Macoura Gadji1,2,*, Youssou Bamar Gueye1,*, David Motto1 and Saliou Diop1,3.
1 National
Centre of Blood Transfusion (NCBT) of Dakar, Senegal,
2
Service of Haematology & Oncology-Haematology (HBOH), Department of
Biology and Applied Pharmaceutical Sciences; Faculty of Medicine,
Pharmacy and Odonto-Stomatology (FMPOS), University Cheikh Anta Diop of
Dakar (UCAD), Dakar, Senegal.
3 Service of Haematology,
Department of Medicine; Faculty of Medicine, Pharmacy and
Odonto-Stomatology (FMPOS), University Cheikh Anta Diop of Dakar
(UCAD), Dakar, Senegal.
* The authors contributed equally to this work..
Correspondence to: :
Prof. Macoura Gadji: PharmD., Ph.D. Head of HBOH/FMPOS/UCAD. Address 1:
CNTS BP 5002 Dakar Fann PtE, Dakar, Senegal. Tel. (221) 776813160.
E-mail:
macoura.gadji@ucad.edu.sn
Published: January 01, 2024
Received: October 26, 2023
Accepted: December 10, 2023
Mediterr J Hematol Infect Dis 2024, 16(1): e2024008 DOI
10.4084/MJHID.2024.008
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Blood transfusion is a supportive therapy improperly performed in sub-Saharan Africa (SSA).[1]
The World Health Organization (WHO) recommends establishing national
blood transfusion systems based on voluntary unpaid blood donors.
Unfortunately, countries of SSA continue to struggle with inadequate
resources and infrastructure for a safer blood supply despite the
important need for blood transfusion to treat severe and chronic anemia
resulting from tropical diseases, sickle cell disease and other
haemoglobinopathies, severe parasitic infections, nutritional anaemia
in a condition of low or moderate safety of transfusion.[1,2]
As a routine practice in front of a deficit of blood products,
prescribers appeal to the patient's family members to donate to
minimize the impact of blood shortages on patient care. This type of
family-aware blood donors, known as familial/replacement donors (FRBD),
despite the risk of transfusion safety, account for 20% of blood
donation in Senegal, for 88.6% in Nigeria,[3] and in
several other African countries (69.5% in Yaounde and 80.2% in Sierra
Leone). During the COVID-19 pandemic, the supply of safe blood was
threatened by the measures taken to fight this virus spread, like the
advice to stay at home and the fear of infection at the blood
transfusion centers, limiting donors' access to blood services. These
measures to prevent the spreading of the COVID-19 pandemic have led to
a sharp decline in stocks of blood products and to an increase of the
number of FRBD. To evaluate the impact of this COVID-19 pandemic on the
infectious safety of blood transfusion, we performed a descriptive and
analytical study carried out during the first period of COVID-19,
aiming to compare the seroprevalence of HIV, HBV, HCV, and syphilis in
FRBD versus voluntary unpaid
blood donors (VUBD). The goal is to evaluate the threats to familial
blood donation during catastrophic periods such as pandemics, wars, and
so on and to help define a policy in improving the recruitment,
retention, and medical screening of blood donors in SSA. After
answering a pre-donation questionnaire, a social worker received the
blood donor, who opened the donor file with an identifier in the donor
management software (Inlog®). At this stage, the blood donor indicates
whether he has come for a voluntary or family/replacement donation.
Subsequently, the donor was interviewed by the medical practitioner for
the pre-donation medical screening, based on questionnaires of
effective blood donors, to verify if the serological results were
indeterminate or discordant in our analysis. The donors' serology and
blood grouping results were taken from the Inlog® software. The
serological tests for HBV, HIV, and HCV were performed with AlinityTM automated, which uses ChemiflexTM
(ABBOTT, Germany) chemiluminescence technology to screen for infectious
markers. According to the manufacturer's instructions, the Rapid Plasma
Reagent test was used to find treponemal antibodies. The determination
of ABO and Rh, blood group typing, was performed with the standard
methods as a globular method with monoclonal antibodies of blood
grouping antisera and serologic method with red blood cells (globule
tests) on a plate. Data analysis was performed using Epi-info software
(version 3.5.4). This software allows the application of the Chi2 test
to accept or reject the statistical hypotheses posed (p < 0.05)
and to give the odd ratio (OR) between the dependent variables and the
independent variables, as well as their 95% confidence interval (CI).
During this pandemic period, 5002 blood donors were collected at the
fixed location of the National Centre of Blood Transfusion. The mean
age of the donors was 32.23 ± 9.9 years. Young people aged from 25 to
34 years constitute the majority of blood donors (35.7%). Male donors
represented 75%; new donors (52.6%) and FRBD (54%) were the majority of
blood donors (Table 1). Analysis of donor status by type of donation showed more FRBD donors among new donors (66.7%) (p˂0.001). Voluntary donors were more represented in the regular known donor group (63.8%) (p<0.001).
Blood group O Rh+ was more represented in this population (49.4%),
followed by group A+ (20.6%) and B+ (17.8%); Rh-negative donors
represented only 8.8%. This study revealed a higher number of FRBD than
VUBD (p <0.001) during the
blood shortage due to the COVID-19 pandemic. This was the case in
Nigeria, where 61.7% of paid donors and 30.6% of family/replacement
blood donors were reported. All these results highlight that family
replacement blood donation is still a common practice in Africa and is
exacerbated during times of blood shortage such as COVID-19 pandemic
period. The prevalence of transfusion transmissible infections (TTIs)
was statistically higher in the FRBD group (9.2%) compared to VUBD
(4.3%) (p<0.001). The prevalence of infectious markers was higher in new unknown donors (10.6%) than in regular known donors (2.9%) (p<0.001, OR=1.9) (Table 2).
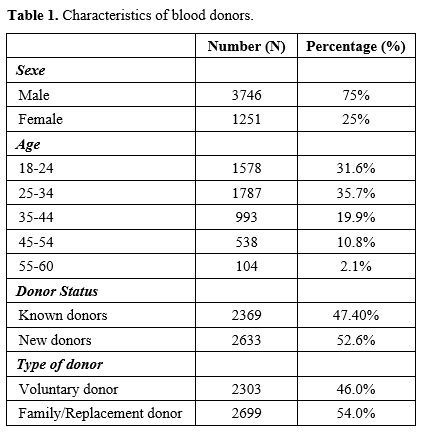 |
Table 1. Characteristics of blood donors. |
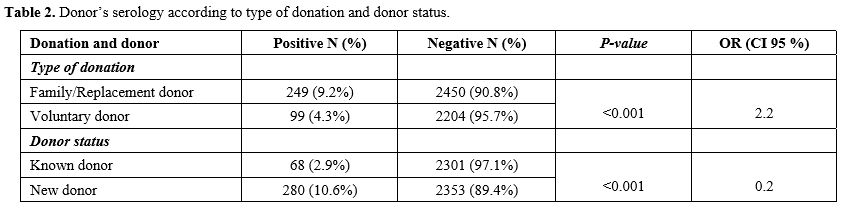 |
Table 2. Donor’s serology according to type of donation and donor status.
|
The
prevalence of TTI markers was statistically higher in the new FRBD
group compared to the new VUBD population (11.7% vs. 8.3%) (p=0.003,
OR=1.4). The comparison of HIV, HCV and syphilis marker
seroprevalences, only in new donors, showed no statistically
significant difference between both categories of new FRBD and new VUBD
(p>0.05). However, for HBV, the prevalence was higher in new FRBD with a statistically significant difference (p=0.002; Table 3).
Our results showed that FRBD increases the risk of having at least one
positive serological result for one of the infectious markers tested (p ˂ 0.001; OR = 2.2), in line with different studies in the World.[4]
Furthermore, a statistically higher seroprevalence of infectious agents
in new donors was found compared to regular donors in Africa, notably
in Mali and Niger.[5] The comparison of HIV, HCV, and
syphilis seroprevalences between new FRBD and new VUBD showed no
statistically significant difference in the prevalence for these three
markers. However, a statistically higher prevalence among new FRBD for
HIV, HCV, and syphilis markers was found in the Democratic Republic of
Congo (DRC).[6] Previously, in Cameroon, a study found a statistically higher prevalence of HCV and HIV in first-time FRBD.[7]
In our study, the lack of statistically significant difference between
voluntary and replacement donors for these three markers could be
explained by the effectiveness of medical screening and the low
prevalence of these infectious markers, especially HIV, in the general
population. However, in our study, the prevalence of HBV is
significantly higher in new FRBD (6.4%) than in new VUBD (2.9%; (p<0.001). These results are similar to those of the study in the DRC, with higher values in the new FRBD.[6]
Nonetheless, in Tanzania, there is no statistical difference in the
prevalence of HIV, HCV, and syphilis, but the prevalence of HBV was
significantly higher in new FRBD.[8] This higher
prevalence of HBsAg in FRBD could be explained by the risk factor of
transmission linked to living in a common household with a person
infected with HBV. Indeed, previous studies revealed that the HBV
virus can be transmitted between people living in the same household.[9,10]
Finally, it is obvious that HBV-carried parents increase the risk of
virus transmission to their children and relatives. The COVID-19
pandemic impacted the proper supply of blood products by increasing
more than 2X the number of FRBD. Thus, replacement donations have
played an important role in limiting the damage observed with blood
shortages despite the increased risk of TTIs. Our study highlights and
strengthens the WHO recommendations for selecting voluntary unpaid
donors. Our results will allow to continue collecting
family/replacement donors in blood shortage situations while taking
into account the prevalence of infectious blood markers in the new
donor population.
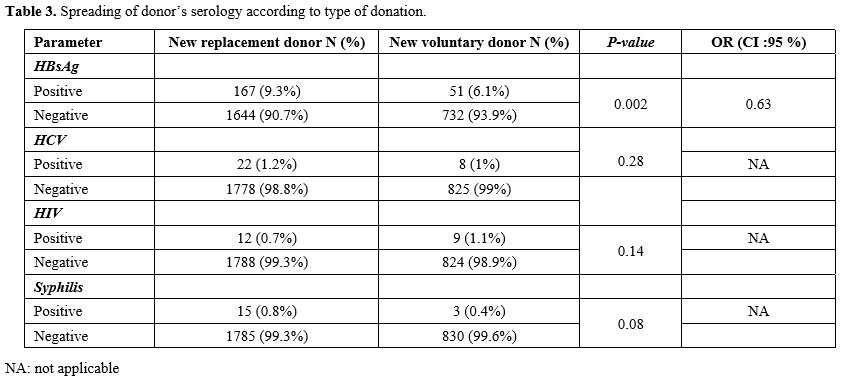 |
- Table 3. Spreading of donor’s serology according to type of donation.
|
Acknowledgments
The authors would like to thank the blood donors who made this study feasible.
References
- Gadji M, Cobar G, Thiongane A, Senghor AB, Seck R,
Faye BF, et al. Red blood cell alloantibodies in pediatric transfusion
in sub‐Saharan Africa: A new cohort and literature review. eJ Haem.
2023 May;4(2):315-23. https://doi.org/10.1002/jha2.645 PMid:37206261 PMCid:PMC10188460
- Ogar
CO, Okoroiwu HU, Obeagu EI, Etura JE, Abunimye DA. Assessment of blood
supply and usage pre- and during COVID-19 pandemic: A lesson from
non-voluntary donation. Transfus Clin Biol. févr 2021;28(1):68‑72. https://doi.org/10.1016/j.tracli.2020.10.004 PMid:33080420 PMCid:PMC7836417
- Shittu
AO, Olawumi HO, Omokanye KO, Ogunfemi MK, Adewuyi JO. The True Status
of Family Replacement Blood Donors in a Tertiary Hospital Blood Service
in Central Nigeria. Africa Sanguine Dec 2019 , 21(2) : 11-14. https://api.semanticscholar.org/CorpusID:214503320
- Ibrahim
Y. A, Mona A. I, Abeer A. S, Mary R. The degree of safety of family
replacement donors versus voluntary non-remunerated donors in an
Egyptian population: a comparative study. Blood Transfus. 2014 Apr;
12(2): 159-165. https://doi.org/10.2450/2012.0115-12. PMID: 23245714; PMCID: PMC4039696.
- Mayaki
Z, Dardenne N, Kabo R, Moutschen M, Sondag D, Albert A, and al.
Séroprévalence des marqueurs infectieux chez les donneurs de sang à
Niamey (Niger). Rev d'Épidémiologie Santé Publique. 2013 June
;61(3):233‑40. https://doi.org/10.1016/j.respe.2012.12.018 PMid:23642899
- Lushamba
C J-PC, Bukombe WB, Kachelewa KB, Maheshe TB, Bisangamo CK. Transfusion
Safety at Dr Rau/Ciriri Hospital, East of Democratic Republic of Congo.
Annales des Sciences de la Santé.2018 , 1(19): 25-35. https://api.semanticscholar.org/CorpusID:216870995
- Ankouane
F, Noah Noah D, Atangana MM, Kamgaing SR ,Guekam PR , M. Biwolé MS.
Seroprevalence of hepatitis B and C virus, HIV-1/2 et la syphilis chez
les donneurs de sang à l'hôpital central de Yaoundé dans la région du
centre du Cameroun. Transfus Clin Biol 2016, ;23(2):68‑72. https://doi.org/10.1016/j.tracli.2015.11.008 PMid:26791918
- Mohamed
Z, Kim JU, Magesa A, Kasubi M, Feldman SF, Chevaliez S, and al. High
prevalence and poor linkage to care of transfusion-transmitted
infections among blood donors in Dar-es-Salaam, Tanzania. J Viral
Hepat. 2019 June; 26(6):750. https://doi.org/10.1111/jvh.13073 PMid:30712273 PMCid:PMC6563112
- Chakravarty R.
, Chowdhury A., Chaudhuri S., Santra A., Neogi M., Rajendran K.. and al. Hepatitis B infection in Eastern Indian families:
need for screening of adult siblings and mothers of adult index cases.
Public Health, 119 (2005), pp. 647-654. https://doi.org/10.1016/j.puhe.2004.09.007 PMid:15925680
- Dumpis U., Charles Holmes E., Mendy M., Hill A., Thursz M., Hall A., and
al. Transmission of hepatitis B virus infection in Gambian families
revealed by phylogenetic analysis.J Hepatol, 35 (2001), pp. 99-104. https://doi.org/10.1016/S0168-8278(01)00064-2 PMid:11495049


