Kang Sun, Chaofan Wu, Qi Kong,
Junxia Hu, Lin Shi, Yubo Pi, Dina Suolitiken, Tingting Cui, Leilei
Chen, Xiaodan He, Zhengyang Song, Lin Wu, Jingshi Wang and Zhao Wang.
Department of Hematology, Beijing Friendship Hospital, Capital Medical University, Beijing, China.
Correspondence to:
Zhao Wang, Department of Hematology, Beijing Friendship Hospital,
Capital Medical University, Beijing, 100000, China. E-mails:
wangzhao@ccmu.edu.cn
Published: May 01, 2024
Received: November 12, 2023
Accepted: April 08, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024037 DOI
10.4084/MJHID.2024.037
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objectives: Chronic
active Epstein-Barr virus disease (CAEBV) is a proliferative disease of
EBV+ T or natural killer (NK) cells with an unclear pathogenesis. This
study aimed to examine the frequency and exhaustion levels of
lymphocyte subsets in patients with CAEBV to further investigate the
pathogenesis.
Methods: Using
flow cytometry, we detected the frequency, expression levels of
programmed cell death 1 (PD-1) and programmed death ligand 1 (PD-L1),
and EBV infection status of peripheral T subsets and NK cells in
patients with CAEBV and healthy individuals.
Results: 24
patients and 15 healthy individuals were enrolled in this study.
Patients showed notably higher expression levels of PD-1 and PD-L1 in
peripheral T subsets and NK cells compared to healthy individuals (P
< 0.05). EBV+ lymphocytes exhibited significantly higher PD-L1
expression levels than EBV- lymphocytes. Additionally, the frequency of
effector memory T (Tem) cells was significantly increased in patients,
and the PD-L1 expression level was positively correlated with the EBV
load. Besides, helper T cell 2 (Th2) immune bias, also favoring EBV
amplification, was found in patients, including increased Th2 cell
frequency, enhanced response capacity, and elevated serum levels of
associated cytokines. The distribution and PD-1 expression levels of
peripheral T subsets returned to normal in patients who responded to
PD-1 blockade therapy.
Conclusions: The
up-regulation of the PD-1/PD-L1 pathway of peripheral T and NK cells
and Th2 immune predominance jointly promoted EBV replication and the
development of CAEBV. PD-1 blockade therapy reduced the PD-1 expression
level of lymphocytes and helped normalize the distribution of the T
subsets.
|
Introduction
Epstein-Barr
virus (EBV), a gamma human herpes virus, infects over 95% of the
population. The majority of these infections occur during childhood,
typically asymptomatically, and infections in adolescents often present
as transient infectious mononucleosis (IM).[1-3]
Chronic active EBV disease (CAEBV) is a progressive disease of ≥3
months duration, characterized by significantly elevated blood levels
of EBV-DNA and the infiltration of organs by EBV-infected lymphocytes
in the absence of identified immunodeficiency.[4]
Owing to the fact that cases affecting B cells are typically associated
with primary immunodeficiency, the spectrum of CAEBV disease has been
reconfigured to encompass only T and NK cell disorders.[5]
The clinical manifestations of CAEBV are heterogeneous, including
fever, lymphadenopathy, and hepatosplenomegaly, sometimes associated to
thrombocytopenia.[6] Compared to patients with
EBV-infected NK cells, those with T-cell infection exhibit shorter
survival, more severe systemic symptoms, and elevated blood EBV titers.
Conversely, patients with NK cell infections frequently present with
severe mosquito bite allergy and elevated serum IgE levels.[4]
Furthermore, CAEBV may present concurrently with hemophagocytic
lymphohistiocytosis (HLH) or lymphoproliferative disease (LPD)/lymphoma
during the disease. CAEBV carries a dismal prognosis, with an overall
survival rate of merely 58% at three years post-diagnosis.[7]
To date, allogeneic hematopoietic stem cell transplantation (allo-HSCT)
remains the exclusive curative approach for CAEBV despite the high
treatment-related mortality rates.[7] Consequently,
comprehensive and in-depth explorations of the pathogenesis and further
discovery of new effective treatments for CAEBV are paramount.
So
far, the pathogenesis of CAEBV remains obscure. It is well known that
lymphocytes play a crucial role in viral infection defense, however,
the investigation of lymphocyte subsets in patients with CAEBV is
limited. Lin et al. reported that the frequency of peripheral naïve T
(Tn) cells and CD28+ T cells was diminished, while the frequency of effector memory T (Tem) cells, regulatory T (Treg) cells, and CD38+
T cells was elevated in patients with CAEBV (n=64) compared to healthy
individuals (n=64) in a retrospective study, suggesting that the
distribution of T cell subsets might be implicated in the pathogenesis
of CAEBV.[9] Nonetheless, the potential modifications
of lymphocyte subsets other than those aforementioned in patients with
CAEBV remain unclear.
In recent years, an increasing focus has
been placed on the role of immune checkpoints (ICs) in EBV immune
escape. The programmed cell death protein 1/programmed cell
death-ligand 1 (PD-1/PD-L1) axis plays a pivotal role in maintaining
immune tolerance by down-regulating inflammatory responses and
inhibiting T-cell activation.[10] However, aberrant
upregulation of PD-1/PD-L1 expression has been observed in various
EBV-associated diseases, such as NK/T-cell lymphoma, gastric cancer
(GC), and nasopharyngeal carcinoma (NPC), and is correlated with poor
prognosis.[11-13] Previously, we effectively reduced
the EBV-DNA copies in peripheral blood mononuclear cells (PBMCs) of
patients with CAEBV through the PD-1 blockade and lenalidomide
combination therapy with an overall response rate (ORR) of 54.2%,
demonstrating the considerable potential of PD-1 blockade therapy in
CAEBV.[14] Interestingly, in that study, we found that the frequency of CD8+Tem cells in the response (R)
group was significantly higher than that in the non-response (NR)
group, indicating that blocking the PD-1/PD-L1 pathway may affect the
distribution of T-cell subsets, which needs to be verified.
Therefore,
this study was designed to investigate the distribution of peripheral
T-cell subsets and the expression levels of PD-1/PD-L1 in T and NK
cells in CAEBV patients before and after PD-1 blockade therapy.
Patients and Methods
Study Population.
This prospective observational study enrolled a total of 24 patients
diagnosed with CAEBV and 15 healthy individuals who were admitted to
Beijing Friendship Hospital, Capital Medical University, between
November 2022 and August 2023. All patients met the diagnostic criteria
outlined in the revised 2017 World Health Organization classification:
(1) IM-like symptoms persisting for more than three months; (2)
elevated levels of EBV-DNA in peripheral blood (PB) or histological
evidence of organ involvement as well as detection of virus RNA or
proteins in affected tissues; (3) Exclusion of IM, congenital
immunodeficiency including X-linked lymphoproliferative diseases (XLP),
familial hemophagocytic lymphohistiocytosis (FHL), human
immunodeficiency virus (HIV) infection, autoimmune disease, or other
underlying conditions requiring immunosuppressive therapy, coinfected
with other viruses such as hepatitis B virus (HBV), hepatitis C virus
(HCV), and cytomegalovirus (CMV).[15] Before
enrollment, of 24 patients, 10 were untreated, 8 had taken
dexamethasone, 4 had received final L-DEP (PEG-asparaginase together
with liposomal doxorubicin, etoposide, and methylprednisolone) regimen
one month earlier, 2 had received final DEP (liposomal doxorubicin,
etoposide, and methylprednisolone) regimen one month earlier, and no
one had received HSCT. The 15 healthy individuals were HSCT donors who
tested negative for EBV-DNA in the week prior to enrollment and
exhibited no fever or any signs of infectious disease or history of
medications within the past two weeks. To detect the frequency and
PD-1/PD-L1 expression levels of peripheral lymphocyte subsets in
patients with CAEBV after PD-1 block therapy, 5 patients who responded
to PD-1 blockade combined with lenalidomide therapy were included in
the study. Of these, 3 had received 6 courses of treatment and 2 had
received 3 courses of treatment. The detailed regimen was shown in our
previous published study.[14]
This study has
been registered with ClinicalTrials.gov under the identifier
NCT05841342. The protocol was approved by the Ethics Committee of
Beijing Friendship Hospital (ID: 2022-P2-333-02), and informed consent
was obtained from all patients prior to participation in the study.
This study was funded by the National Natural Science Foundation of
China (No. 82370185).
Analysis of Lymphocyte Subsets by Flow Cytometry.
Peripheral blood samples were collected and anticoagulated with
ethylenediaminetetraacetic acid (EDTA). Within 2 h, PBMCs were isolated
using a lymphocyte isolation solution (P8610, Solarbio, Beijing, China)
and subsequently incubated with Fc Block reagent (564219, BD
Biosciences, Franklin Lakes, NJ USA) to prevent nonspecific staining.
To concurrently assess the EBV infection status along with the
detection of frequencies and ICs levels of lymphocyte subsets, we
employed the primerFlow RNA assay kit (88-18005-210, Invitrogen,
Waltham, MA, USA) containing AF647 fluorescent probes for EBERs 1-2
(337601-000, Invitrogen). The following operations were conducted
following the instructions provided with the kit. PBMCs were equally
divided into five tubes, four of which were stained with different
surface antibodies. Tube 1 (Blank): No antibodies were added. Tube 2 (
T, NK, NKT and Treg cells): CD3-BV510 (317332, Biolegend, San Diego,
CA, USA), CD4-BB700 (566392, BD), CD25-PE-Cy7 (302612, Biolegend),
CD56-APC-Cy7 (318332, Biolegend), PD-1-BV421 (562516, BD), PD-L1-BB515
(564554, BD); Tube 3 (naïve-memory T cells): CD3-BV510 (317332,
Biolegend), CD4-BB700 (566392, BD), CD8-APC-Cy7 (557834, BD), CD45RO-PE
(304206, Biolegend), CCR7-PE-Cy7 (557648, BD), PD-1-BV421 (562516, BD),
PD-L1-BB515 (564554, BD); Tube 4 (Th1, Th2, Th17 cells and PD-1):
CD3-BV510 (317332, Biolegend), CD4-PE-Cy7 (557852), CD8-APC- Cy7
(557834, BD), CXCR3-BB700 (566532, BD), CCR4-PE (359412, BD),
CCR6-BB515 (564479, BD), PD-1-BV421 (562516, BD); Tube 5 (Th1, Th2,
Th17 cells and PD-L1): Antibodies to CD3, CD4, CD8, CXCR3, CCR4, and
CCR6 were all consistent with those in the tube 4, PD-L1-BV421 (563738,
BD). After incubation at 4°C for 30 minutes under light protection, all
tubes were sequentially washed, fixed, and permeabilized, followed by
the application of Foxp3-PE (560046, BD) intracellular staining only to
tube 2. After the second fixation, all tubes, excluding the blank tube,
were incubated with the EBERs 1-2 probe for incubation at 40°C for 2 h
under light protection. Following the washing process, the samples were
subsequently stored in a light-free environment at 4°C for an overnight
duration. On the second day, the signal amplification steps were
executed. The samples were sequentially incubated first with pre-amp
mix solution for 1.5 h, then with amp mix solution for 1.5 h, and
finally with the label probes for 2 h in the dark at 40°C. At last,
cells were resuspended and detected using a FACSCantoⅡflow cytometer
(BD). The obtained data was subjected to analysis using the Flowjo
software.
The cell subpopulation labeling schemes are as follows: NKT cells (CD3+CD56+), Treg cells (CD3+CD4+CD25+Foxp3+), CD4+ Tn cells (CD3+CD4+CD45RO-CCR7+), CD4+ central-memory T (Tcm) cells (CD3+CD4+CD45RO+CCR7+), CD4+ Tem cells (CD3+CD4+CD45RO+CCR7-), CD8+ Tn cells (CD3+CD8+CD45RO-CCR7+), CD8+ Tcm cells (CD3+CD8+CD45RO+CCR7+), CD8+ Tem cells (CD3+CD8+CD45RO+CCR7-), helper T cell 1 (Th1) (CD3+CD4+CXCR3+CCR4-CCR6-), Th2 cells (CD3+CD4+CXCR3-CCR4+CCR6-), Th17 cells (CD3+CD4+CXCR3- CCR4+CCR6+).
Detection of Cytokines.
In this study, we investigated the serum levels of 9 cytokines in both
healthy individuals and patients, including tumor necrosis factor-alpha
(TNF-α), interferon-gamma (IFN-γ), C-X-C chemokine ligand 9 (CXCL9),
interleukin 18 (IL-18), cluster of differentiation 163 (CD163),
suppression of tumorigenicity 2 (ST2), interleukin-1 receptor
antagonist (IL-1RA), interleukin-10 (IL-10), interleukin-17A (IL-17A).
The employed methods comprised the cytokine microsphere assay from
Becton Dickinson and the Luminex Assay Platform System.
Immune Response Capacity of Th Cell Subsets to PHA Stimulation.
PBMCs from patients of CAEBV or healthy individuals were seeded
in 24-well plates at a density of 106 cells/mL and then stimulated with
PHA (00-4977-93, eBioscience, MA, USA) for 72 h. GolgiPlug™ Protein
Transport Inhibitor (555029, BD) was added 9h before the end of
stimulation. After stimulation, PBMCs were collected and centrifuged at
1200 rpm for 5 min. After being washed twice using PBS buffer, PBMCs
were stained with surface antibodies, including CD3-BV510 and
CD4-BB700. Intracellular staining was performed after the cells were
fixed and permeabilized using the Fixation/Permeabilization Kit
(554714, BD) according to the instructions. Antibodies used for
intracellular staining included IFN-γ-PE-Cy7 (557643, BD), IL-4-APC
(560671, BD), IL-17A-BV421 (562933, BD), IL-10-PE (506804, BD).
Subsequently, cells were washed and resuspended according to the
instructions. Finally, the percentage of cytokine-positive cells among
CD4+ T cells in the samples was detected using the FACSCantoⅡflow cytometer.
Statistical Analysis.
Statistical analysis was executed using GraphPad Prism 8 software. The
measurement data conforming to the normal distribution and homogeneity
of variance were represented by the mean ± standard deviation (SD) and
analyzed with a two-sided Student’s t-test. The measurement data that
did not conform to the normal distribution were represented by M (P25,
P75), and a comparison between the two groups was conducted using the
Mann-Whitney U test. The χ2 test was employed for the processing of
categorical data. The statistical significance was determined when the
P value was less than 0.05.
Results
General Characteristics of Participants.
The CAEBV group consisted of 13 females and 11 males, with a median age
of 28 years. The healthy control group included 9 females and 6 males,
and the median age is 37 years. Age and sex did not differ
significantly between the two groups. In the CAEBV group, 10/24
(41.67%) had a previous history of HLH, 21/24 (87.5%) had fever, 19/24
(79.17%) presented with lymphadenopathy, 14/24 (58.33%) exhibited
splenomegaly, and hepatomegaly was observed in 2 patients (8.33%).
Leukopenia, anemia, and thrombocytopenia were common findings in
patients with CAEBV. The clinical characteristics of all participants
are shown in Table 1.
 |
- Table 1. Clinical characteristics of the study population.
|
Patients with CAEBV Exhibited Increased PD-1/PD-L1 Expression Levels on Peripheral T Cell Subsets and NK Cells.
The gating strategy to set CD3+ total T, CD3+CD4+ Th, CD3+CD8+
cytotoxic T (Tc), CD3+CD56+ NKT, and CD3-CD56+ NK cells by flow
cytometry was illustrated in Figure 1a, b.
There were no significant differences in the frequencies of T cells and
NK cells in peripheral lymphocytes between healthy individuals and
patients with CAEBV, and there were no significant differences in the
frequencies of Th, Tc, and NKT cells in T cells between the two groups (Figure 2a, b).
Patients exhibited significantly elevated expression levels of PD-1 in
T, Th, Tc, and NK cells compared to those in the control group (44.2%
[30.93-55.2%] vs. 20.8% [12.5-35.4%], 50% [35.58-58.78%] vs. 24.2%
[16.4, 26.6%], 31.95% [22.65-49.5%] vs. 21.6% [14.4-26.7%], 4.64%
[2.31-10.86%] vs. 1.57% [0.69-3.68%], Figure 2c, d).
In addition, PD-L1 expression levels showed a similar trend in T, Th,
Tc, NKT, and NK cells across both groups (2.2% [1.28-4.04%] vs. 0.64%
[0.1-0.97%], 1.49% [0.91- 5.28%] vs. 0.22% [0.19-2.57%], 2.37%
[1.26-3.7%] vs. 0.28% [0.18-1.01%], 4.91% [3.6-7.72%] vs. 0.96%
[0.47-2.8%], 11.85% [5.45-24.45%] vs. 1.11% [0.29-2.43%], Figure 2c, e).
 |
Figure 1. The gating strategy to set T-cell subsets and NK cells by flow cytometry.
(a) Representative dot plot showing the gating strategy for CD3-CD56+
NK, CD3+CD56+ NKT, CD3+ total T, and CD3+CD4+CD25+Foxp3+ Treg cells.
(b) Gate strategy to set CD3+CD4+ Th, CD3+CD8+ Tc, CD45RO-CCR7+ Tn,
CD45RO+CCR7+ Tcm, and CD45RO+CCR7- Tem cells. (c) Gate strategy to set
CXCR3+CCR4- CCR6- Th1, CXCR3-CCR4+CCR6- Th2, and CXCR3-CCR4+CCR6+ Th17
cells. |
 |
Figure 2. Patients with CAEBV showed significantly higher expression levels of PD-1 and PD-L1 in T cell subsets and NK cells.
Percentages of T (a) and NK (b) cells in lymphocytes in the CAEBV group
and the control group. n=15 for the control group, and n=24 for the
CAEBV group. Representative flow profiles (c) and summarized positive
percentages of PD-1 (d) and PD-L1 (e) in T, Th, Tc, NKT, and NK cells
in the CAEBV group and the control group. *P < 0.05, **P < 0.01,
***P < 0.001, ****P < 0.0001. ns, not significant.
|
To
investigate the potential association between the increased expression
level of PD-1 or PD-L1 on lymphocytes and EBV infection, we categorized
patients’ lymphocytes into EBER+ and EBER-
and compared the expression levels of PD-1/PD-L1 between two groups.
Results showed that there was no significant difference in PD-1
expression levels between EBER+ T cells and EBER- T cells (38.75%
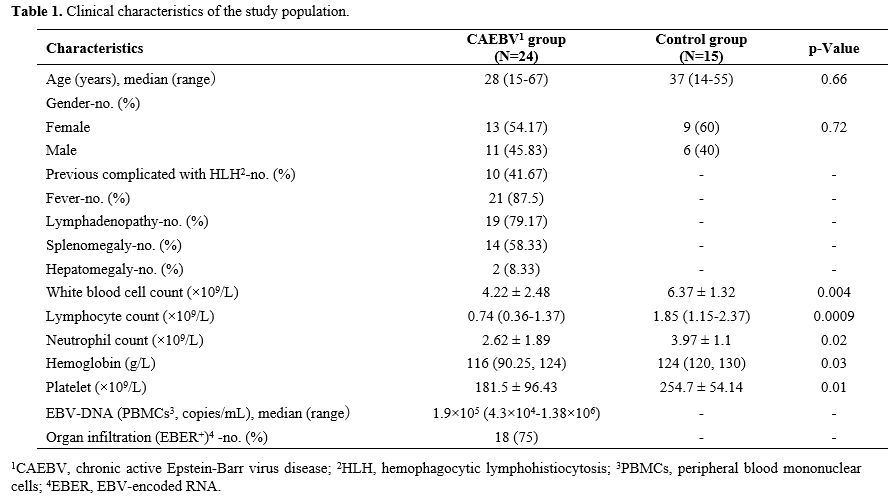
[8.32-57.13%] vs. 47.55% [35.29- 55.49%], Figure 3a, c), or EBER+ NK cells and EBER- NK cells (1.32% [0.19-8.97%] vs. 4.47% [2.58-8.4%], Figure 3a, c). Notably, PD-L1 expression levels of EBER+ T and EBER+ NK cells were significantly higher than those of the corresponding EBER- cells (28.6% [13.39-53.02%] vs. 2.54% [0.9-4.46%], 21% [7.39-44.27%] vs. 10.33% [4.48-11.88%], Figure 3b, d), indicating that EBV induces PD-L1 expression in host lymphocytes in CAEBV.
Next, we distinguished EBER+ T cells based on the surface marker CD45RO. We found that 58.75% of the infected T cells exhibited a CD45RO+ phenotype, suggesting that EBV may ensure its long-term existence by infecting memory T (Tm) cells (Figure 3e, f).
 |
- Figure 3. Elevated PD-L1 expression levels of patients with CAEBV were associated with direct EBV infection.
Representative flow profiles (a) and summarized positive percentages
(c) of PD-1 in EBER- and EBER+ T and NK cells in patients with CAEBV.
Representative flow profiles (b) and summarized positive percentages
(d) of PD-L1 in EBER- and EBER+ T and NK cells in patients with CAEBV.
n=16 for the EBER- and EBER+ T group, n=21 for the EBER- and EBER+ NK
group. (e) Representative dot plot showing the gating strategy for the
frequencies of CD45RO+ memory and CD45RO- non-memory cells in EBER+CD3+
T cells. (f) Summarized frequencies of CD45RO+ memory and CD45RO-
non-memory cells in EBER+CD3+ T cells. n=16 for the CD45RO- and CD45RO+
group. *P < 0.05, ****P < 0.0001. ns, not significant.
|
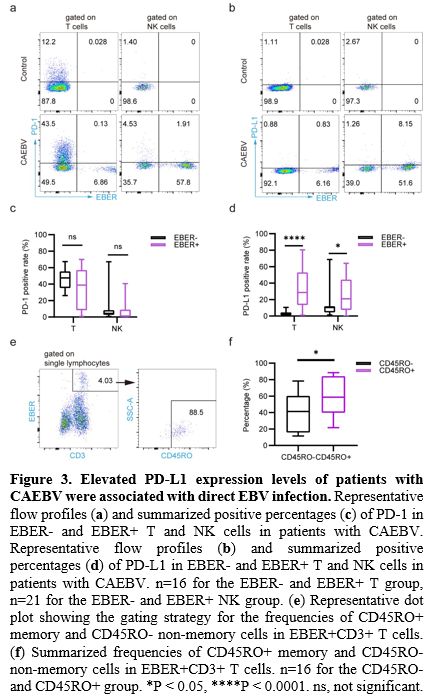
Patients with CAEBV Showed Elevated Frequency and PD-L1 Expression Levels of Tem Cells.
We investigated the frequency and the PD-1/PD-L1 expression level of
naive-memory subpopulations of T cells in the PB of patients with CAEBV
and healthy donors. The results demonstrated a significant reduction in
the frequencies of both CD4+ Tn and CD8+
Tn cells (10.44% [4.32-17.55] vs. 33.8% [29.2-41.2], 16.65% [5.51-30.2]
vs. 42.3% [29.4-48.8]), concurrent with a significant increase in the
frequencies of both CD4+ Tem and CD8+
Tem cells (52.3% [42.7-61.35] vs. 24.5% [18.3-31.1], 29.25%
[24.33-42.58] vs. 22.6% [15.6-25.3]) in patients compared to healthy
individuals (Figure 4a, b). Besides, patients showed significantly elevated frequencies of CD8+ Tcm cells (3.75% [2.37-4.92] vs. 2.71% [1.87-3.37], Figure 4b).
Moreover, the PD-1 expression levels of CD4+ Tcm, CD4+ Tem, and CD8+
Tn cells were markedly elevated in patients with CAEBV than in healthy
individuals (53.35% [42.73-65.68] vs. 39.7% [29.9-42.1], 60.75%
[51.48-75.63] vs. 51.8% [42.8-56.7], 4.65% [2.17-12.39] vs. 1.82%
[0.83-2.5], Figure 4c). Similarly, the PD-L1 expression levels of CD4+ Tcm, CD4+ Tem, CD8+ Tn, CD8+ Tcm, and CD8+
Tem cells were significantly higher in patients with CAEBV than in
healthy individuals (2.15% [1.3-6.21] vs. 0.38% [0.21-3.34], 1.7%
[1.23-5.81] vs. 0.68% [0.4-3.46], 1.47% [0.54-2.67] vs. 0.27%
[0.16-0.54],
5.5% [2.49-9.9] vs. 0% [0-3.85], 3.93% [2.2-6.91] vs. 0.43% [0.17-0.99], Figure 4d). Of note, the PD-L1 expression levels of CD4+ and CD8+ Tem cells were both positively correlated with EBV-DNA copies in PBMCs (r=0.37, p=0.07 and r=0.41, p<0.05, respectively, Figure 4e, f).
 |
- Figure 4. Patients
with CAEBV showed notably decreased frequency of Tn cells and increased
frequency of Tem cells, the PD-L1 expression level of Tem cells was
positively correlated with the EBV-DNA copies in PBMCs.
Frequencies of Tn, Tcm, and Tem cells in CD4+ Th (a) and CD8+ Tc (b)
cells in the CAEBV and control groups. Positive rates of PD-1 (c) and
PD-L1 (d) in CD4+ and CD8+ Tn, Tcm, and Tem cells in the CAEBV and the
control group. The correlation between PD-L1 positive rates of CD4+ Tem
(e) or CD8+ Tem (f) cells and EBV DNA copies in PBMCs. n=15 for the
control group, and n=24 for the CAEBV group. *P < 0.05, **P <
0.01, ***P < 0.001, ****P < 0.0001. ns, not significant.
|
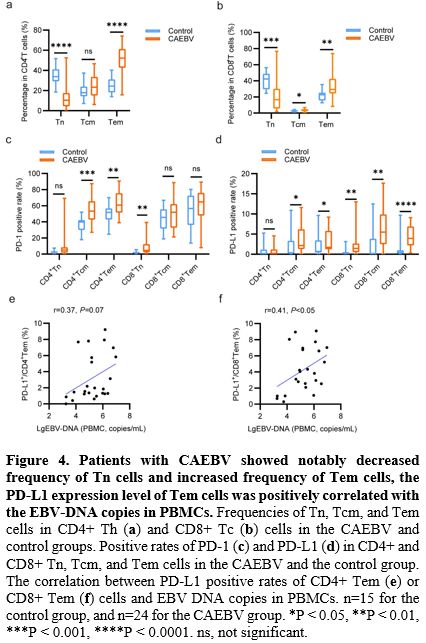
Reduced Th1/Th2 Ratio Was Positively Correlated with The EBV-DNA Copies in PBMCs in CAEBV.
Additionally, we measured the frequency and PD-1/PD-L1 expression
levels of Th1, Th2, Th17, and Treg cells in PB between patients with
CAEBV and healthy individuals. As presented in Figure 5a,
patients exhibited a notable decrease in the frequency of Th1 cells
(2.92% [1.51-4.33] vs. 6.02% [1.89-11.33]) and a significant increase
in the frequency of Th2 cells (25.43% [18.35-29.35] vs. 16.39%
[10.93-21.68]) compared to healthy individuals. Consequently, the
Th1/Th2 ratio in patients with CAEBV was significantly lower than that
in healthy individuals (0.13 [0.06-0.19] vs. 0.49 [0.12-0.77], Figure 5b).
Furthermore, the EBV-DNA copies in PBMCs were found to be negatively correlated with the Th1 frequency (r=-0.4, p<0.05, Figure 5c) and the Th1/Th2 cell ratio (r=-0.41, p<0.05, Figure 5e), while positively correlated with the Th2 frequency (r=0.23, p=0.27, Figure 5d) in the CAEBV group.
Moreover,
the PD-1 expression levels of Th1, Th2, and Treg cells in the CAEBV
group were significantly elevated compared to the control group (53.25%
[42.63-67.35] vs. 43.8% [37.3-53.1], 42.25% [33.6-51.78] vs. 34%
[25.1-44.3], 44.9% [39.4-61.68] vs. 25.2% [16.7-41.7], Figure 5f).
As for the PD-L1 expression levels, no significant differences were
detected in Th1 or Th2 cells between the two groups. However, the PD-L1
expression level of Treg cells was significantly increased in the
patients with CAEBV compared to healthy individuals (2.81% [1.7-11.05]
vs. 1.25% [0-3.08], Figure 5g). There was no significant difference in frequency, PD-1, or PD-L1 expression levels of Th17 cells between the two groups.
 |
- Figure 5. The reduced Th1/Th2 ratio was positively correlated with EBV-DNA copies in PBMCs in CAEBV.
(a) Frequencies of Th1, Th2, Th17, and Treg cells in CD4+ Th cells in
the CAEBV and the control group. (b) The Th1/Th2 ratio and the
Th17/Treg ratio in the CAEBV and control groups. The correlation
between Th1 cell frequency (c), Th2 cell frequency (d), Th1/Th2 ratio
(e), and EBV DNA copies in PBMCs. Positive rates of PD-1 (f) and PD-L1
(g) in Th1, Th2, Th17, and Treg cells in the CAEBV and control groups.
n=15 for the control group, and n=24 for the CAEBV group. *P < 0.05,
**P < 0.01, ***P < 0.001. ns, not significant.
|
Patients with CAEBV Showed Enhanced Response Capacity of Th2 Cells after PHA Stimulation.
To assess the response capacity of Th1, Th2, Th17, and Treg cells, we
measured the frequency of IFN-γ, IL-4, IL-17A, and IL-10 positive cells
in CD4+
T cells of healthy individuals and patients with CAEBV after 72 h
of stimulation with PHA. The results revealed that the percentage of
IL-4+ cells in CD4+ T cells was significantly higher in patients than in healthy people after PHA stimulation (26.95±9.41% vs. 14.71±4.5%, Figure 6b, e).
However, the proportions of IFN-γ, IL-17A, and IL-10. Positive
cells in patients' CD4+ T cells were not significantly different from
those of healthy individuals (Figure 6a, c, d, e).
These results suggest that the response capacity of Th2 cells in
patients with CAEBV is significantly higher than that of healthy
individuals. The Th2 immune bias in patients with CAEBV included both
elevated frequency and enhanced response capacity of Th2 cells.
 |
- Figure 6. Patients with CAEBV showed significantly increased percentages of IL-4+ T cells after PHA stimulation.
Classic dot plot showing the IFN-γ (a), IL-4 (b), IL-17A (c), and IL-10
(d) positive rate in CD4+ Th cells in patients with CAEBV and healthy
individuals before and after PHA stimulation for 72 h. (e) Summarized
positive percentages of IFN-γ, IL-4, IL-17A, and IL-10 cells in CD4+ Th
cells in two groups. *P < 0.05. ns, not significant.
|
Serum CD163 Levels Were Positively Correlated with Th2 Cell Frequency and EBV-DNA Copies in PBMCs in CAEBV.
To better characterize the immune profile of patients with CAEBV, we
measured the serum levels of 9 cytokines, including TNF-α, IFN-γ,
CXCL9, IL-18, CD163, ST2, IL-1RA, IL-10, and IL-17A, in 21 patients
using a Luminex 200 instrument (Table 2).
Based on the biological functions, these cytokines were categorized as
Th1-associated (TNF-α, IFN-γ, CXCL9), M1 macrophage-associated (IL-18),
Th2-associated (ST2, IL-10), M2 macrophage-associated (CD163, IL-1RA),
and Th17- associated (IL-17A). The percentages of patients exhibiting
abnormally elevated Th1/M1-related cytokines were as follows:
 |
- Table 2. Serum cytokine levels in patients with CAEBV.
|
IL-18
(85.71%), IFN-γ (14.29%), TNF-α (4.76%), and CXCL9 (9.52%), while the
proportions of patients with elevated Th2/M2-related cytokines were as
follows: ST2 (66.67%), CD163 (57.14%), IL-1RA (52.38%), and IL-10
(23.81%). None of these patients showed abnormally elevated serum
IL-17A. Notably, 4 cytokines were found abnormally elevated in more
than half of these patients: IL-18, CD163, ST2, and IL-1RA, and the
latter 3 were associated with Th2/M2. In particular, serum CD163 levels
were negatively correlated with Th1 cell frequency (r=-0.45, p<0.05,
Figure 7a), and positively correlated with both Th2 cell frequency (r=0.48, p<0.05, Figure 7b) and EBV-DNA copies in PBMCs (r=0.57, p<0.05, Figure 7c).
Additionally, as presented in Figure 7d and Figure 7e,
both the serum levels of CD163 and IL-1RA were positively associated
with the serum levels of ST2 (r=0.75, p = 0.0001; r = 0.53, p <
0.05, respectively).
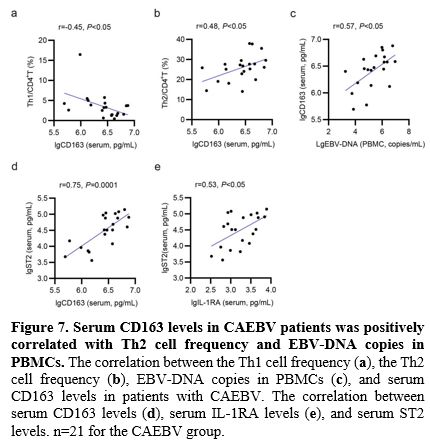 |
- Figure 7. Serum CD163 levels in CAEBV patients was positively correlated with Th2 cell frequency and EBV-DNA copies in PBMCs.
The correlation between the Th1 cell frequency (a), the Th2 cell
frequency (b), EBV-DNA copies in PBMCs (c), and serum CD163 levels in
patients with CAEBV. The correlation between serum CD163 levels (d),
serum IL-1RA levels (e), and serum ST2 levels. n=21 for the CAEBV group.
|
CAEBV Patients Showed Normalized PD-1 Expression Levels and Distribution of Lymphocyte Subsets after PD-1 Blockade.
Our team has previously investigated the efficacy of PD-1 blockade in
combination with lenalidomide in patients with CAEBV and found that
this regimen achieved an ORR of 54.2% and significantly reduced the
EBV-DNA copies number in PB. In order to investigate whether the
frequency and the PD-1/PD-L1 expression levels of lymphocyte subsets in
patients return to the normal range after PD-1 blockade therapy, the
above indicators were detected in 5 patients with CAEBV who received
this regimen and then compared with the results of healthy individuals.
All 5 patients achieved partial response (PR), of which 3 had received
6 courses of treatment, and 2 received 3 courses of treatment.
First,
the PD-1 expression levels of lymphocyte subpopulations were all
decreased in patients with CAEBV after PD-1 blockade therapy. The PD-1
expression levels of several T cell subsets were significantly lower
than those of healthy individuals, including T, Th, Tc (2.39%
[1.34-15.59] vs. 20.8% [12.5-35.4], 4.21% [2.58-17.17] vs. 24.2%
[16.4-26.6], 3.51% [2.07-19.29] vs. 21.6% [14.4-26.7], Figure 8a, b), CD4+
Tcm, CD4+ Tem, CD8+ Tem (5.89% [3.18-26.1] vs. 39.7% [29.9-42.1], 5.4%
[3-31.92] vs. 51.8% [42.8-56.7], 2.07% [1.34-36.15] vs. 56.5%
[36.1-72.5], Figure 9a), Th1,
Th2, and Th17 cells (4.61% [2.99-30.55] vs. 43.8% [37.3-53.1], 0.41%
[0.18-16.37] vs. 34% [25.1-44.3], 1.19% [0.44-15.64] vs. 40.5%
[33.9-50], Figure 9c). The
PD-1 expression levels of other lymphocyte subsets were not
significantly different from those of healthy individuals. These
results suggest that the anti-PD-1 monoclonal antibody (mAb) can
effectively bind to PD-1 expressed on the surface of lymphocytes and
thus block the PD-1/PD-L1 pathway. However, the expression levels of
PD-L1 in several T cell subsets of patients were still significantly
higher than those of healthy individuals, including T, NKT (1.49%
[0.87-4.41] vs. 0.64% [0.1-0.97], 1.79% [1.38-9.74] vs. 1.11%
[0.29-2.43], Figure 8a, c), Th1 and Treg cells (6.75% [5.12-12] vs. 3.04% [0.74-5.61], 13% [6.35-19.25] vs. 1.25% [0-3.08], Figure 9d), which is justified by the fact that the anti-PD-1 mAb could not bind PD-L1 expressed on the surface of lymphocytes.
 |
Figure 8. The PD-1 expression level of T cells was significantly decreased in patients with CAEBV responded to the PD-1 blockade therapy.
(a) Classic dot plot showing the PD-1 and PD-L1 positive rates in T,
Th, Tc, NKT, and NK cells of healthy individuals and patients with
CAEBV who were responded to the PD-1 blockade therapy. Summarized
positive percentages of PD-1 (b) and PD-L1 (c) in T, Th, Tc, NKT, and
NK cells of healthy individuals and patients with CAEBV who were
responded to the PD-1 blockade therapy. n=15 for the control group. n=5
for the CAEBV (post) group. *P < 0.05. ns, not significant. |
 |
Figure 9. The
PD-1 expression levels of T cell subsets was significantly decreased in
patients with CAEBV responded to the PD-1 blockade therapy.
Summarized positive percentages of PD-1 (a) and PD-L1 (b) positive
rates in CD4+ and CD8+ Tn, Tcm, and Tem cells of healthy individuals
and patients with CAEBV who were responded to the PD-1 blockade
therapy. Summarized positive percentages of PD-1 (c) and PD-L1 (d) in
Th1, Th2, Th17, and Treg cells of healthy individuals and patients with
CAEBV who were responded to the PD-1 blockade therapy. n=15 for the
control group. n=5 for the CAEBV (post) group. *P < 0.05, **P <
0.01, ***P < 0.001. ns, not significant.
|
Second,
the frequencies of peripheral lymphocyte subsets in patients with CAEBV
returned to normal after PD-1 blockade treatment (Figure 10).
It is worth noting that in the naïve-memory T cell subsets, patients
exhibited elevated frequencies of Tn cells and decreased frequencies of
Tem cells after PD-1 blockade treatment; all results were not
significantly different from healthy individuals (Figure 10c, d).
In addition, it is noteworthy that the peripheral Th1/Th2 cell ratio
returned to normal in patients with CAEBV after PD-1 blockade therapy (Figure 10e, f).
The exact mechanism by which PD-1 blockade therapy affects the
distribution of peripheral lymphocyte subsets in patients with CAEBV is
unknown and requires in-depth study.
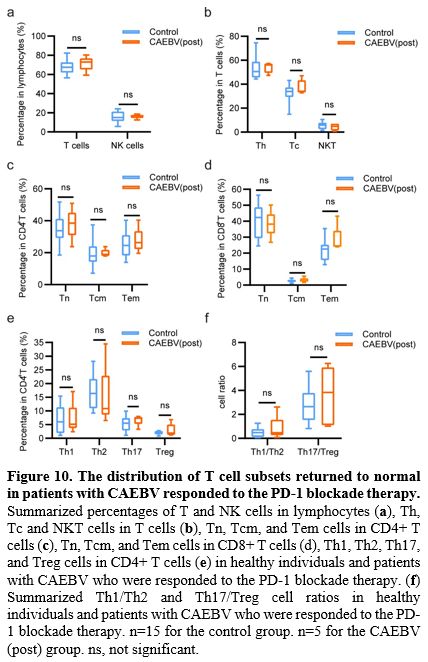 |
- Figure 10. The distribution of T cell subsets returned to normal in patients with CAEBV responded to the PD-1 blockade therapy.
Summarized percentages of T and NK cells in lymphocytes (a), Th, Tc and
NKT cells in T cells (b), Tn, Tcm, and Tem cells in CD4+ T cells (c),
Tn, Tcm, and Tem cells in CD8+ T cells (d), Th1, Th2, Th17, and Treg
cells in CD4+ T cells (e) in healthy individuals and patients with
CAEBV who were responded to the PD-1 blockade therapy. (f) Summarized
Th1/Th2 and Th17/Treg cell ratios in healthy individuals and patients
with CAEBV who were responded to the PD-1 blockade therapy. n=15 for
the control group. n=5 for the CAEBV (post) group. ns, not significant.
|
Discussion
We
observed a significant alteration in the distribution of peripheral T
cell subsets and a notable increase in the expression levels of PD-1
and PD-L1 in T cell subsets and NK cells in patients with CAEBV
compared to healthy individuals, implying the abnormal distribution of
T cell subsets and the exacerbated depletion of T and NK cells might
jointly contribute to the pathogenesis of CAEBV.
Lin et al. reported a decreased frequency of Tn cells (CD45RA+CD62L+) and an increased frequency of Tem (CD45RO+CD62L-) and Treg cells (CD4+CD25+)
in patients with CAEBV compared to healthy individuals in a
retrospective study.[9] Although different staining methods were
employed, the present study also observed a reduction in the frequency
of Tn cells (CD45RO-CCR7+) and an increase in the frequency of Tem cells (CD45RO+CCR7-)
in PB of patients with CAEBV. Nonetheless, the Treg frequency of
patients did not display any significant disparity compared to that of
healthy individuals within this study, which may be partly due to
variations in case numbers and staining protocols. Thus, further
investigation is necessary to reveal the exact rationale behind the
difference. Especially, a decrease in the frequency of Th1 cells and an
increase in the frequency of Th2 cells in patients with CAEBV were found for the
first time in the present study. The reversed Th1/Th2 ratio was
positively correlated with the EBV-DNA copies number in PBMCs,
suggesting that the Th2 immune bias contributes to the development of
CAEBV.
In addition, we observed significantly elevated expression
levels of PD-1 and PD-L1 in peripheral T cell subsets and NK cells of
patients with CAEBV, and the increased expression of PD-L1 was partly
due to the direct EBV infection. By leveraging the innovative approach
introduced by Fournier et al., we classified patients' lymphocytes into
EBER+ and EBER- subgroups and comparatively assessed the expression levels of PD-1 and PD-L1 in two groups.[16] The findings unraveled a significant upregulation of PD-L1 expression in EBER+
T cells and NK cells compared to their EBER- counterparts, suggesting
that EBV promotes the expression of PD-L1 in host cells after
infection. A previous study reported that EBV-miR-BART11 and
EBV-miR-BART17-3p upregulated the expression of PD-L1 through
respectively inhibiting FOXP1 and PBRM1, inhibitors of the expression
of PD-L1 in NPC and GC cell lines in vitro.[11]
EBV-encoded circBART2.2 promoted transcription of PD-L1 by binding the
helicase domain of retinoic acid-inducible gene I (RIG-I) and
activating transcription factors IRF3 and NF-κB in NPC cell line in
vitro.[13] Whether EBV promotes PD-L1 expression of
host T cells and NK cells through the above mechanisms in patients with
CAEBV requires further study and validation.
Although Fournier et al. initially reported that the majority of EBER+ T cells display an effector memory phenotype (CD27- CD45RA-) in two patients with CAEBV, a more substantial cohort of participants is required for further validation and confirmation.[16] Upon dividing EBER+ T cells into CD45RO+ memory and CD45RO- non-memory types from 16 patients with CAEBV, we observed a significantly higher proportion of CD45RO+
memory Tm cells in the infected cells, which verified the correctness of
the results reported by Fournier et al.. We provided a laboratory basis
for the pathophysiological study of CAEBV. EBV predominantly infects
memory Tm cells, presumably to ensure its long-term persistence in
patients with CAEBV. Consequently, EBV escapes the immune response by
upregulating PD-1 and PD-L1 expression in T and NK cells and
potentially achieves long-term presence by infecting memory Tm cells in
patients with CAEBV.
The
underlying mechanisms contributing to the
alteration in the frequency of navïe-memory T cells in patients with
CAEBV could be attributed to the following two aspects: Firstly, since
Tcm and Tem cells are differentiated from Tn cells, the decreased
frequency of Tn cells and increased frequency of Tem cells may be due
to the continued differentiation of Tn cells into Tem cells caused by
prolonged exposure to EBV antigens, which could be regarded as immune
compensatory changes. Patients with other viral infections, such as HCV
and COVID-19, also exhibit a diminished frequency of Tn cells in PB,[17-20]
which might be able to corroborate our speculation. Secondly, the
frequency of Tn cells might be curtailed by Th2 cells. The crucial role
of thymic epithelial cells and the thymic microenvironment in the
proliferation and differentiation of Tn cells has been extensively
demonstrated.[21] Shen et al. demonstrated that Th2
cells play a vital role in inhibiting the development of embryonic
thymocytes through the key factor IL-4 and that even the lowest
concentration of Th2 cells significantly reduces the total number of
thymocytes in mice.[22] ]Inspired by this, we calculated and discovered a negative correlation between the CD3+ Tn cell frequency and the Th2 cell frequency in patients with CAEBV in this study (Supplementary Figure 1),
suggesting Th2 cells may also have an inhibitory effect on the
development of thymocytes and Tn cells in humans, which need to be
further corroborated. The decline in the frequency of Tn cells may
result in decreased T cell counts among patients with CAEBV, and the
exclusive differentiation into EBV-specific T cells may lead to a
diminished ability to cope with infections by other pathogens. A
positive correlation was observed between the PD-L1 expression level in
CD8+ Tem cells and the EBV-
DNA copies in PBMCs, indicating that despite the presence of a
compensative elevated frequency, the severe depletion status of Tem
cells still caused a compromised capacity to combat EBV infection.
It
is widely acknowledged that Th1 cells and their corresponding factors,
notably TNF-α and IFN-γ, play a crucial role in combating viral
infections, while Th2 cells and their factors, including IL-4, IL-5,
and IL-13, are primarily engaged in countering helminths and venoms and
facilitating tissue repair.[23,24] These two cell
types exhibit antagonistic interactions. We observed a Th2 immunity
bias concerning cell frequency, response capacity, and serum levels of
related cytokines in CAEBV: Firstly, the Th1/Th2 cell ratio was
negatively correlated with the EBV-DNA copies in PBMCs in patients with
CAEBV, suggesting the reduced Th1/Th2 ratio contribute to the EBV
replication. Secondly, after PHA stimulation, the proportion of IL-4+ cells in CD4+
T cells of patients with CAEBV was significantly higher than that of
healthy individuals, indicating an enhanced immune response capacity of
Th2 cells in patients. Last, among the 21 CAEBV serum samples, a
relatively lower proportion of patients presented abnormal elevations
of Th1 cell-related cytokines, whereas a relatively higher proportion
of patients exhibited abnormal elevations of Th2 cell-related
cytokines. IL-18, an IFN-γ inducer secreted by M1 macrophages, is known
to trigger Th1 immunity and is frequently employed as a marker to
assess the activity and severity of HLH.[25] In this
study, 18/21 (85.71%) patients with CAEBV exhibited abnormally elevated
serum IL-18 levels, and those concurrent with HLH exhibited
significantly higher serum levels of IL-18 compared to those without
(5161±5378 vs. 1517±1520, P < 0.05, Supplementary Figure 2).
However, its downstream cytokine, IFN-γ, was abnormally elevated in
only 3/21 (14.29%) patients. The proportion of abnormally elevated
TNF-α is even lower (1/21, 4.76%). CXCL9, the recruitment factor for
CXCR3+
Th1 cells, was also observed to be abnormally elevated in only 2/21
(9.52%) patients. Combined with the increased PD-1 expression level of
Th1 cells, these findings suggest a deficiency in Th1 immunity in
patients with CAEBV.
In contrast, serum levels of Th2 cell-related
factors were generally elevated. ST2, the specific receptor of IL-33,
is selectively expressed on the surface of various cells including Th2
cells. ST2 can promote the secretion of IL-5 and IL-13 by Th2 cells, as
well as IL-13-induced M2 macrophages polarization upon binding to
IL-33.[26-30] Also, the IL-33/ST2 axis could be
activated by HBV X protein (HBx) to inhibit the secretion of TNF-α and
IFN-γ from human Th1 cells in vitro.[31] 66.67%
(14/21) of patients showed abnormally elevated serum levels of ST2,
indicating the IL-33/ST2 axis may be activated and contribute to the
Th2 immunity bias in CAEBV. CD163, a specific marker for M2
macrophages, was found abnormally elevated in 12/21 (57.14%) serum
samples from patients with CAEBV. Meanwhile, CD163 serum levels were
negatively correlated with Th1 frequency and positively correlated with
Th2 cell frequency, serum ST2 levels, and EBV-DNA copies in PBMCs, more
directly indicating that the Th2/M2 immune bias favoring EBV
amplification in patients with CAEBV. IL-1RA, secreted by M2
macrophages, is also an important anti-inflammatory factor.[32]
IL-1RA was found abnormally elevated in 11/21 (52.38%) patients and was
also positively correlated with serum levels of ST2. Previous studies
showed that blocking IL-1 enhanced the Th2 immunity in mice, indicating
a potential promotion of IL-1RA to the Th2 immunity,[33,34]
however, its effect on Th2 immunity in humans requires further
investigation. Therefore, although the mechanism of Th2 immunity
predominance in CAEBV remains unclear, targeted suppression of Th2
immunity could potentially serve as a novel treatment approach for
CAEBV.
There was no significant difference in the frequency,
response capacity, and PD-1/PD-L1 expression levels of peripheral Th17
cells between patients and healthy individuals, and serum IL-17A levels
of patients were not elevated abnormally. Consequently, the
contribution of Th17 cells in the pathogenesis of CAEBV might be
minimal. Tregs possess immunosuppressive properties. In the current
study, we observed a significant increase in expression of PD-1 and
PD-L1 in Treg cells from patients with CAEBV compared to healthy
people, indicating that the impaired immunosuppressive function of
Tregs might be contributing to the persistence of inflammation in CAEBV.
It
was satisfying that the expression levels of PD-1 in peripheral T cell
subsets of patients with CAEBV were significantly decreased, even
obviously lower than those of healthy individuals after PD-1 blockade
therapy, demonstrating that anti-PD-1 mAb can effectively bind PD-1 and
block PD-1/P-L1 pathway, which is the biological basis for the
effectiveness of PD-1 blockade therapy. It is worth noting that the
distribution of peripheral T cell subsets of patients with CAEBV
returned to normal after PD-1 blockade treatment. The normalized
frequencies of Tn and Tem cells in patients after PD-1 blockade may be
mainly due to the weakened stimulation of EBV antigen in Tn
differentiation caused by the elimination of the depletion state of
lymphocytes and subsequent enhancement of anti-EBV ability. Besides,
the potential role of the PD-1/PD-L1 pathway in the differentiation of
Tm cells is unclear; whether blocking the PD-1/PD-L1 pathway affects
the frequency of Tm cells deserves further investigation. In addition,
the Th1/Th2 cell ratio returned to normal in CAEBV patients after PD-1
blockade therapy, which may be related to the suppression of the
EBV-induced Th2 immune predominance caused by the enhanced anti-EBV
ability of patients after PD-1 blockade, which needs to be further
verified. Further investigation to understand the mechanisms is
important for improving the efficacy of PD-1 blockade therapy and the
application of combination therapy.
Although the current study
characterized the distribution and the PD-1/PD-L1 expression levels of
peripheral T cell subsets in patients with CAEBV, there are still
several subsets and ICs were not detected due to the limited amount of
blood drawn from patients and the maximum 8-color limit of the flow
cytometry. Furthermore, the precise mechanism by which EBV triggers
PD-L1 expression in host T and NK cells after infection remains
obscure, as does how EBV induces PD-1 expression in uninfected T cells.
Additionally, due to the constraints of the existing platform, we were
unable to assess the serum levels of other Th2 cell-related factors,
including IL-4, IL-5, IL-13, and IL-33. Determining the effects of
these cytokines on the Th1/Th2 balance holds great significance for
further elucidating the pathogenesis of CAEBV.
Conclusions
This
study revealed significantly elevated PD-1/PD-L1 expression in
peripheral T cell subsets and NK cells, and Th2 immunity predominance
jointly facilitates the EBV proliferation and the development of CAEBV.
Most EBV-infected T cells are Tm cells, which may contribute to the
long-term persistence of EBV. Anti-PD-1 mAb effectively blocked the
PD-1/PD-L1 pathway in T cells and contributed to the normalization of T
cell subset distribution in some CAEBV patients. These findings
provide insights into a novel pathogenesis of CAEBV and pave the
way for the development of innovative therapeutic approaches.
Author Contributions.
Conceptualization,
Zhao Wang, Kang Sun; methodology, Chaofan Wu, Kang Sun; software, Kang
Sun; resources, Qi Kong, Junxia Hu, Lin Shi, Yubo Pi, Deli Song,
Tingting Cui, Leilei Chen, Xiaodan He, Zhengyang Song, Lin Wu, Jingshi
Wu; writing, Kang Sun; supervision, Jingshi Wang, Zhao Wang; funding
acquisition, Zhao Wang. All authors have read and agreed to the
published version of the manuscript.
Acknowledgments
Thanks to Dr. Qingyun Sun for providing technical support for submission.
References
- Okuno Y, Murata T, Sato Y, et al. Defective
Epstein-Barr virus in chronic active infection and haematological
malignancy. Nat Microbiol. 2019; 4(3): 404-413. https://doi.org/10.1038/s41564-018-0334-0 PMid:30664667
- Chakravorty
S, Afzali B, Kazemian M. EBV-associated diseases: Current therapeutics
and emerging technologies. Front Immunol. 2022; 13:1059133. https://doi.org/10.3389/fimmu.2022.1059133 PMid:36389670 PMCid:PMC9647127
- Cui
X, Snapper CM. Epstein Barr Virus: Development of Vaccines and Immune
Cell Therapy for EBV-Associated Diseases. Front Immunol. 2021;
12:734471. https://doi.org/10.3389/fimmu.2021.734471 PMid:34691042 PMCid:PMC8532523
- Leticia
QM, Steven HS, Thomas T, et al. New concepts in EBV-associated B, T,
and NK cell lymphoproliferative disorders. Virchows Arch. 2023; 482(1):
227-244. https://doi.org/10.1007/s00428-022-03414-4 PMid:36216980 PMCid:PMC9852222
- Elias
C, Elaine SJ, James RC, et al. The International Consensus
Classification of Mature Lymphoid Neoplasms: a report from the Clinical
Advisory Committee. Blood. 2022;140(11):1229-1253. https://doi.org/10.1182/blood.2022015851 PMid:35653592 PMCid:PMC9479027
- Páez-Guillán
E.M., Campos-Franco J., Alende R., Gonzalez-Quintela A. Hematological
abnormalities beyond lymphocytosis during infectious mononucleosis:
epstein-barr virus-induced thrombocytopenia. Mediterr J Hematol Infect
Dis 2023, 15(1): e2023023, https://doi.org/10.4084/MJHID.2023.023 PMid:36908863 PMCid:PMC10000900
- Yonese
I, Sakashita C, Imadome KI, et al. Nationwide survey of systemic
chronic active EBV infection in Japan in accordance with the new WHO
classification. Blood Adv. 2020; 4(13): 2918-2926. https://doi.org/10.1182/bloodadvances.2020001451 PMid:32598475 PMCid:PMC7362364
- Austen
JJW, Charlotte JH, Claire B. Severe Epstein-Barr virus infection in
primary immunodeficiency and the normal host. Br J Haematol.
2016;175(4): 559-576. https://doi.org/10.1111/bjh.14339 PMid:27748521
- Lin
J, Chen X, Wu H, Chen X, Hu X, Xu J. Peripheral blood lymphocyte counts
in patients with infectious mononucleosis or chronic active
Epstein-Barr virus infection and prognostic risk factors of chronic
active Epstein-Barr virus infection. Am J Transl Res. 2021; 13 (11):
12797-12806.
- Ming
Y, Dechao J, Hanxiao X, et al. Biomarkers for predicting efficacy of
PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17(1):129-143. https://doi.org/10.1186/s12943-018-0864-3 PMid:30139382 PMCid:PMC6107958
- Xi-Wen
B, Hua W, Wen-Wen Z, et al. PD-L1 is upregulated by EBV-driven LMP1
through NF-κB pathway and correlates with poor prognosis in natural
killer/T-cell lymphoma. J Hematol Oncol. 2016; 9(1):109-121. https://doi.org/10.1186/s13045-016-0341-7 PMid:27737703 PMCid:PMC5064887
- Jie
W, Junshang G, Yian W, et al. EBV miRNAs BART11 and BART17-3p promote
immune escape through the enhancer-mediated transcription of PD-L1. Nat
Commun. 2022;13(1):866-885. https://doi.org/10.1038/s41467-022-28479-2 PMid:35165282 PMCid:PMC8844414
- Junshang
G, Jie W, Fang X, et al. Epstein-Barr Virus-Encoded Circular RNA
CircBART2.2 Promotes Immune Escape of Nasopharyngeal Carcinoma by
Regulating PD-L1. Cancer Res. 2021; 81(19):5074-5088. https://doi.org/10.1158/0008-5472.CAN-20-4321 PMid:34321242 PMCid:PMC8974435
- Yue
S, Jingshi W, Yini W, et al. PD-1 blockade and lenalidomide combination
therapy for chronic active Epstein-Barr virus infection. Clin Microbiol
Infect. 2023; 29(6): e7-e13 https://doi.org/10.1016/j.cmi.2023.01.017 PMid:36702399
- Sebastian
FP, Oscar S, Yasodha N. Defining the elusive boundaries of chronic
active Epstein-Barr virus infection. Haematologica. 2018; 103(6):
924-927. https://doi.org/10.3324/haematol.2018.193714 PMid:29866887 PMCid:PMC6058790
- Benjamin
F, David B, Julie B, et al. Rapid identification and characterization
of infected cells in blood during chronic active Epstein- Barr virus
infection. J Exp Med. 2020; 217(11): e20192262. https://doi.org/10.1084/jem.20192262 PMid:32812031 PMCid:PMC7596820
- Ann
WNA, Carey LS, Alyssa L, et al. Naïve CD4+ T Cell Lymphopenia and
Apoptosis in Chronic Hepatitis C Virus Infection Is Dri ven by the
CD31+ Subset and Is Partially Normalized in Direct-Acting Antiviral
Treated Persons. Front Immunol. 2021; 12: 641230. https://doi.org/10.3389/fimmu.2021.641230 PMid:33912168 PMCid:PMC8075159
- Ann
WNA, Carey LS, Lenche K, et al. Variable Normalization of Naïve CD4+
Lymphopenia and Markers of Monocyte and T Cell Activation over the
Course of Direct-Acting Anti-Viral Treatment of Chronic Hepatitis C
Virus Infection. Viruses. 2021; 14(1): 50-60. https://doi.org/10.3390/v14010050 PMid:35062255 PMCid:PMC8780994
- Poonam
S, Bhagyashri T, Prachi A, et al. Lymphopenia with Altered T Cell
Subsets in Hospitalized COVID-19 Patients in Pune, India. Viral
Immunol. 2023; 36(3): 163-175.
- Quirin
N, Marc S, Florian W, et al. Pro- and Anti-Inflammatory Responses in
Severe COVID-19-Induced Acute Respiratory Distress Syndrome-An
Observational Pilot Study. Front Immunol. 2020; 11: 581338. https://doi.org/10.3389/fimmu.2020.581338 PMid:33123167 PMCid:PMC7573122
- Zhanfeng
L, Xue D, Zhaoqi Z, et al. Age‐related thymic involution: Mechanisms
and functional impact. Aging Cell. 2022; 21(8): e13671 https://doi.org/10.1111/acel.13671 PMid:35822239 PMCid:PMC9381902
- Hui
S, Chen Y, Ya-Nan G, et al. Recirculating Th2 cells induce severe
thymic dysfunction via IL-4/STAT6 signaling pathway. Biochem Biophys
Res Commun. 2018; 501(1): 320-327. https://doi.org/10.1016/j.bbrc.2018.05.030 PMid:29738764
- Francesco
A, Chiara R, Sergio R. The 3 major types of innate and adaptive
cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135(3):
626-35. https://doi.org/10.1016/j.jaci.2014.11.001 PMid:25528359
- Weihang
L, Jindong H, Weifang X, et al. Distinct spatial and temporal roles for
Th1, Th2, and Th17 cells in asthma. Front Immunol. 2022; 13: 974066. https://doi.org/10.3389/fimmu.2022.974066 PMid:36032162 PMCid:PMC9411752
- Charles AD. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019;15(10): 612-632. https://doi.org/10.1038/s41584-019-0277-8 PMid:31515542
- Punniyakoti
VT, Srinivasan R, Poojitha M, et al. Modulation of IL-33/ST2 signaling
as a potential new therapeutic target for cardiovascular diseases.
Cytokine Growth Factor Rev. 2023; 71-72: 94-104. PMid: 37422366 https://doi.org/10.1016/j.cytogfr.2023.06.003 PMid:37422366
- Jochen
S, Alexander O, Elizabeth O, et al. IL-33, an interleukin-1-like
cytokine that signals via the IL-1 receptor-related protein ST2 and
induces T helper type 2-associated cytokines. Immunity. 2005; 23(5):
479-90. https://doi.org/10.1016/j.immuni.2005.09.015 PMid:16286016
- Jaewoo
H, Soohyun K, P CL. Interleukin-33 and ST2 Signaling in Tumor
Microenvironment. J Interferon Cytokine Res. 2019; 39(1): 61-71. https://doi.org/10.1089/jir.2018.0044 PMid:30256696 PMCid:PMC6350413
- Corinne
C, Jean-PG. Interleukin-33 (IL-33): A critical review of its biology
and the mechanisms involved in its release as a potent extracellular
cytokine. Cytokine. 2022; 156: 155891-155906. https://doi.org/10.1016/j.cyto.2022.155891 PMid:35640416
- Wenyi
J, Jingyao L, Ying Y, et al. IL‐33/ST2 as a potential target for tumor
immunotherapy. Eur J Immunol. 2021; 51(8): 1943-1955 https://doi.org/10.1002/eji.202149175 PMid:34131922
- Siyan
C, Luxi W, Lirong P, et al. Hepatitis B virus X protein (HBx) promotes
ST2 expression by GATA2 in liver cells. Mol Immunol. 2020; 123: 32-39 https://doi.org/10.1016/j.molimm.2020.04.024 PMid:32413787
- Tiago
LF, Andreas HG, Christian L, et al. Macrophage: A Potential Target on
Cartilage Regeneration. Front Immunol. 2020; 11(11):111- 120. https://doi.org/10.3389/fimmu.2020.00111 PMid:32117263 PMCid:PMC7026000
- Anna
B, Slaven C, Valentina B, et al. IL-1 receptor blockade skews
inflammation towards Th2 in a mouse model of systemic sclerosis. Eur
Respir J. 2019; 54(3): 1900154-1900165. https://doi.org/10.1183/13993003.00154-2019 PMid:31320452 PMCid:PMC6860995
- Carl
RH, Bojana SM, Crissy F, et al. The role of Interleukin 1 receptor
antagonist in mesenchymal stem cell‐based tissue repair and
regeneration. Biofactors. 2020; 46(2): 263-275. https://doi.org/10.1002/biof.1587 PMid:31755595











