 |
|
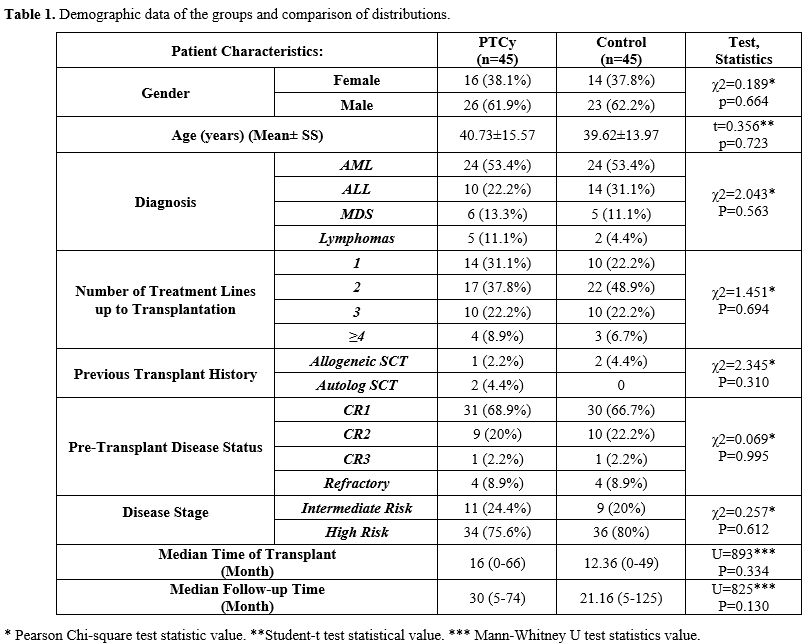
Comparison of Transplantation Data. Table 2 illustrates that there was no statistically significant difference in the mean CD34+ cell count between the PTCy group (5.24±1.41) and the control group (5.32±1.39) (p=0.871). In addition, there was also no statistically significant difference in the mean TNC value between the PTCy group (9.91±4.58) and the control group (10.69±3.89) (p=0.175). The distribution of stem cell sources was found to be similar in both the PTCy and control groups (p=0.699), with the majority of patients in both groups receiving a "peripheral" stem cell source.
 |
|
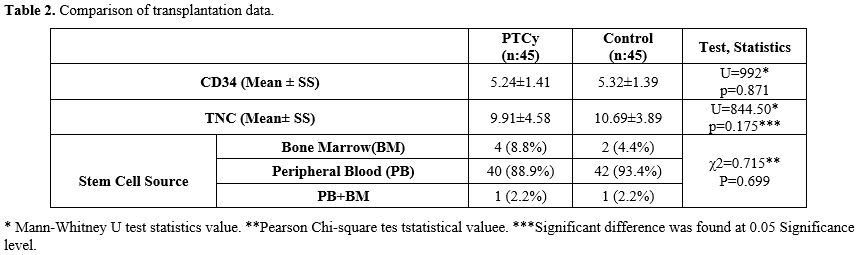
Comparison of Pre-Transplant Viral Serologies. The distribution of CMV-IgG and EBV IgG status in donor and recipient in both Post-Cy and control groups was similar. It was observed that both donor and recipient CMV IgG positivity constituted the majority of the frequency (75.6%-84.5%). The same was true for EBV IgG serology (77.8%, 64.5%). Anti-HBc-IgG status distributions were similar in the PTCy and control groups (P=0,667), and the rate of both donor and recipient negative status were majority in both groups (PTCy; 36 (80.0%), control; 34 (76.6%)) (Table 3). HBsAg, Anti HCV, and Anti HIV serology results of all patients or donors were negative. Preemptive antiviral treatment (tenofovir or entecavir) was started if any donor or recipient had a positive AntiHBcIgG serology. Hepatitis B reactivation was not observed in any patient during the transplant period and the following one-year follow-up.
 |
|
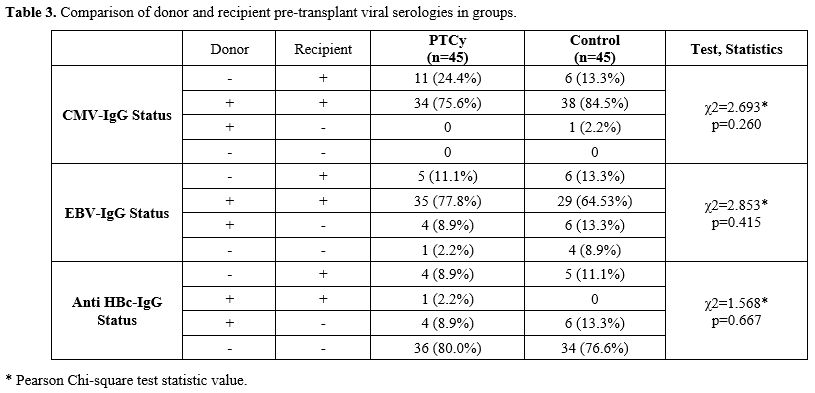
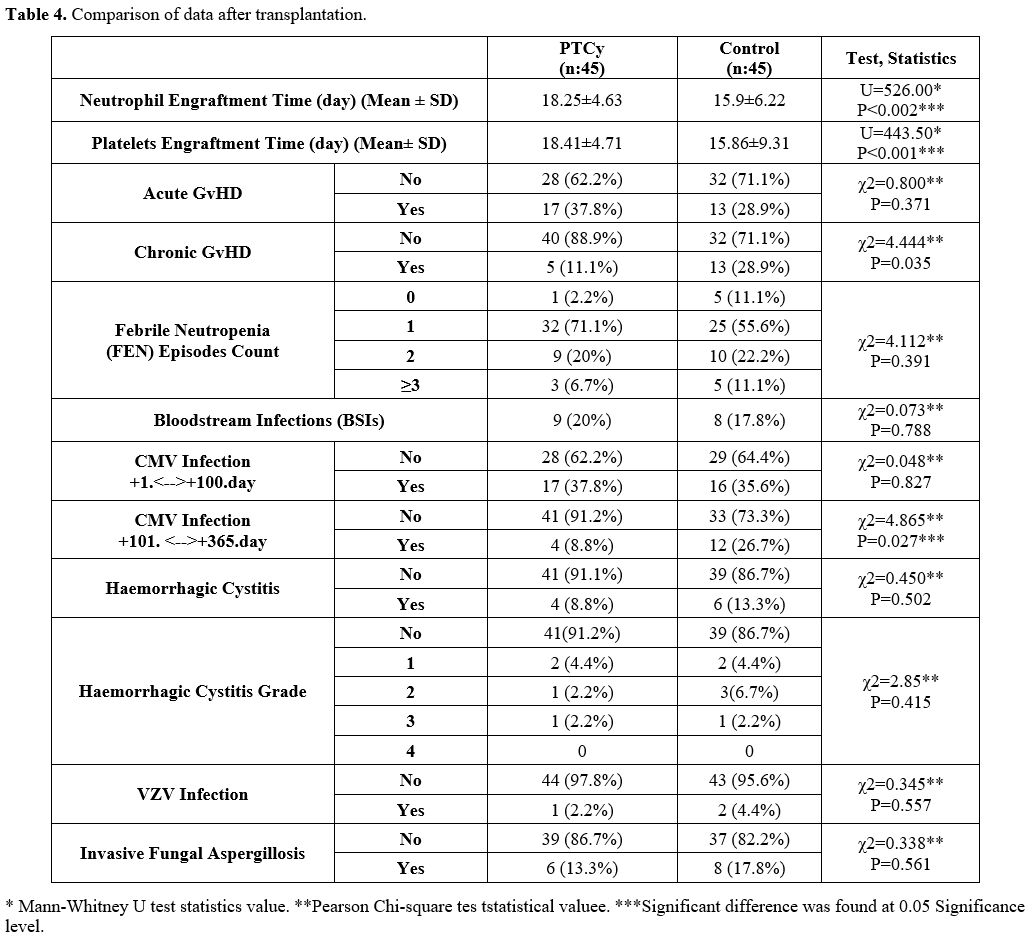
Comparison of GVHD rates, Viral Reactivation, Infections, and Survival Data After Transplantation. Frequency distributions of aGVHD in the PTCy and control groups were similar (p=0.037). Moreover, the incidence of cGVHD was significantly higher in the control group (28.9%, n=13) compared to the PTCy group (11.1%, n=5). Frequency distributions of febrile neutropenic (FEN) in the PTCy and control groups were similar (p=0.391). It was observed that those who had ‘’1’’FEN in the first 30 days in both groups were in the majority (PTCy; n:32 (71.1%) and control; 25 (55.6%). There was no statistically significant difference in BSI frequencies between the PTCy arm and the control arms. The distribution of CMV infection in the first 100 days was similar in the PTCy and control groups (p=0.827). The distribution of CMV infection rate between the 100th and 365th days in the PTCy and control group was statistically significant, and it was observed more frequently in the control group. (PTCy; 4(8.8%), control; n:12(26.7%) (p=0.027). HC rates and their grades were similar in the PTCy and control groups (p=0.502). The rate of HC was n:4 (8.8%) in the PTCy group and n:6 (13.3%) in the control group. The rates of VZV infection and invasive aspergillosis were similar in the PTCy and control groups (Table 4).
 |
|
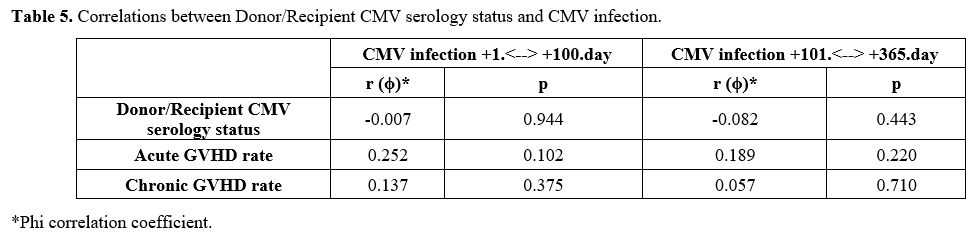
No statistically significant association was observed between CMV infection observed both within the first 100 days and between days 101-365 and donor/recipient CMV serology status, aGVHD, and cGVHD (Table 5).
 |
|
In the PTCy arm, 6 patients died due to infections (n:4 bacterial septic shock, n:2 CMV pneumonia. In the control group, 6 patients died due to BSI septic shock and 2 due to Covid-19-related infection. There was no statistically significant difference in IRM between both groups. When evaluated in terms of OS, no statistically significant difference was found between PTCy and control groups. Median OS for PTCy: 56 months (Range 0-66), and control: 38 months (Range 0-49)(p=0.664) (Figure 1). Leukemia Free Survival calculation included 34 patients in the PTCy arm and 38 patients in the control arm, and no statistically significant difference was found between the two arms (P=0.230, 18 months in PTCy vs 7.83 months in the control group). The RI was similar in both groups (P=0.612) and found to be 20.0% vs 24.4% in PTCy and control groups, respectively. Also, NRM rates were similar in both groups (P=0.788) and found to be 17.8% vs. 20.0% in PTCy and control groups, respectively (Table 6). The median GRFS time was 3 months (Range 1-57) and 5 months (Range 1-49) for PTCy and control, respectively, and no statistically significant difference was found between the two arms (p=0.915) (Figure 2).
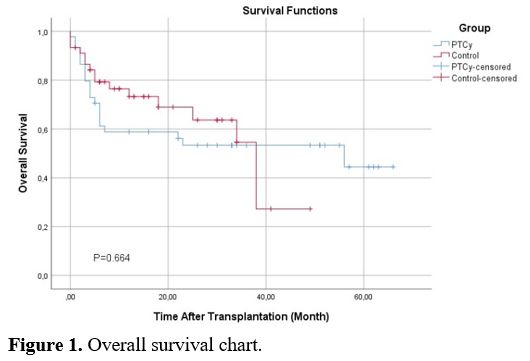 |
Figure 1. Overall survival chart. |
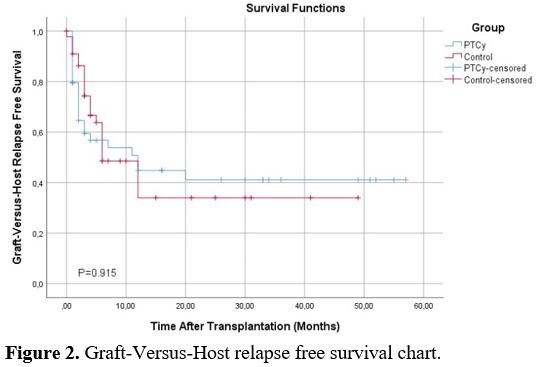 |
Figure 2. Graft-Versus-Host relapse free survival chart. |
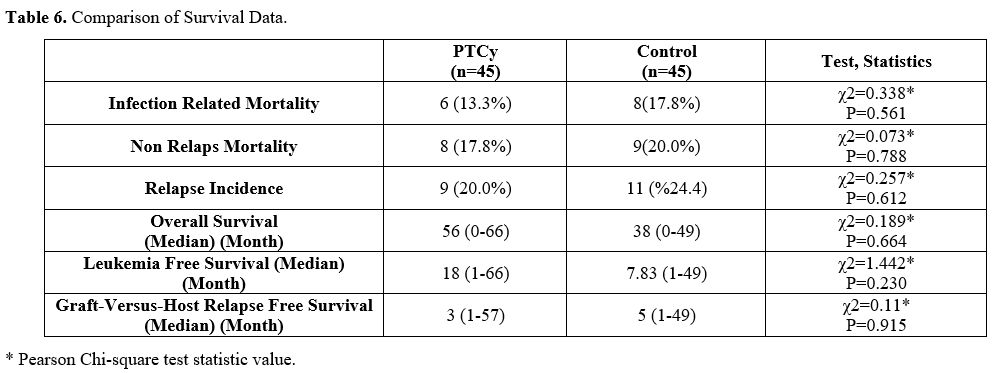 |
Table 6. Comparison of Survival Data. |