Rawand
Polus Shamoon1,2,3,4, Ahmed Khudair Yassin5,6
and Sara Laith Alnuaimy2 .
1
Department of Pathology, College of Medicine, Hawler Medical
University, Erbil, Iraq.
2 Department of Hematology, Nanakali Hospital of
Blood Diseases and Cancer, Erbil, Iraq.
3 Department of Hematology, Thalassemia Care
Center, Erbil, Iraq.
4 Department of Laboratory Medical Sciences,
College of Health Sciences, Catholic University in Erbil, Erbil, Iraq.
5 Department of Internal Medicine, College of
Medicine, Hawler Medical University, Erbil, Iraq.
6 Department of Clinical Hematology, Nanakali
Hospital of Blood Diseases and Cancer, Erbil, Iraq.
Correspondence to:
Dr. Rawand P. Shamoon. Erbil, Iraq. Postal code: 44001. Tel:
+9647504498630. E-mail:
rawand.shamoon@hmu.edu.krd
Published: March 01, 2024
Received: November 19, 2023
Accepted: February 08, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024019 DOI
10.4084/MJHID.2024.019
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Immune
thrombocytopenia (ITP) is an acquired immune-mediated disease that
lacks an underlying etiology. Steroids are the main first-line
treatment of ITP, while the second-line treatment consists primarily of
splenectomy and rituximab. This study aimed to assess and compare the
response to rituximab and splenectomy.
Methods: This
retrospective comparative study reviewed ITP patients treated at a
single private hematology clinic from 2007 to 2019. Seventy-four ITP
patients were recruited, 27 were on rituximab, and 47 had undergone
splenectomy. The initial platelet counts and bleeding symptoms were
recorded, and initial and long-term responses to treatment were
evaluated based on the American Society of Hematology guidelines.
Results: The
mean age of the patients was 42.1 years with a male-to-female ratio of
1:1.8. The initial mean platelet count was comparable between the
rituximab and splenectomy groups (p = 0.749). The initial complete
response (CR) differed significantly between the rituximab and
splenectomy groups (44.4% versus 83%, p = 0.002). The five-year
response rate was significantly higher in the splenectomy than in the
rituximab group (74% versus 52%, log-rank 0.038). Splenectomy was the
only significant predictive factor for long-term response (OR = 0.193,
p = 0.006).
Conclusion: The
overall response revealed that splenectomy appeared superior to
rituximab as a second-line treatment of ITP. Splenectomy was the only
positive prognostic indicator of sustained response.
|
Introduction
Immune
thrombocytopenia (ITP) is an acquired hematological disorder
characterized by a shortened platelet life span, attributed to
autoimmune destruction, and impaired thrombopoiesis, resulting in
isolated thrombocytopenia with an increased risk of bleeding.[1,2]
Patients with ITP may be asymptomatic or experience varying severity of
bleeding, ranging from mild mucocutaneous bleeding to life-threatening
hemorrhage.[3,4] The annual incidence of ITP among
adults ranges between 1.6 and 3.9/100,000, with women being slightly
more affected than men.[5]
ITP in adults is
usually chronic, lasting for more than 12 months. The principal goal of
treatment is achieving a safe platelet count to prevent major bleeding
rather than correcting the platelet count. Major bleeding in ITP
patients is possible with a <30 ×109/L
platelet count. Steroids are the standard initial treatment option for
ITP. They usually achieve a response in 60-80% of patients.[4]
Nevertheless, some ITP patients experience relapse during dose tapering
or following the cessation of steroids, necessitating further
treatment.[6] Over the past three decades, splenectomy
has been the primary second-line treatment for relapsed and
steroid-refractory ITP patients. Although splenectomy has an
outstanding response rate, it is associated with nearly 1% surgical
mortality and a lifelong higher risk of infections. Moreover, ITP
recurs in approximately one-third of splenectomized patients; thus, due
to ongoing debates over the procedure, a trend has emerged to avoid or
delay splenectomy.[7] With the development of various
medical treatments, there is a tendency toward exhausting all possible
therapeutic options before undergoing such an irreversible procedure.
Consequently, the splenectomy rate has dropped from more than 60% to
approximately 20% in ITP treatment.[7]
Rituximab
is a monoclonal antibody medication directed against CD20, an antigen
found on the surface of B-lymphocytes, which are known to play a
significant role in the development of ITP by producing anti-platelet
glycoprotein antibodies.[8] It was originally
developed to treat B-cell lymphoma and effectively treats various
autoimmune illnesses by reducing circulating B-cell levels through
three different mechanisms: antibody-dependent cellular cytotoxicity,
complement-mediated cytotoxicity, and induction of apoptosis in the
target cell. Furthermore, it has been found that rituximab increases
regulatory T-cell activity. In ITP, rituximab showed a response rate of
up to 60%, making it a good alternative to splenectomy with fewer side
effects.[9] However, studies have reported that the
effect of rituximab is better in newly diagnosed ITP than in persistent
or chronic ITP. Thus, strategies to improve its efficacy in chronic ITP
are of value.[8,6]
Many studies
have assessed and compared the response of chronic ITP patients managed
via variable treatment regimes, including rituximab, TPO-RA, and
splenectomy. The debate on the efficacy of rituximab or splenectomy
outcome in chronic ITP remains unresolved. In this study, we assessed
the efficacy of rituximab compared to splenectomy as a second-line
treatment option for chronic ITP by determining the initial and
long-term response rates in the enrolled patients.
Patients and
Methods
This
retrospective study reviewed records of ITP patients who were
registered at a single private hematology clinic in Erbil, northern
Iraq. From 2007 to 2019, 356 patients were diagnosed with ITP at this
center, of which 214 had chronic ITP. The diagnosis of ITP was made
based on the International Working Group criteria: platelet count
<100 ×109/L without any other causes of thrombocytopenia. Chronic ITP was defined as ITP lasting more than 12 months.[10]
Cases of secondary ITP and those who received steroids only or had been
treated with multiple agents, such as rituximab with immunomodulators
and/or TPO-RA, prior to splenectomy were excluded. Only 74 cases
fulfilled our inclusion criteria and were analyzed. Access to patient
data extended from January to the end of April 2023, and data were
fully anonymized before being accessed. The study was approved by the
ethics committee of Hawler Medical University.
The patients'
demographic, clinical, and laboratory data were retrieved from the
medical records. The platelet count at the time of diagnosis and the
initial bleeding symptoms before treatment were recorded. Bleeding
symptoms were categorized as skin bleeding, including petechiae and
ecchymosis; mucosal bleeding, including nasal, gingival, GIT, GUT, and
vaginal bleeding; CNS bleeding; and bleeding from multiple sites. The
WHO bleeding scale was adopted for grading bleeding.[11] The patients were treated based on the American Society of Hematology guidelines for managing chronic ITP.[12]
All patients received steroids as a first-line therapy. Intravenous
immunoglobulin was additionally used in 13 cases. The second line of
therapy included rituximab, which was used in 27 patients, while open
splenectomy was performed for the remaining 47 patients. The majority
of the rituximab-treated ITP patients received 4 to 6 cycles of
therapy; each cycle comprised intravenous rituximab 375mg/m2 weekly for four weeks, followed by 8 weeks off therapy.
Response
to treatment was evaluated based on the platelet count and bleeding
events. Complete response (CR): platelet count >100 ×109/L, response (R): platelet count 30-100 ×109/L, and no response (NR): platelet count <30 ×109/L.[10]
The overall response rate (ORR) included both CR+R. The initial
response was assessed eight weeks after the initiation of therapy for
the rituximab-treated group and ten weeks after the surgery for the
splenectomy group. The long-term response was checked in the follow-up
with the platelet counts of all patients within both groups.
Statistical
analyses were performed using SPSS version 25. Numerical variables were
expressed as mean (SD). Comparisons between numerical variables were
made using the student's t-test. Categorical variables were compared
using chi-square and Fisher’s exact tests. The Kaplan-Meier test was
performed to assess the long-term response, and the log-rank was used
to determine the difference in long-term responses between the two
groups. Regression analysis using a binary logistic regression table
was used to predict factors associated with sustained CR. A p-value of
≤0.05 was considered statistically significant.
Results
Seventy-four
patients with chronic ITP were enrolled in this study; 27 received
rituximab, and 47 underwent splenectomy as second-line treatment. The
mean age of the patients was 42.10 years; females constituted 64.9%
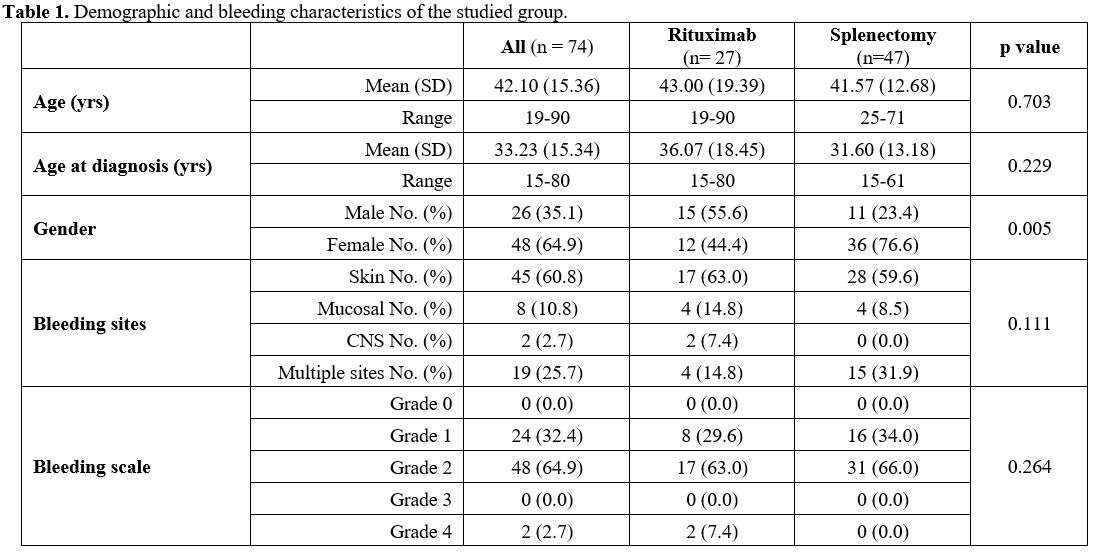
with a male-to-female ratio of 1:1.8. The mean age of the patients
within the two groups was comparable (p = 0.703), while the gender
difference was significant between the two groups (p = 0.005). The
bleeding pattern and grades showed no significant difference within the
groups. Table 1 shows the demographic and bleeding characteristics of the studied groups.
 |
- Table
1. Demographic and bleeding characteristics of the studied group.
|
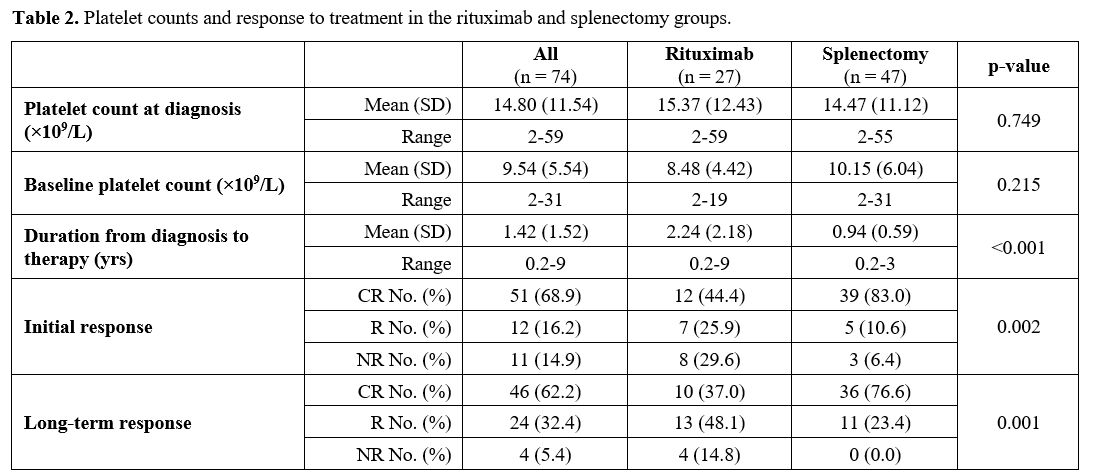
The patients’ mean platelet count at diagnosis was 14.8 ×109/L
(±11.54). The mean platelet count of the rituximab-treated and
splenectomy groups showed no significant variation, 15.37±12.43 and
14.47±11.12, respectively; p=0.749. Twenty-eight patients (37.8%) had
platelet counts <10 ×109/L and 71 patients (95.9%) had platelet counts <50 ×109/L.
The mean baseline platelet counts were not different within the two
groups (p=0.215). The mean duration from diagnosis to commencement of
second-line therapy was significantly longer in the rituximab-treated
group than in the splenectomy group (p <0.001). The average number
of cycles of the rituximab-treated patients received was 4.2, ranging
from 2 to 8. Regarding the initial and long-term
responses, the splenectomized ITP patients showed significantly higher CR than the
rituximab-treated group; p values 0.002 and 0.001, respectively (Table 2).
 |
- Table
2. Platelet counts and response to treatment in the rituximab and
splenectomy groups.
|
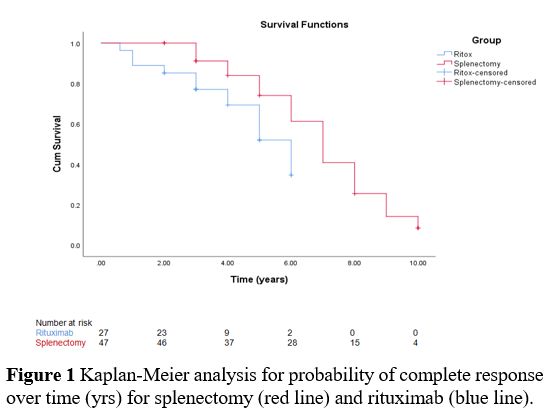
The
Kaplan-Meier analysis showed that the splenectomized ITP patients
maintained a response significantly higher than the rituximab-treated
group as the 5-year CR reached 74% and 52%, respectively, with the
log-rank difference being significant (p=0.038) (Figure 1).
 |
- Figure
1. Kaplan-Meier analysis for probability of complete response over time
(yrs) for splenectomy (red line) and rituximab (blue line).
|
Table 3
shows the factors that predict sustained CR. Among the four analyzed
predictive parameters of age, gender, delay in commencing therapy, and
treatment options, only splenectomy emerged as a statistically
significant predictive factor of CR (OR =0.193, 95% CI = 0.060-0.619, p
= 0.006).
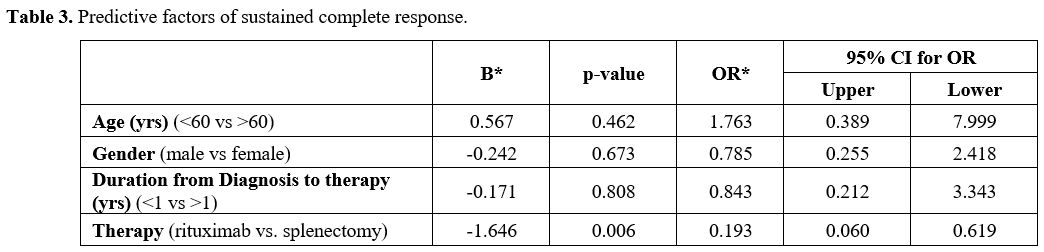 |
- Table
3. Predictive factors of sustained complete response.
|
Discussion
Achieving a safe platelet count to evade bleeding is the main aim of treating ITP.[8]
The first-line treatment of ITP relies mainly on steroids, which have a
good initial response but a high relapse rate, necessitating
second-line treatment. Formerly, splenectomy was the only second-line
treatment option for chronic and refractory ITP cases. Nevertheless,
surgery is not recommended in the early phase of the disease, and the
concern of potential surgical complications has caused clinicians to
postpone the surgical approach. Accordingly, treatments such as
rituximab and TPO-RA have emerged to provide new treatment options for
ITP.[13]
In the current study, 27 ITP patients
were treated with rituximab, while 47 had undergone splenectomy. The
majority of the splenectomized patients (36/47) were old ITP cases who
were diagnosed between 2007 and 2012 and had no or very limited
opportunity to be treated with rituximab or the TPO-RAs. The recent ITP
cases are consistently treated with either rituximab or a TPO-RA when
they require second-line therapy unless the patient disagrees. The mean
age of the enrolled patients was 42.1 years, comparable to many other
studies.[1,13,14] In our cohort,
nearly two-thirds of the patients were females, and most had undergone
splenectomy. Female predominance is a consistent finding across studies[13,15]
because the disease affects more women than men, which is mostly
attributed to the role of sex hormones in immune system disorders,
thereby increasing susceptibility to ITP in women.[16]
All
ITP patients experienced symptoms ranging from minor skin bleeding to
severe intracranial hemorrhage. More than half of the patients had skin
bleeding, and a considerable proportion had bleeding in multiple sites.
Platelet transfusions were carried out for only two patients with
intracranial hemorrhage prior to treatment with rituximab. In general,
bleeding symptoms are less noticeable in ITP than in other forms of
thrombocytopenia, and platelet count is not a reliable predictor of
bleeding because other factors, such as age and comorbidities, might
contribute to bleeding risk. In some cases, autoantibodies react with
platelet glycoproteins, resulting in impaired adhesion or aggregation,
thereby causing severe bleeding for the level of platelet count.[17]
Concerning immunodeficiency-related side effects during the disease,
none of our enrolled ITP patients encountered any significant
immunodeficiency-related side effects. It is worth mentioning here that
all splenectomized patients received prophylactic pneumococcal
vaccination. In literature, many studies on ITP patients reported
variable immunodeficiency complications, though such complications are
rarely observed in this locality. The exact reason for this
inconsistency is unknown, but in addition to prophylactic vaccination,
environmental factors may be contributing. Two ITP patients developed
portal venous thrombosis following splenectomy; they were treated with
anticoagulants for six months with no long-term consequences.
The mean initial platelet count at diagnosis was 14.8 ×109/L; the mean platelet count prior to starting second-line therapy, baseline count, was even less (8.9 ×109/L).
The difference in platelet counts between the rituximab and splenectomy
groups were not significant. At diagnosis, 95.9% of the patients had a
platelet count <50 ×109/L
which is slightly more than what was reported by Koylu et al., who
reported that 82.6% of ITP patients had a platelet count of <50 ×109/L.[1]
Considering
the response to treatment, the initial response of the splenectomy arm
showed significantly higher CR than that of the rituximab arm (83%
versus 44.4%; p=0.002). The overall response rate (ORR) was also higher
among the splenectomy group (93.6% versus 70.3% for the splenectomy and
rituximab groups, respectively). Moulis et al. reported that CR after
splenectomy was significantly higher than rituximab (82.8% versus
39.5%, p <0.001).[18] Koylu et al. reported 87.7%
initial CR in splenectomized patients, while the initial CR to
splenectomy in an Indian study was 74.4%.[1,15]
On the other hand, a study in France revealed that patients on
rituximab had a CR of 56.4%, which is higher than in our cohort;
however, their overall response was similar to ours (71.8%).[19] An American study reported approximate response figures;[20] one meta-analysis over 368 patients showed a CR of 41% after rituximab with an ORR of 57%.[7]
In the current analysis, the long-term response was significantly
higher in the splenectomy group than in the rituximab group (CR = 76.6%
versus 37%; p value =0.001). A study by Chater et al. found that the CR
after 30 months was significantly higher in splenectomy than in
rituximab (75.7% versus 30%, p = 0.001).[21] In
contrast, a study by Alaskar et al. found that the sustained response
did not differ significantly between rituximab and splenectomy
(p=0.549).[2] In this study, the probability of
maintaining CR five years after splenectomy was 74% but only 52% for
the rituximab-treated patients. Two studies reported comparable
results; Ahmed et al. reported 76.5% 5-years of sustained CR after
splenectomy,[15] and Zaja et al. estimated the 5-year sustained response rate after rituximab at 41%.[14]
However, Patel et al., who reviewed data from 17 published studies
including 376 adult ITP patients, found less impressive long-term
outcomes following rituximab as the 5-year response rate was only 21%.[22]
In
our study, splenectomy was the only significant predictor of sustained
CR. Other factors, including age, gender, and duration between
diagnosis and inception of second-line therapy, did not significantly
influence the outcome. Chater et al. reported that female gender and
splenectomy, as second-line treatment, were significant predictive
factors of CR in the univariate analysis, while only splenectomy
reached statistical significance in the multivariate analysis.[21] Other studies have reported that splenectomy and younger age are positive prognostic factors for a long-lasting response.[14,18]
In
the current cohort, relapse following treatment with rituximab and
splenomegaly was encountered in four patients: three patients in the
rituximab arm and one patient in the splenectomy group. They were all
treated with steroids and later with a TPO-RA. Based on our
observations, which were consistent with most prior studies, the
splenectomy outcome was superior to that of rituximab. Despite
rituximab's impressive results when administered earlier in the course
of the disease, its long-term effect is not promising, as it acts
exclusively on B-cells without affecting other immune cells, such as
T-cells and plasma cells, causing either persistence of long-lived
plasma cells in the spleen and bone marrow or abnormal activation of
T-cells.[9] Accordingly, some studies revealed that
combining rituximab with other medications was intriguing in terms of
targeting plasma cells and T-cells in addition to B-cells. The addition
of dexamethasone to rituximab was investigated in a cohort of 67 ITP
patients, resulting in an initial response rate of 75% and a nearly 50%
long-term response rate at five years.[8] Another
study explored adding cyclosporine to rituximab with dexamethasone in
20 patients to target plasma cells and T-cells in addition to B-cells,
revealing a higher response rate at six months (60%).[23]
All of these findings point to the possibility of utilizing rituximab
early in the disease course as a pre-splenectomy alternative,
particularly for patients at risk for surgical complications or
unwilling to undergo surgery.[14]
Conclusions
Although
rituximab is an effective second-line treatment for ITP, splenectomy
still has better outcomes. However, early administration of rituximab
in the disease course may provide a better outcome. The only positive
predictive factor of sustained response was splenectomy. Larger
multicenter studies are recommended to assess and compare the outcomes
of splenectomy and rituximab as second-line treatment options in ITP.
References
- Koylu A, Pamuk GE,
Uyanik MS, et al. Immune thrombocytopenia: epidemiological and clinical
features of 216 patients in northwestern Turkey. Ann Hematol.
2015;94:459-466. https://doi.org/10.1007/s00277-014-2220-z
PMid:25238800
- Al
Askar AS, Shaheen NA, Al Zahrani M, et al. Splenectomy vs. rituximab as
a second-line therapy in immune thrombocytopenic purpura: a single
center experience. Int J Hematol. 2018;107:69-74. https://doi.org/10.1007/s12185-017-2325-y
PMid:28895035
- Stasi
R, Newland A, Thornton P, Pabinger I. Should medical treatment options
be exhausted before splenectomy is performed in adult ITP patients? A
debate. Ann Hematol. 2010;89:1185-1195. https://doi.org/10.1007/s00277-010-1066-2
PMid:20842501
- Cooper
N, Ghanima W. Immune Thrombocytopenia. N Engl J Med. 2019; 381:945-955.
https://doi.org/10.1056/NEJMcp1810479 PMid:31483965
- Abrahamson
PE, Hall SA, Feudjo-Tepie M, et al. The incidence of idiopathic
thrombocytopenic purpura among adults: a population-based study and
literature review. Eur J Haematol. 2009;83:83-89. https://doi.org/10.1111/j.1600-0609.2009.01247.x
PMid:19245532
- Feng
R, Zhang HX, Chen CY. Rituximab should be used earlier in ITP patients:
a meta-analysis of randomized controlled trials. Int J Clin Exp Med
2016;9:918-926.
- Auger
S, Duny Y, Rossi JF, et al. Rituximab before splenectomy in adults with
primary idiopathic thrombocytopenic purpura: a meta-analysis. Br J
Haematol. 2012;158:386-398. https://doi.org/10.1111/j.1365-2141.2012.09169.x
PMid:22612239
- Bussel
JB, Lee CS, Seery C, et al. Rituximab and three dexamethasone cycles
provide responses similar to splenectomy in women and those with immune
thrombocytopenia of less than two years duration. Haematologica.
2014;99:1264-1271. https://doi.org/10.3324/haematol.2013.103291
PMid:24747949 PMCid:PMC4077090
- Lucchini
E, Zaja F, Bussel J. Rituximab in the treatment of immune
thrombocytopenia: what is the role of this agent in 2019?
Haematologica. 2019;104:1124-1135. https://doi.org/10.3324/haematol.2019.218883
PMid:31126963 PMCid:PMC6545833
- Rodeghiero
F, Stasi R, Gernsheimer T, et al. Standardization of terminology,
definitions and outcome criteria in immune thrombocytopenic purpura of
adults and children: report from an international working group. Blood.
2009;113:2386-2393. https://doi.org/10.1182/blood-2008-07-162503
PMid:19005182
- Kaufman
R, Djulbegovic B, Gernsheimer T, et al. Platelet Transfusion: A
Clinical Practice Guideline From the AABB. Ann Intern Med.
2015;162:205-213. https://doi.org/10.7326/M14-1589 PMid:25383671
- Neunert
C, Lim W, Crowther M, et al. The American Society of Hematology 2011
evidence-based practice guideline for immune thrombocytopenia. Blood.
2011;117:4190-4207. https://doi.org/10.1182/blood-2010-08-302984
PMid:21325604
- Palandri
F, Polverelli N, Sollazzo D, et al. Have splenectomy rate and main
outcomes of ITP changed after the introduction of new treatments? A
monocentric study in the outpatient setting during 35 years. Am J
Hematol. 2016;91:E267-272. https://doi.org/10.1002/ajh.24310
- Zaja
F, Volpetti S, Chiozzotto M, et al. Long-term follow-up analysis after
rituximab salvage therapy in adult patients with immune
thrombocytopenia. Am J Hematol. 2012;87:886-889. https://doi.org/10.1002/ajh.23272
PMid:22718483
- Ahmed
R, Devasia AJ, Viswabandya A, et al. Long-term outcome following
splenectomy for chronic and persistent immune thrombocytopenia (ITP) in
adults and children : Splenectomy in ITP. Ann Hematol. 2016;95:1429-34.
https://doi.org/10.1007/s00277-016-2738-3
PMid:27370992
- Andrès
E. What impact for sex difference on immune thrombocytopenic purpura?
Women Health Open J. 2016;2:e1-3. https://doi.org/10.17140/WHOJ-2-e004
- Provan
D, Newland AC. Current management of primary immune thrombocytopenia.
Adv Ther. 2015;32:875-887. https://doi.org/10.1007/s12325-015-0251-z
PMid:26499177 PMCid:PMC4635183
- Moulis
G, Sailler L, Sommet A, et al. Rituximab versus splenectomy in
persistent or chronic adult primary immune thrombocytopenia: an
adjusted comparison of mortality and morbidity. Am J Hematol.
2014;89:41-46. https://doi.org/10.1002/ajh.23580
PMid:24038066
- Brah
S, Chiche L, Fanciullino R, et al. Efficacy of rituximab in immune
thrombocytopenic purpura: a retrospective survey. Ann Hematol.
2012;91:279-285. 3 https://doi.org/10.1007/s00277-011-1283-3
PMid:21710166
- Dabak
V, Hanbali A, Kuriakose P. Can rituximab replace splenectomy in immune
thrombocytopenic purpura? Indian J Hematol Blood Transfus. 2009;25:6-9.
https://doi.org/10.1007/s12288-009-0002-x
PMid:23100964 PMCid:PMC3453478
- Chater
C, Terriou L, Duhamel A, et al. Reemergence of Splenectomy for ITP
Second-line Treatment? Ann Surg. 2016;264:772-777. https://doi.org/10.1097/SLA.0000000000001912
PMid:27741009
- Patel
VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to
rituximab therapy in children and adults with immune thrombocytopenia.
Blood. 2012;119:5989-5995. https://doi.org/10.1182/blood-2011-11-393975
PMid:22566601 PMCid:PMC3383014
- Choi
PYI, Roncolato F, Badoux X, et al. A novel triple therapy for ITP using
high-dose dexamethasone, low-dose rituximab, and cyclosporine (TT4).
Blood. 2015;126:500-503. https://doi.org/10.1182/blood-2015-03-631937
PMid:25972158 PMCid:PMC4560338



