, Candelli M, Covino M, Franceschi F.
Proadrenomedullin in Sepsis and Septic Shock: A Role in the Emergency
Department. Medicina (Kaunas). 2021;57:920. https://doi.org/10.3390/medicina57090920
PMid:34577843 PMCid:PMC8472723
- Corr
P, Fairley D, McKenna JP, Shields MD, Waterfield T. Diagnostic value of
midregional pro-Adrenomedullin as a biomarker of invasive bacterial
infection in children: a systematic review. BMC Pediatr.
2022;22(1):176. https://doi.org/10.1186/s12887-022-03255-9
PMid:35379203 PMCid:PMC8977188
- Milas
GP, Issaris V. Proadrenomedullin and neonatal sepsis: a systematic
review and meta-analysis of diagnostic accuracy. Eur J Pediatr.
2022;181(1):59-71. https://doi.org/10.1007/s00431-021-04214-9
PMid:34342678
- Peñalver
Penedo R, Rupérez Lucas M, Álvarez-Sala Walther LA, Torregrosa.
Benavent A, Casas Losada ML, Bañuelos Andrio L, Rebolledo Poves AB,
Bueno Campaña M. MR-Proadrenomedullin as biomarker of renal damage in
urinary tract infection in children. BMC Pediatr. 2021;21(1):292. https://doi.org/10.1186/s12887-021-02765-2
PMid:34187408 PMCid:PMC8240321
- Benninga
R., van den Bogaard P., Rebeaud F. Abionic's PSP 'Sepsis Test' on the
AbioSCOPE® Device: 5 Minutes to Save Lives. Abionic SA. 2019
- https://resources.novusbio.com/manual/Manual-NBP2-82495-65554905.pdf?_ga=2.172681509.782560015.1697549965-1777041038.1697549965&_gac=1.220721642.1697550127.Cj0KCQjw4bipBhCyARIsAFsieCw6S7da4Ll8_S8EkVLQF0Zzide9hf4w3oP1Q4CKm6QESWymF4Wi0t0aAumiEALw_wcB
- Goldstein
B, Giroir B, Randolph A; International Consensus Conference on
Pediatric Sepsis. International pediatric sepsis consensus conference:
definitions for sepsis and organ dysfunction in pediatrics. Pediatr
Crit Care Med. 2005;6(1):2-8. https://doi.org/10.1097/01.PCC.0000149131.72248.E6
PMid:15636651
- Freifeld
AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II,
Rolston KV, Young JA, Wingard JR; Infectious Diseases Society of
America. Clinical practice guideline for the use of antimicrobial
agents in neutropenic patients with cancer: 2010 update by the
infectious diseases society of america. Clin Infect Dis. 2011 Feb
15;52:e56-93. https://doi.org/10.1093/cid/cir073
PMid:21258094
- García
de Guadiana-Romualdo L, Jiménez-Santos E, Cerezuela-Fuentes P,
Español-Morales I, Berger M, Esteban-Torrella P, Hernando-Holgado A,
Albaladejo-Otón MD. Analyzing the capability of PSP, PCT and sCD25 to
support the diagnosis of infection in cancer patients with febrile
neutropenia. Clin Chem Lab Med. 2019;57:540-548. https://doi.org/10.1515/cclm-2018-0154
PMid:30240355
- Llewelyn
MJ, Berger M, Gregory M et al. Sepsis biomarkers in unselected patients
on admission to intensive or high-dependency care. Crit. Care
2013;17:R60. https://doi.org/10.1186/cc12588
PMid:23531337 PMCid:PMC3672658
- Gukasjan
R, Raptis DA, Schulz H-U, Halangk W, Graf R . Pancreatic stone protein
predicts outcome in patients with peritonitis in the ICU. Crit. Care
Med. 2013;41:1027-36. https://doi.org/10.1097/CCM.0b013e3182771193
PMid:23399938
- Klein
HJ, Csordas A, Falk V et al. Pancreatic stone protein predicts
postoperative infection in cardiac surgery patients irrespective of
cardiopulmonary bypass or surgical technique. PLoS ONE
2015;10:e0120276. https://doi.org/10.1371/journal.pone.0120276
PMid:25793700 PMCid:PMC4368752
- García
de Guadiana-Romualdo L, Berger M, Jiménez-Santos E et al. Pancreatic
stone protein and soluble CD25 for infection and sepsis in an Emergency
Department. Eur. J. Clin. Invest. 2017;47:297-304. https://doi.org/10.1111/eci.12732
PMid:28155994
- Boeck
L, Graf R, Eggimann P et al. Pancreatic stone protein: a marker of
organ failure and outcome in ventilator-associated pneumonia. Chest
2011;140:925 - 32. https://doi.org/10.1378/chest.11-0018
PMid:21835904
- Que
Y-A, Delodder F, Guessous I et al. Pancreatic stone protein as an early
biomarker predicting mortality in a prospective cohort of patients with
sepsis requiring ICU management. Crit. Care 2012;16:R114. https://doi.org/10.1186/cc11406
PMid:22748193 PMCid:PMC3580689
- Que
Y-A, Guessous I, Dupuis-Lozeron E. Prognostication of mortality in
critically ill patients with severe infections. Chest 2015;148:674-82. https://doi.org/10.1378/chest.15-0123
PMid:26065577
- Arıkan
K, Karadag-Oncel E, Aytac S, Cetin M, Cengiz AB, Gümrük F, Kara A,
Ceyhan M. Usage of Plasma Presepsin, C-Reactive Protein, Procalcitonin
and Proadrenomedullin to Predict Bacteremia in Febrile Neutropenia of
Pediatric Hematological Malignancy Patients. Lab Med. 2021;52:477-84. https://doi.org/10.1093/labmed/lmab002
PMid:33851202
- Florin
TA, Ambroggio L, Brokamp C, Zhang Y, Nylen ES, Rattan M, Crotty E,
Belsky MA, Krueger S, Epperson TN, Kachelmeyer A, Ruddy RM, Shah SS.
Proadrenomedullin Predicts Severe Disease in Children With Suspected
Community-acquired Pneumonia. Clin Infect Dis. 2021;73:e524-e530. https://doi.org/10.1093/cid/ciaa1138
PMid:32761072 PMCid:PMC8326530
- Agnello
L, Bivona G, Parisi E, Lucido GD, Iacona A, Ciaccio AM, Giglio RV,
Ziino O, Ciaccio M. Presepsin and Midregional Proadrenomedullin in
Pediatric Oncologic Patients with Febrile Neutropenia. Lab Med.
2020;51:585-91. https://doi.org/10.1093/labmed/lmaa011
PMid:32221546
- Solé-Ribalta
A, Bobillo-Pérez S, Valls A, Girona-Alarcón M, Launes C, Cambra FJ,
Jordan I, Esteban E. Diagnostic and prognostic value of procalcitonin
and mid-regional pro-adrenomedullin in septic paediatric patients. Eur
J Pediatr. 2020;179:1089-96. https://doi.org/10.1007/s00431-020-03587-7
PMid:31974673
- Alcoba
G, Manzano S, Lacroix L, Galetto-Lacour A, Gervaix A. Proadrenomedullin
and copeptin in pediatric pneumonia: a prospective diagnostic accuracy
study. BMC Infect Dis. 2015;15:347. https://doi.org/10.1186/s12879-015-1095-5
PMid:26286191 PMCid:PMC4543464
- Al
Shuaibi M, Bahu RR, Chaftari AM, Al Wohoush I, Shomali W, Jiang Y,
Debiane L, Raad S, Jabbour J, Al Akhrass F, Hachem RY, Raad I.
Pro-adrenomedullin as a novel biomarker for predicting infections and
response to antimicrobials in febrile patients with hematologic
malignancies. Clin Infect Dis. 2013;56:943-50. https://doi.org/10.1093/cid/cis1029
PMid:23288950
- Kesik
V, Ataş E, Gülcan Kurt Y, Aydın FN, Babacan O, Gülgün M, Korkmazer N.
Adrenomedullin predicts high risk and culture positivity in children
with solid tumors suffering from neutropenic fever. J Infect Chemother.
2016;22:617-21. https://doi.org/10.1016/j.jiac.2016.06.007
PMid:27400951
- Demirkaya
M, Tugcu D, Akcay A, Aydogan G, Akıcı F, Salcioglu Z, Ekmekci H,
Sevinir B, Balci Ekmekci O. Adrenomedullin--A New Marker in Febrile
Neutropenia: Comparison With CRP and Procalcitonin. Pediatr Hematol
Oncol. 2015;32:482-9. https://doi.org/10.3109/08880018.2015.1057310
PMid:26271020
- Oikonomopoulou
N, Míguez-Navarro C, Rivas-García A, García Gamiz M, López-López R,
Oliver-Sáez P, Riaño-Méndez B, Farfan-Orte T, Lobato-Salinas Z,
Rúbies-Olives J, Llena-Isla P, Lancho-Monreal EM; PROADM-DOLOR
ABDOMINAL of the research net of the Spanish Society of Pediatric
Emergencies (RISEUP-SPERG). Assessment of proadrenomedullin as
diagnostic or prognostic biomarker of acute appendicitis in children
with acute abdominal pain. Am J Emerg Med. 2019;37:1289-94. https://doi.org/10.1016/j.ajem.2018.09.038
PMid:30287129
- Míguez
C, Tomatis Souverbielle C, Haro A, Guerrero G, Pérez-Egido L,
García-Gamiz M, Marañon R. Evaluation of proadrenomedullin as a
diagnostic or prognostic biomarker of acute appendicitis in children.
Am J Emerg Med. 2016;34:2298-305. https://doi.org/10.1016/j.ajem.2016.08.032
PMid:27609121
- Rey
C, García-Hernández I, Concha A, Martínez-Camblor P, Botrán M, Medina
A, Prieto B, López-Herce J. Pro-adrenomedullin, pro-endothelin-1,
procalcitonin, C-reactive protein and mortality risk in critically ill
children: a prospective study. Crit Care. 2013;17:R240. https://doi.org/10.1186/cc13064
PMid:24131732 PMCid:PMC3840693






