Silvia Maria Trisolini1, Alessandro Laganà2 and Saveria Capria1.
1 Hematology, Department of Translational and Precision Medicine; "Sapienza" University of Rome, Italy.
2 Resident Doctor at the Department of Hematology, University “Sapienza” of Rome, Rome, Italy.
Correspondence to:
Silvia Maria Trisolini, Hematology, Department of Translational and
Precision Medicine, Sapienza University, Via Benevento 6, 00161 Rome,
Italy. Tel. +390649974431, Fax +390644241984. E-mail:
trisolini@bce.uniroma1.it
Published: July 01, 2024
Received: November 21, 2023
Accepted: June 19, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024060 DOI
10.4084/MJHID.2024.060
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Immune
thrombotic thrombocytopenic purpura (iTTP) is a life-threatening
thrombotic microangiopathy characterized by microangiopathic hemolytic
anemia, thrombocytopenia, and ischemic end-organ injury due to
microvascular platelet-rich thrombi. iTTP pathophysiology is based on a
severe ADAMTS13 deficiency, the specific von Willebrand factor
(vWF)-cleaving protease, due to anti-ADAMTS13 autoantibodies. Early
diagnosis and treatment reduce the mortality. Frontline therapy
includes daily plasma exchange (PEX) with fresh frozen plasma
replacement and immunosuppression with corticosteroids. Caplacizumab
has recently been added to frontline therapy. Caplacizumab is a
nanobody that binds to the A1 domain of vWF, blocking the interaction
of ultra-large vWF multimers with the platelet and thereby preventing
the formation of platelet-rich thrombi. Caplacizumab reduces mortality
due to ischemic events, refractoriness, and exacerbations after PEX
discontinuation. Until now, the criteria for response to treatment
mainly took into account the normalization of platelet count and
discontinuation of PEX; with the use of caplacizumab leading to rapid
normalization of platelet count, it has been necessary to redefine the
response criteria, taking into account also the underlying autoimmune
disease. Monitoring of ADAMTS13 activity is important to identify cases
with a low value of activity (<10IU/L), requiring the optimization
of immunosuppressive therapy with the addition of Rituximab. Rituximab
is effective in patients with refractory disease or relapsing disease.
Currently, the use of Rituximab has expanded, both in frontline
treatment and during follow-up, as a pre-emptive approach. Some
patients do not achieve ADAMTS13 remission following the acute phase
despite steroids and rituximab treatment, requiring an individualized
immunosuppressive approach to prevent clinical relapse. In iTTP, there
is an increased risk of venous thrombotic events (VTEs) as well as
arterial thrombotic events, and most occur after platelet
normalization. Until now, there has been no consensus on the use of
pharmacological thromboprophylaxis in patients on caplacizumab because
the drug is known to increase bleeding risk.
|
Case presentation
A
70-year-old man was hospitalized for dysarthria and confusion.
Contrast-enhanced brain CT excluded cerebrovascular events, while blood
routine tests showed normocytic anemia 7.8 g/dL with 14% schistocytes,
thrombocytopenia 10x109/L, total
bilirubin 2.8 mg/dL (indirect 2.5 mg/dl) lactate dehydrogenase (LDH)
1500 units/L and elevated Troponin 75.5 pg/mL without ECG
abnormalities. Thrombotic thrombocytopenic purpura (TTP) was suspected,
and PLASMIC SCORE demonstrated high risk at 7 points. The patient was
started on daily plasmapheresis (PEX), steroids (1 mg/kg
methylprednisolone), and Caplacizumab. ADAMST13 level was later
confirmed to be less than 3 IU/dl with anti-ADAMTS13 autoantibodies
>80 U/ml. PEX was performed for 7 consecutive days, with complete
recovery of neurological symptoms, platelet counts, and haptoglobin
levels, so the patient was discharged on self-administered Caplacizumab
and steroids. Five days after the last PEX, the planned monitoring of
ADAMTS13 activity was still <3 IU/dl, and the patient showed a drop
in platelet counts to 60x109/L
associated with signs of microangiopathy. Therefore, PEX was resumed,
and Rituximab (375 mg/mq) was added to the ongoing treatment, with fast
platelet recovery. After the second dose of Rituximab, we observed a
new episode of aphasia/dysarthria and confusion, but this time,
Brain-MRI was consistent with recent ischemic cerebral lesions. In view
of the onset of an acute cerebrovascular event, although platelet count
was slowly increasing but not yet in the normal range, we considered it
necessary to increase the anti-aggregating therapy by adding ASA 100
mg/day. However, we were afraid to administer it together with
caplacizumab, which was suspended while the rest of the ongoing
treatment was continued. After a rise in platelet counts, up to 150x109/L
PEX was withheld, and steroids were quickly tapered. Ten days later,
after the third rituximab dose, a new fall in platelets count without
signs of microangiopathy was observed. Since ADAMTS13 activity was 60
IU/dl and ADAMTS13 autoantibodies were not detectable, we investigated
for further causes of thrombocytopenia, such as decreased bone marrow
production, consumption processes, and infections. The diagnostic
work-up showed 150.000 gene copies/ml of blood-CMV-DNA. Antiviral
therapy with IV Ganciclovir (5 mg/kg BID) was started, and a
progressive normalization of platelet count was documented.
What
is the appropriate management of iTTP? What are the criteria for
defining refractory disease in the caplacizumab era? What is the most
appropriate time to add Rituximab? Is administration of caplacizumab
and antiplatelets or anticoagulant therapy safe? Are all the drops in
platelets count as a sign of iTTP refractory or exacerbation?
Thrombotic
thrombocytopenic purpura (TTP) is a rare and life-threatening
thrombotic microangiopathy (TMA), first described in 1924 by Dr. Eli
Moschcowitz.[1] This disease has an annual incidence of 1.5–6 per million cases in adults[2,3] and is characterized by microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and organ failure of variable severity.[4]
In patients with TTP, the levels of the von Willebrand factor
(VWF)-cleaving protease ADAMTS13 are severely decreased. Acquired
immune-mediated thrombotic thrombocytopenic purpura (iTTP) is caused by
the development of anti-ADAMTS13 autoantibodies targeting ADAMTS13,
resulting in a lowered ADAMTS13 function or an increase in the
metalloprotease’s clearance.[5]
TTP is a medical emergency with life-threatening complications and a 90% mortality rate if left untreated.
Pathophysiology
Progress has been made in recent years in understanding the pathophysiology of the disease. ADAMTS13 is the 13th member of the ADAMTS protein family identified for the first time in 2001.[6]
It is a metalloprotease that cleaves the ultra-large vWF multimers
secreted by Weibel-Palade bodies of endothelial cells and α-granules of
platelets linked to the endothelium. Arterial shear-stress and
reciprocal interaction induce a change in both vWF and ADAMTS13
conformations that allow ADAMTS13 to cleave vWF into smaller and less
adhesive multimers.[7-10] Hence, severe ADAMTS13
deficiency (< 10 IU/dl) leads to the accumulation of unusually
ultra-large vWF multimers in the bloodstream and subsequent platelets
adhesion, agglutination, and formation of occlusive thrombi in small
arterioles and capillaries, inducing widespread microvascular ischemia.[11,12]
Nevertheless,
a severe enzyme deficiency is necessary, but not sufficient on its own
to cause an episode of TTP. It has been suggested that another stressor
factor in conjunction with this severe deficiency is usually required
to develop a clinical evident TTP, such as activation of the complement
system.[13-15]
Anti-ADAMTS13 autoantibodies can
be divided into two groups: inhibitory and non-inhibitory. Inhibitory
antibodies neutralize the proteolytic activity of the enzyme, while
non-inhibitory antibodies accelerate its clearance from plasma by
binding to the protease and increasing its uptake by the reticular
endothelial system (RES).[16,17] Even if inhibitory
antibodies have always been considered the major cause of ADAMTS13
deficiency, recent studies have proved that antigen depletion plays an
important role in this deficiency.[18] Immune TTP is
characterized by a polyclonal immune response, as proven by the fact
that autoantibodies have been found against all domains of ADAMTS13.[19,20] However, the spacer domain is the most frequently involved with autoantibodies directed against it in 95% of cases.[21]
The
most common isotype class of anti-ADAMT13 autoantibodies is IgG, but
IgA and IgM have been reported as well (20% of cases). Among the IgG
isotype, the IgG4 subclass is most frequent, followed by IgG1.[22-24]
The autoantibody isotype may contribute to the disease's clinical
phenotype; for example, IgA and IgG1 antibodies are associated with a
higher death rate and IgG4 with an increased risk of relapse.[22-24]
In addition to free anti-ADAMTS13 autoantibodies, circulating
ADAMTS13-specific immune complexes have also been reported during acute
iTTP.[25-27] These complexes may lead to complement
activation; in fact, during an acute iTTP episode, it has been found
that C3a and C5a are elevated, suggesting a complement activation
through the classic pathway; nevertheless, elevated levels of factor Bb
have been detected, evoking activation of the alternative pathway.[13-15,21,28]
Complement activation in TTP may play the role of the "second hit",
acting as another stressor in combination with severe ADAMTS13
deficiency to induce the clinical syndrome.[15]
Molecular mimicry between ADAMTS13 and certain pathogens infection
might be considered one of the triggers to evoke an immune response,
although no bacterial or viral infections are directly linked with
iTTP.[21,29-33] Cytomegalovirus
(CMV) infection can also be considered a possible trigger of TTP and
may cause refractoriness at iTTP treatment.[34]
COVID-19 infection has been associated with endotheliopathy, and it is
also associated with TTP. Recently, de novo and relapsed iTTP have been
reported during SARS-Cov-2 infection.[35] Cases of iTTP following the administration of vaccines have been described in the literature.[36-37] Recently, de novo and relapsed iTTP have been reported after COVID-19 vaccination, mainly with adenoviral and rarely with mRNA vaccines.[38-39]
Some studies have investigated the possible correlation between
COVID-19 vaccines and the iTTP new onset or recurrence. Results showed
that COVID-19 vaccination does not increase the risk of de novo or
relapsed iTTP, except in individuals in hematologic remission with
extremely low ADAMTS13 activity (<20%), requiring closer monitoring
in these patients.[40-43]
Clinical Manifestation and Diagnosis
The
TMA syndromes (TMAs) are a group of different diseases united by common
clinical and pathological features. They occur in children or in
adults. The clinical manifestations include microangiopathic hemolytic
anemia, thrombocytopenia, and organ damage. Although they are different
entities, they have in common a pathogenic mechanism involving
endothelial injury and thrombus formation. Therefore, laboratory tests
are important alongside a thorough history and examination. Prognosis
and treatment depend on the nature of the underlying disease. In TTP
and Hemolytic Uremic Syndrome (HUS), there is an underlying
abnormality, such as ADAMTS13 deficiency or a complement mutation, that
may not be clinically expressed until pregnancy, surgery, or an
inflammatory disorder precipitates an acute TMA episode. The treatment,
in these patients, is focused on the cause of the primary TMA syndrome,
not the precipitating condition. These patients are distinct from many
other patients who have microangiopathic hemolytic anemia and
thrombocytopenia that are manifestations of an underlying disorder,
such as systemic infections, systemic cancer, severe preeclampsia or
HELLP syndrome, severe hypertension, autoimmune disorders and
hematopoietic stem cell or organ transplantation. The treatment of such
patients is focused on the underlying disorder. A thorough diagnostic
evaluation will usually reveal the underlying etiology and guide
treatment. As many of the investigations will not be available at the
initial presentation, the initial focus should be on the consideration
of TTP, given the high mortality if untreated. The ADAMTS13 activity
test should be applied to any diagnosis of thrombotic microangiopathy.
Anemia
with schistocytes >1%, elevated serum LDH, reticulocyte count, total
bilirubin, predominantly unconjugated, decreased haptoglobin,
thrombocytopenia, and microvascular ischemia are the typical clinical
manifestations in TMAs. Direct and indirect antiglobulin tests are
negative, and coagulation parameters are normal. In TTP, although all
organs can be affected, central nervous system, heart, and digestive
tract involvement are more frequent. Conversely, renal damage is
usually mild, in contrast to other forms of TMA, such as HUS. Signs and
symptoms are variable at presentation. More than 60% of cases present
with neurological manifestations, which widely range from mild
confusion or altered sensorium to headache, transient focal brain
defect, stroke, seizures, or coma.[2,3,44] Abdominal pain, nausea, and diarrhea are due to gastrointestinal ischemia, which can be evident in 35% of patients.[3]
Evidence of myocardial ischemia highlighted either by an abnormal
electrocardiogram or, more commonly, by elevated cardiac troponin-I
measurements can be found in around a quarter of acute TTP patients.
Most frequently, this myocardial ischemia is asymptomatic. Benhamou et
al. have found that even if asymptomatic, a cardiac troponin-I (CTnI)
level of > 0.25 μg/L at presentation in patients with TTP appears to
be an independent factor associated with a three-fold increase in the
risk of death or refractoriness.[45] Renal injury is not uncommon in iTTP. Hence, most patients present with creatinine below 2 mg/dL.[3,46] The main causes of morbidity and mortality in iTTP are thrombotic and ischemic complications.
Early diagnosis and treatment reduce the mortality rate, which, however, remains around 10–15%.[47]
Severe deficiency of ADAMTS13 activity (<10 IU/dl) with detectable
inhibitory autoantibodies against ADAMTS13 confirms the diagnosis. Due
to the rarity of TTP, ADAMTS13 assays are not widely performed and
remain mainly confined to specialized laboratories, therefore, the
initial diagnosis is a clinical diagnosis.[48] Scoring systems developed using data from TMA registries, such as the French score and Plasmic score,[49-51]
may help decide on the urgency of ADAMTS13 testing and the likelihood
of a positive TTP diagnosis. The PLASMIC and French scores turned out
to be useful predictors of a significant reduction in ADAMTS13
activity, and in the high-risk group (scores 6–7) of the PLASMIC score (Table 1),
patients who received treatment had meaningfully higher overall
survival than those who did not. However, a recent meta-analysis
demonstrated that the PLASMIC score can support differential diagnosis
by excluding TTP, but due to low specificity and positive predictive
value, it is insufficient to confirm TTP diagnosis.[52]
Older iTTP patients have an atypical clinical presentation and a poorer
response to treatment and prognosis. Renal and cardiac involvement are
more frequent and severe in older patients, whereas hematologic
features such as thrombocytopenia and anemia are less pronounced. These
differences translate into poorer performances of both the French and
PLASMIC scores.[53]
Hence, ADAMTS13 activity measurement remains necessary for an accurate differential diagnosis and management of TTP.
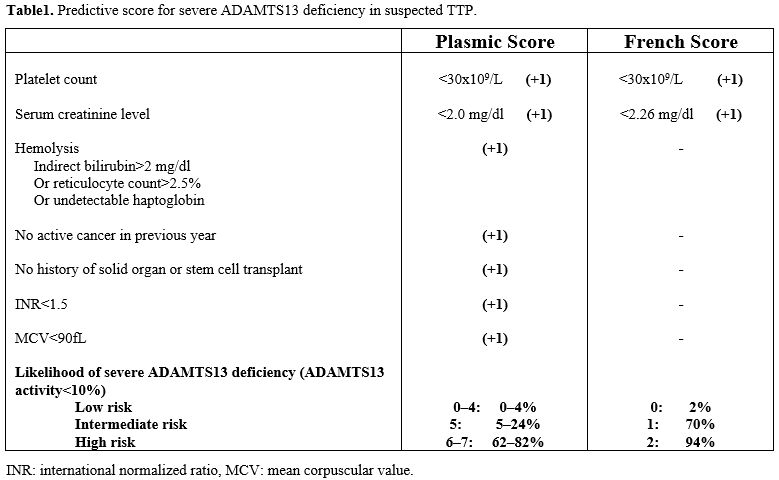 |
- Table 1. Predictive score for severe ADAMTS13 deficiency in suspected TTP.
|
Management of Acute Phase
When
a TTP is suspected, the patient's blood sample for ADAMTS13 activity
testing should be collected immediately, but treatment must be
initiated before the result is available. According to the ISTH
guidelines, the results of ADAMTS13 activity should ideally be
available within 72 hours, though a result within seven days is
acceptable.[54] Prompt initiation of therapy reduces mortality to 10-15%.
Until
recently, the standard treatment of acute iTTP consisted of daily
therapeutic plasma exchange (PEX) and immunosuppressive therapy. In
1991, the Canadian randomized clinical trial documented the
effectiveness of PEX over plasma infusion alone.[55]
However, in situations where PEX cannot be quickly performed, plasma
infusion may be used temporarily as a temporizing measure.[56]
PEX removes anti-ADAMTS13 antibodies and replaces ADAMTS13. It should
be initiated as soon as possible and not later than 6 hours of
presumptive clinical diagnosis.[57] The expert panel
suggests one PEX session daily; the usual volume for exchange is 40
ml/kg (1 plasma volume), but in patients with severe disease, such as
those with neurological manifestations or who do not readily respond to
treatment, the PEX volume can be increased to 60 ml/kg (1.5 plasma
volume), or PEX may be performed more than once daily. According to
2022 guidelines, PEX may be discontinued soon after a clinical
response, defined by a sustained platelet count ≥150x109/L
and LDH <1.5 times the upper limit of normal and no clinical
evidence of new or progressive ischemic organ injury, is achieved.[58]
Corticosteroid therapy is commonly used in conjunction with PEX to suppress the production of anti-ADAMTS13 autoantibodies.[54,57]
Although the corticosteroid dosage, dose adjustment, or tapering were
not well determined in randomized clinical trials, high-dose
corticosteroids (e.g., prednisone, 1 mg/kg per day, orally, or
methylprednisolone, 125 mg, IV, two to four times) are usually used as
the initial regimen. Balduini et al. compared high-dose
methylprednisolone (10 mg/kg/day for 3 days followed by 2.5 mg/kg/day)
to standard dose methylprednisolone (1 mg/kg/day) and found that
remission rates were significantly higher in the high dose group (76.6%
vs. 46.6%).[59] Therefore, high-dose steroids bolus
with methylprednisolone may be used as the first-line, especially in
patients with severe presentations or neurological symptoms.[59]
Anti-vWF
therapy with caplacizumab was recently approved for the initial
treatment of iTTP in conjunction with PEX and steroids. Caplacizumab is
a nanobody (a bivalent humanized immunoglobulin fragment) that binds to
the A1 domain of vWF, blocking the interaction of ultra-large vWF
multimers with the platelet GpIb-IX-V receptor and thereby preventing
the formation of platelet-rich thrombi.[60] The current
ISTH guidelines recommend its use in acute iTTP because
immunosuppressive therapy requires a certain period to obtain a
response, and fatal thrombosis is very common in the first 10 days
after diagnosis.[54] Caplacizumab is started at 10 mg
intravenously immediately after diagnosis, followed by 10 mg
subcutaneously (s.c.) after each PEX, and subsequently followed by
daily s.c. injections until stable recovery of ADAMTS13 activity >
10-20 IU/dl. Its efficacy and safety have been demonstrated in two
randomized controlled trials: the phase II (TITAN) and phase III
(HERCULES) studies.[61-62] In the integrated analysis
of data from both trials, a significant reduction in the number of
deaths (0 vs. 4; P <0.05) and a significantly lower incidence of
refractory TTP (0 vs. 8; P <0.05) were observed in patients who
received caplacizumab versus placebo.[63] Also,
caplacizumab significantly reduced the time to platelet count
normalization (P < .001), significantly reduced the time to
normalization of the organ damage marker LDH (P .03) and induced a
faster normalization of troponin and serum creatinine. During the
overall treatment period, there was a 33.3% reduction in the median
number of PEX days with caplacizumab vs placebo (5.0 days vs 7.5 days,
respectively). The trials also demonstrated a reduction in hospital and
ICU length of stay. The most common adverse event associated with
caplacizumab was mucocutaneous bleeding. However, these events were
mild or moderate in severity and resolved spontaneously in most
patients.
Recent studies provide real-world data on the efficacy
and safety of caplacizumab, confirming its therapeutic benefits when
used as initial treatment. The time to platelet count normalization and
clinical remission was much shorter among patients who received
caplacizumab within 3 days of the first PEX, and the early prevention
of microcirculation occlusion and ischemic organ damage seemed to avoid
long-term complications and eventually death.[64-68]
Caplacizumab
is an expensive drug, but the costs are balanced by reduced
hospitalization and long-term effects. It has been described that
patients with TTP in the pre-caplacizumab era, in the long run, suffer
from disabling conditions related to cerebral microthrombosis.[69]
Our
patient, based on clinical suspicion, was treated with PEX, steroids,
and caplacizumab, achieving improvement of neurological symptoms and
normalization of platelet count. Seven PEX were performed, and then the
patient was discharged, continuing home steroids and caplacizumab. Five
days after the last PEX, a reduction in platelet count was noted. This
event is possible during caplacizumab administration but is not
frequent. Training had been performed to teach her the correct
administration of caplacizumab at home. However, we do not know whether
the patient was compliant with the dosing and administration, and no
anti-vWF tests were performed to demonstrate the caplacizumab activity.[70]
Plasma ADAMTS13 activity was less than 3 IU/dl, and ADAMTS13
autoantibodies were still present. Therefore, PEX was resumed, and
immunosuppressive treatment was intensified with the addition of
Rituximab.
In registration trials, more relapses occurred with
caplacizumab compared with placebo (14 vs 0 participants) and occurred
within 10 days of stopping caplacizumab in patients with persistent
ADAMTS13 levels <10 IU/dl, highlighting the importance of monitoring
weekly ADAMTS13 activity and continuing caplacizumab treatment until
resolution of the underlying autoimmune disease, possibly optimizing
immunosuppressive therapy.[61-62] An ADAMTS13
threshold above which anti-vWF therapy can be safely discontinued
remains to be defined, although a level that is increasing >20 IU/dl
for at least 2 consecutive weeks has been suggested.[71]
In the recently reported German post-marketing experience with
caplacizumab, there were no iTTP recurrences when the drug was
discontinued in patients with ADAMTS13 activity >10 IU/dl.[72]
Until
now, the criteria for response to treatment mainly took into account
the normalization of platelet count and discontinuation of PEX. Now,
with the use of caplacizumab, leading to rapid normalization of
platelet count, it has been necessary to redefine the response
criteria, taking into account also the underlying autoimmune disease.
Therefore, the International Working Group for TTP proposed revised
consensus outcome definitions that incorporate ADAMTS13 activity and
the effects of anti-VWF therapy on the platelet count, by using an
estimate-talk-estimate approach.[73] The updated
definitions distinguish clinical remission and clinical relapse,
defined primarily by platelet count, from ADAMTS13 remission and
ADAMTS13 relapse, defined by ADAMTS13 activity. The IWG defines
clinical remission as a sustained clinical response with both no TPE
and no anti-vWF therapy in the past 30 days. Clinical exacerbation
occurs when the platelet count decreases to < 150x109/L
(with other causes of thrombocytopenia excluded), with or without
clinical evidence of new or progressive ischemic organ injury, within
30 days of stopping PEX or anti-vWF therapy. Refractory TTP is used to
describe cases where there is no clinical response after five sessions
of PEX or there is an initial response followed by a platelet decline
while receiving standard treatment and requires early intensified
treatment. Partial ADAMTS13 remission as ADAMTS13 activity >20 UI/dl
but less than the lower limit of normal (LLN) and complete ADAMTS13
remission as ADAMTS13 activity >= LLN.[73]
Due
to biological variability in ADAMTS13 activity, it is important to
repeat the measurement to confirm ADAMTS13 activity, relying more on
the trend than on a specific value.
Some patients may achieve
clinical remission without ADAMTS13 remission; therefore, the risk of
clinical relapse is very high in these patients, so it is important to
optimize immunosuppressive therapy to avoid recurrence.
Platelet
transfusions are usually avoided in TTP. However, platelet transfusions
are sometimes used in TTP patients with serious bleeding or in TTP
patients undergoing invasive procedures with a high risk of bleeding.[74]
Rituximab Treatment
Rituximab
is a chimeric anti-CD20 monoclonal antibody. It suppresses
anti-ADAMTS13 antibody production by depleting B lymphocytes via
complement-dependent cytotoxicity and antibody-dependent cell-mediated
cytotoxicity. Rituximab is effective in patients with refractory
disease whose platelet counts do not recover despite conventional
therapy and in those with early relapsed disease. The usual dosage is
375 mg/m2 once a week for four weeks,
modifying the schedule from weekly to every 3-4 days in case of
concurrent PEX treatment considering the accelerated clearance.[75] In refractory patients, Rituximab can normalize platelet count early and prevent short-term relapse.[76]
Currently, the use of Rituximab has expanded, both in front-line
treatment and as a pre-emptive approach in patients in clinical
remission but having low ADAMTS13 levels.
In 2011, the UK group
reported in a prospective trial that front-line treatment with
Rituximab administered within three days of admission resulted in
shorter hospitalization and fewer relapses (10% vs >50% in
historical controls) and was well-tolerated.[77] This
study provided evidence for the benefits of Rituximab as initial
therapy, showing a high remission rate in patients who received the
standard therapy, with nearly half of the patients not relapsing.
Therefore, Rituximab may be suitable as a first-line treatment of iTTP
for some patients; however, a subgroup of patients may receive
unnecessary treatment.
A recent meta-analysis also suggests the
benefit of upfront Rituximab, which documented that its use reduces
mortality and prevents relapse.[78] However, the study has some limitations because none of the included studies was a randomized trial.
In
a recent study, Coppo et al. treated 90 iTTP patients with a frontline
triplet regimen associating PEX, immunosuppression with corticosteroids
and Rituximab, and caplacizumab. Outcomes were compared with 180
historical patients treated with the standard frontline treatment (PEX
and corticosteroids, with Rituximab as salvage therapy). Patients from
the triplet regimen experienced fewer exacerbations (3.4% vs. 44%, P
< .01); they recovered durable platelet count 1.8 times faster than
historical patients (P < .01), with fewer PEX sessions (P < .01)
and the number of days in hospital was 41% lower in the triplet regimen
than in the historical cohort (13 vs 22 days; P < .01). In addition,
the use of Rituximab in frontline resulted in more rapid improvement in
ADAMTS13 activity (>20 IU/dl) than the historical regimen in which
Rituximab was introduced only later as salvage therapy, with a mean of
28 days compared with 48 days (P .01).[68]
To
date, however, the use of Rituximab in the frontline remains
controversial, and the ISTH guidelines suggest that Rituximab should be
used as part of the first-line treatment of severe TTP, recommending
Rituximab in the frontline therapy for only selected cases such as
patients with comorbid autoimmune disorders due to low levels of
evidence.[54]
Monitoring ADAMTS13 activity could
help identify those patients who do not have an optimal response to
immunosuppressive treatment with steroids as they maintain a low value
of activity (<10 IU/dl) after at least three weeks of steroid
treatment.
Increasing immunosuppression in cases with refractory
iTTP or in those without normalization of ADAMTS13 activity may expose
patients to opportunistic viral or bacterial infections. A literature
review of viral infections after Rituximab conducted by Aksov et al.
showed that hepatitis B virus infection was the most common infection
observed in 39% of the cases. Cytomegalovirus (CMV) infection was
observed in 23% of the cases; CMV usually is a latent infection and
gets reactivated during an immunocompromised state.[79] Thrombocytopenia, due to CMV reactivation, may mimic an iTTP exacerbation as described by Laganà et al. (Figure 1).[80]
Therefore, early identification of CMV is essential for the successful
treatment of refractory/relapsed iTTP or a false iTTP exacerbation to
avoid CMV complications.
In our patient, a second fall in platelet
count was not associated with signs of hemolysis. Since the ADAMTS13
activity was normal, we looked for secondary causes that could explain
the reduction in platelet count. Cytomegalovirus infection was
documented and responsive to antiviral treatment.
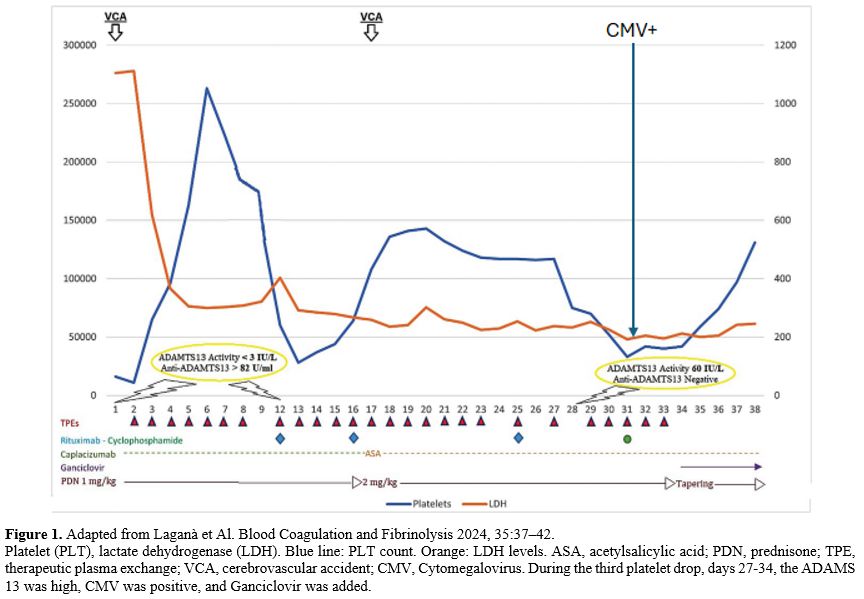 |
- Figure 1. Adapted from Laganà et Al. Blood Coagulation and Fibrinolysis 2024, 35:37–42.
Platelet
(PLT), lactate dehydrogenase (LDH). Blue line: PLT count. Orange: LDH
levels. ASA, acetylsalicylic acid; PDN, prednisone; TPE, therapeutic
plasma exchange; VCA, cerebrovascular accident; CMV, Cytomegalovirus.
During the third platelet drop, days 27-34, the ADAMS 13 was high, CMV
was positive, and Ganciclovir was added.
|
Additional Immunosuppressive Agents
Some
patients do not achieve ADAMTS13 remission following the acute phase
despite steroids and rituximab treatment, requiring an individualized
immunosuppressive approach to prevent clinical relapse. Mycophenolate
mofetil (MMF) or azathioprine are both used in the treatment of
autoimmune diseases by modulating the immune system with suppression of
B and T lymphocyte proliferation and antibody production.[81-82]
Cyclosporin A has been used to treat relapsed and refractory iTTP with
recovery of ADAMTS13 activity and reduction of antibody levels,
suggesting a role in patients with disease recurrence.[83-84] Cyclophosphamide was utilized as salvage therapy for refractory/relapsed patients.[85]
In
patients unable to receive Rituximab due to severe allergic reactions,
alternative anti-CD 20 therapy (including ofatumumab or obinutuzumab)
has also been used.[86-87] Finally, in some cases
where patients do not have an adequate response to anti-CD20 therapy,
other immunomodulating agents (such as bortezomib or daratumumab) can
be considered.[88-89]
Introduction
Caplacizumab
and Thromboprophylaxis. In iTTP, there is an increased risk of venous
and arterial thrombotic events (VTEs). VTE rates range from 3.8% to
13%, and most VTEs occur after platelet normalization, especially in
patients who do not receive thromboprophylaxis.[90]The
effect of caplacizumab on the risk of VTE is unclear. In real-world
studies, the risk of VTE was similar between caplacizumab and control
groups, suggesting that caplacizumab was not effective in preventing
VTE.[91,68] There is no consensus
on the use of pharmacological thromboprophylaxis in patients on
caplacizumab because the drug is known to increase bleeding risk, so
co-administration with antiplatelets or anticoagulant drugs may lead to
major bleeding. In the recent ISTH guidelines for the treatment of TTP,
no recommendations on antithrombotic use either with or without
caplacizumab are included, and there is no statement to withhold or
continue caplacizumab in case of adding an antithrombotic agent due to
a new thrombotic event.[54]When
our patient presented a second episode of aphasia, dysarthria, and
confusion, although there were no signs of cerebral hemorrhage, in
order to treat the new ischemic event, we preferred not to administer
caplacizumab together with acetylsalicylic acid not to increase the
hemorrhagic risk. Recently, Elverdi et al. published a review focusing
on the thrombotic complications and bleeding events observed in TTP
patients both in the pre- and post-capacizumab era.[92]
They suggest that, in the absence of clinical indication, concomitant
use of caplacizumab and antiplatelet drugs should not be encouraged.
However, if strongly indicated, they can be used concomitantly safely,
yet careful monitoring is mandatory. When the platelet counts are
<50 x 109/L, the indication could be assessed on a patient-by-patient basis by weighing the risk of hemorrhage vs. thrombosis.
Introduction
TTP
in Remission. After complete remission, the risk of relapse is between
30 to 50%, exposing patients to death and treatment-related
complications.[93] It has been reported that
persistently undetectable ADAMTS13 activity (<10 IU/dl) in patients
in remission represents an early predictor of clinical relapse and that
the cumulative incidence of relapse increases dramatically with time
(74% at 7 years).[94] Therefore, long-term monitoring of ADAMTS13
activity is necessary during follow-up. In patients in remission,
ADAMTS13 activity testing is usually scheduled every month for the
first 3 months, then every 3 months for the first year, then every 6–12
months if stable, and more frequently if levels begin to drop. It has
been shown that pre-emptive rituximab infusions in patients with
persistently undetectable ADAMTS13 activity or when the activity falls
from normal levels to <20 IU/dl allow, in most cases, the rapid
recovery of ADAMTS13 activity.[95-97] However, this
recovery may not be sustained, and a substantial number of patients
require repeated rituximab infusions to maintain a detectable enzyme
activity over time. Consequently, the systematic pre-emptive use of
Rituximab in this setting is still debated.[98]In
a recent retrospective study, the French TMA group reported the
long-term outcome of 92 patients with iTTP in clinical remission who
received pre-emptive Rituximab after identification of severe ADAMTS13
deficiency (activity <10 IU/dl) during the follow-up and presented
an improved relapse-free survival, compared with a historical control
of patients.[99] There was no increased incidence of
adverse effects with long-term use of Rituximab and no loss of response
with repeated courses. Dosing regimens, apart from the standard weekly
375 mg/m2 dose, have been used, ranging from 100 mg to 500 mg/m2
weekly with 1 o 2 o 4 infusions per course. They found that half of the
patients treated with pre-emptive Rituximab required repeated courses
for subsequent recurrences of ADAMTS13 relapses, and retreated patients
usually responded again to Rituximab. However, they observed that the
interval between treatments was twice as long after a preemptive course
of 4 infusions compared with a course with 1 or 2 infusions.
Retrospective evidence suggests that although low-dose Rituximab (200
mg x4 weekly) prevents iTTP relapses, it is associated with higher
re-treatment rates than the standard dose.[96]Also,
whereas an ADAMTS13 level of >20 IU/dl < ULN may be sufficient to
prevent iTTP relapse, it may not be sufficient to prevent other adverse
clinical outcomes. Interestingly, a recent study demonstrated that,
among patients with a history of iTTP who are in clinical remission,
those with a partial ADAMTS13 remission are at greater risk of ischemic
stroke than those with a complete ADAMTS13 remission.[100]
A recent study showed that a subgroup of anti-ADAMTS13 autoantibodies
from iTTP patients can induce an open ADAMTS13 conformation. Most
importantly, an open ADAMTS13 conformation is present in acute iTTP
patients and in those in clinical remission with decreased ADAMTS13
activity <50 IU/dl. Therefore, open ADAMTS13 may become a novel and
sensitive biomarker to monitor iTTP patients and identify the early
stages of subclinical iTTP, but further investigation is warranted.[101]Finally,
in patients who have recovered from an acute TTP episode, the Guideline
panel acknowledged the importance of monitoring for the development of
mood disorders, neurocognitive symptoms (including short-term memory
issues), and hypertension, which may develop during remission. Specific
recommendations regarding screening for long-term complications cannot
be made at this time. However, serial follow-up and monitoring for
these complications should be considered part of routine follow-up.[97]
The Future: Recombinant Adamts13
Another novel therapy currently under investigation in clinical trials is recombinant ADAMTS13 (BAX930/SHP655/TAK755).[102]
A Phase II trial is assessing the role of rADAMTS13 in treating acute
presentations of iTTP in addition to standard care (NCT03922308). The
potential inhibitory effect of autoantibodies on the recombinant
ADAMTS13 will need to be evaluated.
Concluding Remarks
Major
advances in iTTP pathophysiology and management have occurred over the
last few decades, leading to a significant improvement in patient
outcomes. Early diagnosis and improved therapeutic management have
reduced mortality and prolonged survival. Plasma exchange and
immunosuppressive therapy remain the standard treatments.
Caplacizumab
reduces mortality due to ischemic events, refractoriness, and
exacerbations after treatment discontinuation; for maximum efficacy, it
should be started as early as possible with the first PEX.
However,
there are still open issues. When can the PEX be discontinued with the
use of caplacizumab, which induces rapid normalization of platelet
count, whether sufficient to rely only on platelet count?
Close
monitoring of ADAMTS13 activity after platelet recovery can guide in
optimizing immunosuppressive therapy with Rituximab, although rituximab
treatment in combination with steroids may induce opportunistic
infections such as pneumocystis infections or viral infections (CMV or
hepatitis). Such infections may also mimic an exacerbation of TTP.
In
case of ischemic events or VTE, concomitant administration of
caplacizumab and antiplatelet or anticoagulant therapy is not
encouraged but can be done very cautiously if necessary.
Targeted immune treatments should be performed in remission to reduce relapses during follow-up.
Author Contributions
SMT
and AL wrote and edited the manuscript. SC revised and corrected the
manuscript. All authors have read and agreed to the published version
of the manuscript.
References
- Moschcowitz, E. Hyaline Thrombosis of the Terminal
Arterioles and Capillaries: A Hitherto Undescribed Disease. Proc. N. Y.
Pathol. Soc. 1924, 24, 21-24
- Scully,
M.; Yarranton, H.; Liesner, R.; Cavenagh, J.; Hunt, B.; Benjamin, S.;
Bevan, D.; Mackie, I.; Machin, S. Regional UK TTP registry: Correlation
with laboratory ADAMTS 13 analysis and clinical features. Br. J.
Haematol. 2008, 142, 819-826. https://doi.org/10.1111/j.1365-2141.2008.07276.x PMid:18637802
- Mariotte,
E.; Azoulay, E.; Galicier, L.; Rondeau, E.; Zouiti, F.; Boisseau, P.;
Poullin, P.; de Maistre, E.; Provôt, F.; Delmas, Y.; et al.
Epidemiology and pathophysiology of adulthood-onset thrombotic
microangiopathy with severe ADAMTS13 deficiency (thrombotic
thrombocytopenic purpura): A cross-sectional analysis of the French
national registry for thrombotic microangiopathy. Lancet Haematol.
2016, 3, e237-e245. https://doi.org/10.1016/S2352-3026(16)30018-7 PMid:27132698
- Amorosi
EL, Ultmann JE. Thrombotic thrombocytopenic Purpura: report of 16 cases
and review of the literature. Medicine 1966;45:139-60. https://doi.org/10.1097/00005792-196603000-00003
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014 Aug 14;371(7):654-66. https://doi.org/10.1056/NEJMra1312353 PMid:25119611
- Levy
GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS
gene family cause thrombotic thrombocytopenic purpura. Nature.
2001;413(6855):488-494. https://doi.org/10.1038/35097008 PMid:11586351
- South K, Luken BM, Crawley JTB, et al. Conformational activation of ADAMTS13. Proc Natl Acad Sci USA. 2014;111(52):18578-18583. https://doi.org/10.1073/pnas.1411979112 PMid:25512499 PMCid:PMC4284544
- Muia
J, Zhu J, Gupta G, et al. Allosteric activation of ADAMTS13 by von
Willebrand factor. Proc Natl Acad Sci USA. 2014;111 (52):18584-18589. https://doi.org/10.1073/pnas.1413282112 PMid:25512528 PMCid:PMC4284596
- Deforche
L, Roose E, Vandenbulcke A, et al. Linker regions and flexibility
around the metalloprotease domain account for conformational activation
of ADAMTS-13. J Thromb Haemost JTH.2015;13(11):2063-2075. https://doi.org/10.1111/jth.13149 PMid:26391536 PMCid:PMC4778570
- Crawley
JTB, de Groot R, Xiang Y, et al. Unraveling the scissile bond: how
ADAMTS13 recognizes and cleaves von Willebrand factor. Blood.
2011;118(12):3212-3221 https://doi.org/10.1182/blood-2011-02-306597 PMid:21715306 PMCid:PMC3179391
- Sadler
JE What's new in the diagnosis and pathophysiology of thrombotic
thrombocytopenic purpura. Hematol Educ Program Am Soc Hematol Am Soc
Hematol Educ Program. 2015;2015:631-636 https://doi.org/10.1182/asheducation-2015.1.631 PMid:26637781 PMCid:PMC4777280
- Furlan
M, Robles R, Galbusera M, et al. von Willebrand factor-cleaving
protease in thrombotic thrombocytopenic purpura and the
hemolytic-uremic syndrome. N Engl J Med. 1998;339 (22):1578-1584. https://doi.org/10.1056/NEJM199811263392202 PMid:9828245
- Réti,
M.; Farkas, P.; Csuka, D.; Rázsó, K.; Schlammadinger, Á.; Udvardy,
M.L.; Madách, K.; Domján, G.; Bereczki, C.; Reusz, G.S.; et al.
Complement activation in thrombotic thrombocytopenic purpura. J.
Thromb. Haemost. 2012, 791-8 https://doi.org/10.1111/j.1538-7836.2012.04674.x PMid:22372946
- Turner,
N.; Sartain, S.; Moake, J. Ultralarge von Willebrand factor-induced
platelet clumping and activation of the alternative complement pathway
in thrombotic thrombocytopenic purpura and the hemolytic-uremic
syndromes. Hematol. Oncol. Clin. N.Am. 2015, 29, 509-524 https://doi.org/10.1016/j.hoc.2015.01.008 PMid:26043389
- Miyata, T.; Fan, X. A second hit for TMA. Blood 2012, 120, 1152-1154 https://doi.org/10.1182/blood-2012-06-433235 PMid:22879625
- Scheiflinger,
F.; Knöbl, P.; Trattner, B.; Plaimauer, B.; Mohr, G.; Dockal, M.;
Dorner, F.; Rieger, M. Nonneutralizing IgM and IgG antibodies to von
Willebrand factor-cleaving protease (ADAMTS-13) in a patient with
thrombotic thrombocytopenic purpura. Blood 2003, 102, 3241-3243. https://doi.org/10.1182/blood-2003-05-1616 PMid:12855569
- Rieger,
M.; Mannucci, P.M.; Kremer Hovinga, J.A.; Herzog, A.; Gerstenbauer, G.;
Konetschny, C.; Zimmermann, K.; Scharrer,I.; Peyvandi, F.; Galbusera,
M.; et al. ADAMTS13 autoantibodies in patients with thrombotic
microangiopathies and other immunomediated diseases. Blood 2005, 106,
1262-1267 https://doi.org/10.1182/blood-2004-11-4490 PMid:15890682
- Thomas,
M.R.; de Groot, R.; Scully, M.A.; Crawley, J.T. Pathogenicity of
Anti-ADAMTS13 Autoantibodies in Acquired Thrombotic Thrombocytopenic
Purpura. EBioMedicine 2015, 2, 942-952 https://doi.org/10.1016/j.ebiom.2015.06.007 PMid:26425702 PMCid:PMC4563118
- Zheng,
X.L.; Wu, H.M.; Shang, D.; Falls, E.; Skipwith, C.G.; Cataland, S.R.;
Bennett, C.L.; Kwaan, H.C. Multiple domains of ADAMTS13 are targeted by
autoantibodies against ADAMTS13 in patients with acquired idiopathic
thrombotic thrombocytopenic purpura. Haematologica 2010, 95, 1555-1562.
https://doi.org/10.3324/haematol.2009.019299 PMid:20378566 PMCid:PMC2930958
- Yamaguchi,
Y.; Moriki, T.; Igari, A.; Nakagawa, T.; Wada, H.; Matsumoto, M.;
Fujimura, Y.; Murata, M. Epitope analysis of autoantibodies to ADAMTS13
in patients with acquired thrombotic thrombocytopenic purpura. Thromb.
Res. 2011, 128, 169-173. https://doi.org/10.1016/j.thromres.2011.03.010 PMid:21496883
- Verbij FC, Fijnheer R, Voorberg J, et al. Acquired TTP: ADAMTS13 meets the immune system. Blood Rev. 2014;28(6):227-234 https://doi.org/10.1016/j.blre.2014.07.004 PMid:25213289
- Ferrari,
S.; Scheiflinger, F.; Rieger, M.; Mudde, G.; Wolf, M.; Coppo, P.;
Girma, J.P.; Azoulay, E.; Brun-Buisson, C.; Fakhouri, F.; et al.
Prognostic value of anti-ADAMTS 13 antibody features (Ig isotype,
titer, and inhibitory effect) in a cohort of 35 adult French patients
undergoing a first episode of thrombotic microangiopathy with
undetectable ADAMTS 13 activity. Blood 2007, 109, 2815-2822. https://doi.org/10.1182/blood-2006-02-006064 PMid:17164349
- Ferrari,
S.; Mudde, G.C.; Rieger, M.; Veyradier, A.; Kremer Hovinga, J.A.;
Scheiflinger, F. IgG subclass distribution of antiADAMTS13 antibodies
in patients with acquired thrombotic thrombocytopenic purpura. J.
Thromb. Haemost. 2009, 7, 1703-1710. https://doi.org/10.1111/j.1538-7836.2009.03568.x PMid:19682238
- Bettoni,
G.; Palla, R.; Valsecchi, C.; Consonni, D.; Lotta, L.A.; Trisolini,
S.M.; Mancini, I.; Musallam, K.M.; Rosendaal, F.R.; Peyvandi, F.
ADAMTS-13 activity and autoantibodies classes and subclasses as
prognostic predictors in acquired thrombotic thrombocytopenic purpura.
J. Thromb. Haemost. 2012, 10, 1556-1565. https://doi.org/10.1111/j.1538-7836.2012.04808.x PMid:22672482
- Lotta,
L.A.; Valsecchi, C.; Pontiggia, S.; Mancini, I.; Cannavò, A.; Artoni,
A.; Mikovic, D.; Meloni, G.; Peyvandi, F. Measurement and prevalence of
circulating ADAMTS13-specific immune complexes in autoimmune thrombotic
thrombocytopenic purpura. J. Thromb. Haemost. 2014, 12, 329-336. https://doi.org/10.1111/jth.12494 PMid:24354764
- Ferrari,
S.; Palavra, K.; Gruber, B.; Kremer Hovinga, J.A.; Knöbl, P.; Caron,
C.; Cromwell, C.; Aledort, L.; Plaimauer, B.; Turecek, P.L.; et al.
Persistence of circulating ADAMTS13-specific immune complexes in
patients with acquired thrombotic thrombocytopenic purpura.
Haematologica 2014, 99, 779-787. https://doi.org/10.3324/haematol.2013.094151 PMid:24241492 PMCid:PMC3971089
- Mancini,
I.; Ferrari, B.; Valsecchi, C.; Pontiggia, S.; Fornili, M.; Biganzoli,
E.; Peyvandi, F.; Investigators, I.G.o.T. ADAMTS13- specific
circulating immune complexes as potential predictors of relapse in
patients with acquired thrombotic thrombocytopenic purpura. Eur. J.
Intern. Med. 2017, 39, 79-83 https://doi.org/10.1016/j.ejim.2016.11.003 PMid:27887777
- Westwood,
J.P.; Langley, K.; Heelas, E.; Machin, S.J.; Scully, M. Complement and
cytokine response in acute Thrombotic Thrombocytopenic Purpura. Br. J.
Haematol. 2014, 164, 858-866 https://doi.org/10.1111/bjh.12707 PMid:24372446 PMCid:PMC4155869
- Kosugi,
N.; Tsurutani, Y.; Isonishi, A.; Hori, Y.; Matsumoto, M.; Fujimura, Y.
Influenza A infection triggers thrombotic thrombocytopenic purpura by
producing the anti-ADAMTS13 IgG inhibitor. Intern. Med. 2010, 49,
689-693. https://doi.org/10.2169/internalmedicine.49.2957 PMid:20371960
- Franchini,
M. Thrombotic thrombocytopenic purpura: Proposal of a new pathogenic
mechanism involving Helicobacter pylori infection. Med. Hypotheses
2005, 65, 1128-1131. https://doi.org/10.1016/j.mehy.2005.06.015 PMid:16084670
- Talebi,
T.; Fernandez-Castro, G.; Montero, A.J.; Stefanovic, A.; Lian, E. A
case of severe thrombotic thrombocytopenic purpura with concomitant
Legionella pneumonia: Review of pathogenesis and treatment. Am. J.
Ther. 2011, 18, e180-e185. https://doi.org/10.1097/MJT.0b013e3181d1b4a1 PMid:20216382
- Yagita,
M.; Uemura, M.; Nakamura, T.; Kunitomi, A.; Matsumoto, M.; Fujimura, Y.
Development of ADAMTS13 inhibitor in a patient with hepatitis C
virus-related liver cirrhosis causes thrombotic thrombocytopenic
purpura. J. Hepatol. 2005, 42, 420-421. https://doi.org/10.1016/j.jhep.2004.08.030 PMid:15710227
- Gunther,
K.; Garizio, D.; Nesara, P. ADAMTS13 activity and the presence of
acquired inhibitors in human immunodeficiency virus-related thrombotic
thrombocytopenic purpura. Transfusion 2007, 47, 1710-1716. https://doi.org/10.1111/j.1537-2995.2007.01346.x PMid:17725738
- Boteju
M, Weeratunga P, Sivashangar A, Chang T. Cytomegalovirus induced
refractory TTP in an immunocompetent individual: a case report. BMC
Infect Dis. 2019 May 8;19(1):394.). https://doi.org/10.1186/s12879-019-4037-9 PMid:31068128 PMCid:PMC6507177
- Alhomoud M, Alhobay B, Armitage K COVID-19 infection triggering Thrombotic Thrombocytopenic Purpura IDCases 2021:26 https://doi.org/10.1016/j.idcr.2021.e01256 PMid:34458098 PMCid:PMC8383479
- Dias
PJ, Gopal S. Refractory thrombotic thrombocytopenic purpura following
influenza vaccination. Anaesthesia. 2009;64(4):444-446 https://doi.org/10.1111/j.1365-2044.2008.05823.x PMid:19317713
- Kojima
Y, Ohashi H, Nakamura T, et al.. Acute thrombotic thrombocytopenic
purpura after pneumococcal vaccination. Blood Coagul Fibrinolysis.
2014;25(5):512-514 https://doi.org/10.1097/MBC.0000000000000058 PMid:24469391
- Greinacher
A, Selleng K, Palankar R, et al.. Insights in chAdox1 nCoV-19
vaccine-induced immune thrombotic thrombocytopenia. Blood.
2021;138(22):2256-2268 https://doi.org/10.1182/blood.2021013231 PMid:34587242 PMCid:PMC8483989
- Alislambouli
M, Veras Victoria A, Matta J, Yin F. Acquired thrombotic
thrombocytopenic purpura following Pfizer COVID-19 vaccination. Eur J
Haematol. 2022;3(1):207-210. https://doi.org/10.1002/jha2.342 PMid:34909764 PMCid:PMC8657522
- Picod
A, Rebibou JM, Dossier A, et al.. Immune-mediated thrombotic
thrombocytopenic purpura following COVID-19 vaccination. Blood.
2022;139(16):2565-2569 https://doi.org/10.1182/blood.2021015149 PMid:35271696 PMCid:PMC8916825
- Shah H, Kim A, Sukumar S, et al.. sARS-CoV-2 vaccination and immune thrombocytopenic purpura. Blood. 2022;139(16):2570-2573 https://doi.org/10.1182/blood.2022015545 PMid:35259252 PMCid:PMC8906888
- Trisolini
SM Capria S Artoni A Mancini I Biglietto M Gentile G Peyvandi F and
Test AM Covid-19 vaccination in patients with immune-mediated
thrombotic thrombocytopenic purpura: a single-referral center
experience. Haematol 2023 Jul 1; 108(7): 1957-1959. https://doi.org/10.3324/haematol.2022.282311 PMid:36579445 PMCid:PMC10316277
- Capecchi
M, De Leo P, Abbatista M,Mancini I, Agosti P, Biganzoli M, Suffrritti
C, Ferrari B, LecchiA, La Marca S, Padovan L, Scalambrino E, Clerici M,
Tripodi A, Artoni A, Gualtierotti R, and Peyvandi F. Risk of relapse
after SARS-CoV-2 vaccine in the Milan cohort of thrombotic
thrombocytopenic purpura patients Haematol 2023 Nov 1; 108(11):
3152-3155. https://doi.org/10.3324/haematol.2022.282478 PMid:36951158 PMCid:PMC10620557
- Jang,
M.J.; Chong, S.Y.; Kim, I.H.; Kim, J.H.; Jung, C.W.; Kim, J.Y.; Park,
J.C.; Lee, S.M.; Kim, Y.K.; Lee, J.E.; et al. Clinical features of
severe acquired ADAMTS13 deficiency in thrombotic thrombocytopenic
purpura: The Korean TTP registry experience. Int. J.Hematol. 2011, 93,
163-169. https://doi.org/10.1007/s12185-011-0771-5 PMid:21287408
- Benhamou,
Y.; Boelle, P.Y.; Baudin, B.; Ederhy, S.; Gras, J.; Galicier, L.;
Azoulay, E.; Provôt, F.; Maury, E.; Pène, F.; et al. Cardiac troponin-I
on diagnosis predicts early death and refractoriness in acquired
thrombotic thrombocytopenic purpura. Experience of the French
Thrombotic Microangiopathies Reference Center. J. Thromb. Haemost.
2015, 13, 293-302
- Vesely,
S.K.; George, J.N.; Lämmle, B.; Studt, J.D.; Alberio, L.; El-Harake,
M.A.; Raskob, G.E. ADAMTS13 activity in thrombotic thrombocytopenic
purpura-hemolytic uremic syndrome: Relation to presenting features and
clinical outcomes in a prospective cohort of 142 patients. Blood 2003,
102, 60-68. https://doi.org/10.1182/blood-2003-01-0193 PMid:12637323
- Bell
WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic
thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience
in 108 patients. N Engl J Med 1991; 325:398-403. https://doi.org/10.1056/NEJM199108083250605 PMid:2062331
- Mackie
I, Mancini I, Muia J, Kremer Hovinga J, Nair S, Machin S, Baker R.
International Council for Standardization in Haematology (ICSH)
recommendations for laboratory measurement of ADAMTS13. Int J Lab
Hematol. 2020 Dec;42(6):685-696. https://doi.org/10.1111/ijlh.13295 PMid:32672897
- Coppo
P, Schwarzinger M, Buffet M, Wynckel A, Clabault K, Presne C, et al.
Predictive features of severe acquired ADAMTS13 deficiency in
idiopathic thrombotic microangiopathies: the French TMA reference
center experience. PLoS One 2010;5:e10208 https://doi.org/10.1371/journal.pone.0010208 PMid:20436664 PMCid:PMC2859048
- Bendapudi
P.K., Hurwitz S., Fry A., Marques M.B., Waldo S.W., Li A. Derivation
and external validation of the PLASMIC score for rapid assessment of
adults with thrombotic microangiopathies: a cohort study. Lancet
Haematol. 2017;4:157-164. https://doi.org/10.1016/S2352-3026(17)30026-1 PMid:28259520
- Li
A, Khalighi PR, Wu Q, Garcia DA. External validation of the PLASMIC
score: a clinical prediction tool for thrombotic thrombocytopenic
purpura diagnosis and treatment. J Thromb Haemost 2018;16:164-9 https://doi.org/10.1111/jth.13882 PMid:29064619 PMCid:PMC5760324
- Paydary
K, Banwell E, Tong J, Chen Y, Cuker A. Diagnostic accuracy of the
PLASMIC score in patients with suspected thrombotic thrombocytopenic
purpura: A systematic review and meta-analysis. Transfusion. 2020
Sep;60(9):2047-2057. https://doi.org/10.1111/trf.15954 PMid:32757237
- Joseph
A, Joly BS, Picod A, Veyradier A, Coppo P The Specificities of
Thrombotic Thrombocytopenic Purpura at Extreme Ages: A Narrative
Review. J Clin Med 2023 May; 12(9):3068 https://doi.org/10.3390/jcm12093068 PMid:37176509 PMCid:PMC10179719
- Zheng
XL, Vesely SK, Cataland SR, Coppo P, Geldziler B, Iorio A, Matsumoto M,
Mustafa RA, Pai M, Rock G, Russell L, Tarawneh R, Valdes J, Peyvandi F.
ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J
Thromb Haemost. 2020 Oct;18(10):2496-2502. https://doi.org/10.1111/jth.15010 PMid:32914526 PMCid:PMC8091490
- Rock,
GA; Shumak, K.H.; Buskard, N.A.; Blanchette, V.S.; Kelton, J.G.; Nair,
R.C.; Spasoff, R.A. Comparison of plasma exchange with plasma infusion
in the treatment of thrombotic thrombocytopenic purpura. Canadian
Apheresis Study Group. N. Engl. J.Med. 1991, 325, 393-397 https://doi.org/10.1056/NEJM199108083250604 PMid:2062330
- Coppo
P, Bussel A, Charrier S, Adrie C, Galicier L, Boulanger E, et al.
High-dose plasma infusion versus plasma exchange as early treatment of
thrombotic thrombocytopenic purpura/hemolytic-uremic syndrome. Medicine
(Baltimore) 2003;82:27-38 https://doi.org/10.1097/00005792-200301000-00003 PMid:12544708
- Scully
M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F, Cheung B, Machin
SJ; British Committee for Standards in Haematology. Guidelines on the
diagnosis and management of thrombotic thrombocytopenic purpura and
other thrombotic microangiopathies. Br J Haematol. 2012
Aug;158(3):323-35. https://doi.org/10.1111/j.1365-2141.2012.09167.x PMid:22624596
- Kathrin
Eller, Paul Knoebel, Sevcan A. Bakkaloglu, Jan J. Menne, Paul T.
Brinkkoetter, Leonie Grandt, Ursula Thiem, Paul Coppo, Marie Scully and
Maria C. Haller. European renal best practice endorsement of guidelines
for diagnosis and therapy of thrombotic thrombocytopenic purpura
published by the International Society on thrombosis and Haemostasis.
Nephrol Dial Transplant (2022) 37: 1229-1234 https://doi.org/10.1093/ndt/gfac034 PMid:35195251 PMCid:PMC9217651
- Balduini,
C.L.; Gugliotta, L.; Luppi, M.; Laurenti, L.; Klersy, C.; Pieresca, C.;
Quintini, G.; Iuliano, F.; Re, R.; Spedini, P.; et al. High versus
standard dose methylprednisolone in the acute phase of idiopathic
thrombotic thrombocytopenic purpura: A randomized study. Ann. Hematol.
2010, 89, 591-596 https://doi.org/10.1007/s00277-009-0877-5 PMid:20033409
- Callewaert
F, Roodt J, Ulrichts H, Stohr T, van Rensburg WJ, Lamprecht S, et al.
Evaluation of efficacy and safety of the anti-VWF Nanobody ALX-0681 in
a preclinical baboon model of acquired thrombotic thrombocytopenic
purpura. Blood 2012; 120:3603-10. https://doi.org/10.1182/blood-2012-04-420943 PMid:22948047
- Peyvandi
F, Scully M, Kremer Hovinga JA, Cataland S, Knobl ¨ P, Wu H, et al.
Caplacizumab for acquired thrombotic thrombocytopenic Purpura. N Engl J
Med 2016; 374:511-22. https://doi.org/10.1056/NEJMoa1505533 PMid:26863353
- Scully
M, Cataland SR, Peyvandi F, Coppo P, Knobl ¨ P, Kremer Hovinga JA, et
al. Caplacizumab treatment for acquired thrombotic thrombocytopenic
Purpura. N Engl J Med 2019; 380:335-46 https://doi.org/10.1056/NEJMoa1806311 PMid:30625070
- Flora
Peyvandi, Spero Cataland, Marie Scully, Paul Coppo, Paul Knoebl,
Johanna A. Kremer Hovinga, Ara Metjian, Javier de la Rubia, Katerina
Pavenski, Jessica Minkue Mi Edou, Hilde De Winter, Filip Callewaert;
Caplacizumab prevents refractoriness and mortality in acquired
thrombotic thrombocytopenic purpura: integrated analysis. Blood Adv
2021; 5 (8): 2137-2141. https://doi.org/10.1182/bloodadvances.2020001834 PMid:33881463 PMCid:PMC8095153
- Izquierdo
CP, Mingot-Castellano ME, Fuentes AEK, García-Arroba Peinado J, Cid J,
Jimenez MM, Valcarcel D, Gómez-Seguí I, de la Rubia J, Martin P,
Goterris R, Hernández L, Tallón I, Varea S, Fernández M, García-Muñoz
N, Vara M, Zarzoso MF, García-Candel F, Paciello ML, García-García I,
Zalba S, Campuzano V, Gala JM, Estévez JV, Jiménez GM, López Lorenzo
JL, Arias EG, Freiría C, Solé M, Ávila Idrovo LF, Hernández Castellet
JC, Cruz N, Lavilla E, Pérez-Montaña A, Atucha JA, Moreno Beltrán ME,
Moreno Macías JR, Salinas R, Del Rio-Garma J. Real-world effectiveness
of caplacizumab vs the standard of care in immune thrombotic
thrombocytopenic purpura. Blood Adv. 2022 Dec 27;6(24):6219-6227 https://doi.org/10.1182/bloodadvances.2022008028 PMid:35930694 PMCid:PMC9792393
- Albanell-Fernández
M, Monge-Escartín I, Carcelero-San Martín E, Riu Viladoms G, Ruiz-Boy
S, Lozano M, Soy D, Moreno-Castaño AB, Diaz-Ricart M, Cid J. Real-world
data of the use and experience of caplacizumab for the treatment of
acquired thrombotic thrombocytopenic purpura: Case series. Transfus
Apher Sci. 2023 Jun;62(3):103722. https://doi.org/10.1016/j.transci.2023.103722 PMid:37169697
- Sarode
R. Thrombotic thrombocytopenic purpura in caplacizumab era - An
individualized approach. Transfus Apher Sci. 2023 Apr;62(2):103682. https://doi.org/10.1016/j.transci.2023.103682 PMid:36890095
- Dutt
T, Shaw RJ, Stubbs M, Yong J, Bailiff B, Cranfield T, Crowley MP,
Desborough M, Eyre TA, Gooding R, Graiger J, Hanley J, Haughton J,
Hermans J, Hill Q, Humphrey L, Lowe G, Lyall H, Mohsin M, Nicolson PLR,
Pridde N, Rampotas A, Rayment R, Rhodes S, Taylor A, Thomas W, Tomkins
O, Van Veen JJ, Lane S, Toh CH, Scully M Real-world experience with
caplacizumab in the management of acute TTP Blood 2021 Apr
1;137(13):1731-1740 doi : 10.1182/blood.2020007599. https://doi.org/10.1182/blood.2020007599 PMid:33150355
- Coppo
P, Bubenheim M, Azoulay E, Galicier L, Malot S, Big'e N, et al. A
regimen with caplacizumab, immunosuppression, and plasma exchange
prevents unfavorable outcomes in immune-mediated TTP. Blood
2021;137:733-42. https://doi.org/10.1182/blood.2020008021 PMid:33150928 PMCid:PMC7986049
- Silvia
Riva, Ilaria Mancini, Alberto Maino, Barbara Ferrari, Andrea Artoni,
Pasquale Agosti and Flora Peyvandi. Long-term neuropsychological
sequelae, emotional wellbeing and quality of life in patients with
acquired thrombotic thrombocytopenic purpura. Heamatologica 2020
Jul;105(7):1957-1962. https://doi.org/10.3324/haematol.2019.226423 PMid:31558667 PMCid:PMC7327631
- Bowyer
A, Brown P, Hopkins B, Scully M, Shepherd F, Lowe A, Mensah P, Maclean
R, Kitchen S, van Veen JJ. Von Willebrand factor assays in patients
with acquired immune thrombotic thrombocytopenia purpura treated with
caplacizumab. Br J Haematol. 2022 May;197(3):349-358. https://doi.org/10.1111/bjh.18080 PMid:35262910
- Mazepa
MA, Masias C, Chaturvedi S. How targeted therapy disrupts the treatment
paradigm for acquired TTP: the risks, benefits, and unknowns. Blood.
2019 Aug 1;134(5):415-420. https://doi.org/10.1182/blood.2019000954 PMid:31217190
- Völker
LA, Kaufeld J, Miesbach W, Brähler S, Reinhardt M, Kühne L, Mühlfeld A,
Schreiber A, Gaedeke J, Tölle M, Jabs WJ, Özcan F, Markau S, Girndt M,
Bauer F, Westhoff TH, Felten H, Hausberg M, Brand M, Gerth J, Bieringer
M, Bommer M, Zschiedrich S, Schneider J, Elitok S, Gawlik A, Gäckler A,
Kribben A, Schwenger V, Schoenermarck U, Roeder M, Radermacher J,
Bramstedt J, Morgner A, Herbst R, Harth A, Potthoff SA, von Auer C,
Wendt R, Christ H, Brinkkoetter PT, Menne J. ADAMTS13 and VWF
activities guide individualized caplacizumab treatment in patients with
aTTP. Blood Adv. 2020 Jul 14;4(13):3093-3101. https://doi.org/10.1182/bloodadvances.2020001987 PMid:32634237 PMCid:PMC7362349
- Cuker
A, Cataland SR, Coppo P, de la Rubia J, Friedman KD, George JN, Knoebl
PN, Kremer Hovinga JA, Lämmle B, Matsumoto M, Pavenski K, Peyvandi F,
Sakai K, Sarode R, Thomas MR, Tomiyama Y, Veyradier A, Westwood JP,
Scully M. Redefining outcomes in immune TTP: an international working
group consensus report. Blood. 2021 Apr 8;137(14):1855-1861. https://doi.org/10.1182/blood.2020009150 PMid:33529333
- Swisher
KK, Terrell DR, Vesely SK, Kremer Hovinga JA, Lämmle B, George JN.
Clinical outcomes after platelet transfusions in patients with
thrombotic thrombocytopenic purpura. Transfusion. 2009
May;49(5):873-87. https://doi.org/10.1111/j.1537-2995.2008.02082.x PMid:19210323
- McDonald
V, Manns K, Mackie IJ, Machin SJ, Scully MA. Rituximab pharmacokinetics
during the management of acute idiopathic thrombotic thrombocytopenic
purpura. J Thromb Haemost. 2010 Jun;8(6):1201-8. https://doi.org/10.1111/j.1538-7836.2010.03818.x PMid:20175870
- Kremer
Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K.
Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017 Apr
6;3:17020. https://doi.org/10.1038/nrdp.2017.20 PMid:28382967
- Scully
M, McDonald V, Cavenagh J, Hunt BJ, Longair I, Cohen H, Machin SJ. A
phase 2 study of the safety and efficacy of Rituximab with plasma
exchange in acute acquired thrombotic thrombocytopenic purpura. Blood.
2011 Aug 18;118(7):1746-53. https://doi.org/10.1182/blood-2011-03-341131 PMid:21636861
- Owattanapanich
W, Wongprasert C, Rotchanapanya W, Owattanapanich N, Ruchutrakool T.
Comparison of the long-term remission of Rituximab and conventional
treatment for acquired thrombotic thrombocytopenic Purpura: a
systematic review and meta-analysis. Clin Appl Thromb Hemost 2019;25 https://doi.org/10.1177/1076029618825309 PMid:30808221 PMCid:PMC6714958
- Aksoy
S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundag K, Barista
I. Rituximab-related viral infections in lymphoma patients. Leuk
Lymphoma. 2007 Jul;48(7):1307-12. https://doi.org/10.1080/10428190701411441 PMid:17613758
- Laganà
A. Trisolini SM, Capria S True vs. false immune-mediated thrombotic
thrombocytopenic purpura exacerbations: a clinical case in the
caplacizumab era. Blood Coagulation and Fibrinol 35(1):p 37-42, Jan
2024 https://doi.org/10.1097/MBC.0000000000001266 PMid:37994623
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000 May;47(2-3):85-118. https://doi.org/10.1016/S0162-3109(00)00188-0 PMid:10878285
- Moake
JL, Rudy CK, Troll JH, Schafer AI, Weinstein MJ, Colannino NM, Hong SL.
Therapy of chronic relapsing thrombotic thrombocytopenic purpura with
prednisone and azathioprine. Am J Hematol. 1985 Sep;20(1):73-9. https://doi.org/10.1002/ajh.2830200110 PMid:3875285
- Nosari
A, Redaelli R, Caimi TM, Mostarda G, Morra E. Cyclosporine therapy in
refractory/relapsed patients with thrombotic thrombocytopenic purpura.
Am J Hematol 2009; 84:313-4. https://doi.org/10.1002/ajh.21385
PMid:19306354
- Cataland
SR, Jin M, Lin S, Kraut EH, George JN, Wu HM. Effect of prophylactic
cyclosporine therapy on ADAMTS13 biomarkers in patients with idiopathic
thrombotic thrombocytopenic purpura. Am J Hematol. 2008
Dec;83(12):911-5 https://doi.org/10.1002/ajh.21281 PMid:18821711 PMCid:PMC2824143
- Zappasodi
P, Corso A, Castagnola C, Tajana M, Lunghi M, Bernasconi C. A
successful combination of plasma exchange and intravenous
cyclophosphamide in a patient with a refractory thrombotic
thrombocytopenic purpura. Eur J Haematol. 1999 Oct;63(4):278-9 https://doi.org/10.1111/j.1600-0609.1999.tb01893.x PMid:10530421
- Ahmadpoor
P, Aglae C, Garo F, Cariou S, Renaud S, Reboul P, Moranne O. Humanized
anti CD-20 as an alternative in chronic management of relapsing
thrombotic thrombocytopenic microangiopathy resistant to Rituximab due
to anti chimeric antibody. Int J Hematol. 2021 Mar;113(3):456-460. https://doi.org/10.1007/s12185-020-03020-7 PMid:33067738
- Doyle
AJ, Stubbs MJ, Lester W, Thomas W, Westwood JP, Thomas M, Percy C,
Prasannan N, Scully M. The use of obinutuzumab and ofatumumab in the
treatment of immune thrombotic thrombocytopenic purpura. Br J Haematol.
2022 Jul;198(2):391-396. https://doi.org/10.1111/bjh.18192 PMid:35430727
- Shortt
J, Oh DH, Opat SS. ADAMTS13 antibody depletion by bortezomib in
thrombotic thrombocytopenic purpura. N Engl J Med. 2013 Jan
3;368(1):90-2 https://doi.org/10.1056/NEJMc1213206 PMid:23281998
- Van
den Berg J, Kremer Hovinga JA, Pfleger C, Hegemann I, Stehle G, Holbro
A, Studt JD. Daratumumab for immune thrombotic thrombocytopenic
purpura. Blood Adv. 2022 Feb 8;6(3):993-997. https://doi.org/10.1182/bloodadvances.2021005124 PMid:34551063 PMCid:PMC8945322
- Tse
B, Lim G, Sholzberg M, Pavenski K. Describing the point prevalence and
characteristics of venous thromboembolism in patients with thrombotic
thrombocytopenic purpura. J Thromb Haemost. 2020 Nov;18(11):2870-2877. https://doi.org/10.1111/jth.15027 PMid:33448602
- Dutt
T, Shaw RJ, Stubbs M, Yong J, Bailiff B, Cranfield T, Crowley MP,
Desborough M, Eyre TA, Gooding R, Grainger J, Hanley J, Haughton J,
Hermans J, Hill Q, Humphrey L, Lowe G, Lyall H, Mohsin M, Nicolson PLR,
Priddee N, Rampotas A, Rayment R, Rhodes S, Taylor A, Thomas W, Tomkins
O, Van Veen JJ, Lane S, Toh CH, Scully M. Real-world experience with
caplacizumab in the management of acute TTP. Blood. 2021 Apr
1;137(13):1731-1740. https://doi.org/10.1182/blood.2020007599 PMid:33150355
- Tuğrul
Elverdi, Melis Dila Özer Çerme, Tahacan Aydın & Ahmet Emre
Eşkazan. Do patients with immune-mediated thrombotic thrombocytopenic
purpura receiving caplacizumab need antithrombotic therapy?, Expert
Review of Clinical Pharmacology, 14:10, 1183-1188 https://doi.org/10.1080/17512433.2021.1944102 PMid:34130583
- Selvakumar
S, Liu A, Chaturvedi S. Immune thrombotic thrombocytopenic purpura:
Spotlight on long-term outcomes and survivorship. Front Med (Lausanne).
2023 Feb 28; 10:1137019. https://doi.org/10.3389/fmed.2023.1137019 PMid:36926315 PMCid:PMC10011081
- Peyvandi
F, Lavoretano S, Palla R, Feys HB, Vanhoorelbeke K, Battaglioli T,
Valsecchi C, Canciani MT, Fabris F, Zver S, Réti M, Mikovic D, Karimi
M, Giuffrida G, Laurenti L, Mannucci PM. ADAMTS13 and anti-ADAMTS13
antibodies as markers for recurrence of acquired thrombotic
thrombocytopenic purpura during remission. Haematologica. 2008
Feb;93(2):232-9. https://doi.org/10.3324/haematol.11739 PMid:18223285
- Cuker A. Adjuvant rituximab to prevent TTP relapse. Blood. 2016 Jun 16;127(24):2952-3 https://doi.org/10.1182/blood-2016-04-710475 PMid:27313327
- Westwood
JP, Thomas M, Alwan F, McDonald V, Benjamin S, Lester WA, Lowe GC, Dutt
T, Hill QA, Scully M. Rituximab prophylaxis to prevent thrombotic
thrombocytopenic purpura relapse: outcome and evaluation of dosing
regimens. Blood Adv. 2017 Jun 26;1(15):1159-1166. https://doi.org/10.1182/bloodadvances.2017008268 PMid:29296757 PMCid:PMC5728327
- Zheng,
X.L.; Vesely, S.K.; Cataland, S.R.; Coppo, P.; Geldziler, B.; Iorio,
A.; Matsumoto, M.; Mustafa, R.A.; Pai, M.; Rock, G.; et al. Good
practice statements (GPS) for the clinical care of patients with
thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2020
Oct;18(10):2503-12 https://doi.org/10.1111/jth.15009 PMid:32914535 PMCid:PMC7880820
- Page
EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Rituximab
reduces risk for relapse in patients with thrombotic thrombocytopenic
purpura. Blood. 2016 Jun 16;127(24):3092-4. https://doi.org/10.1182/blood-2016-03-703827 PMid:27060171
- Jestin
M, Benhamou Y, Schelpe AS, Roose E, Provôt F, Galicier L, Hié M, Presne
C, Poullin P, Wynckel A, Saheb S, Deligny C, Servais A, Girault S,
Delmas Y, Kanouni T, Lautrette A, Chauveau D, Mousson C, Perez P,
Halimi JM, Charvet-Rumpler A, Hamidou M, Cathébras P, Vanhoorelbeke K,
Veyradier A, Coppo P; French Thrombotic Microangiopathies Reference
Center. Preemptive Rituximab prevents long-term relapses in
immune-mediated thrombotic thrombocytopenic purpura. Blood. 2018 Nov
15;132(20):2143-2153. https://doi.org/10.1182/blood-2018-04-840090 PMid:30201758
- Upreti
H, Kasmani J, Dane K, Braunstein EM, Streiff MB, Shanbhag S, Moliterno
AR, Sperati CJ, Gottesman RF, Brodsky RA, Kickler TS, Chaturvedi S.
Reduced ADAMTS13 activity during TTP remission is associated with
stroke in TTP survivors. Blood. 2019 Sep 26;134(13):1037-1045. https://doi.org/10.1182/blood.2019001056 PMid:31431443 PMCid:PMC7022317
- Roose
E, Schelpe AS, Tellier E, Sinkovits G, Joly BS, Dekimpe C, Kaplanski G,
Le Besnerais M, Mancini I, Falter T, Von Auer C, Feys HB, Reti M,
Rossmann H, Vandenbulcke A, Pareyn I, Voorberg J, Greinacher A,
Benhamou Y, Deckmyn H, Fijnheer R, Prohászka Z, Peyvandi F, Lämmle B,
Coppo P, De Meyer SF, Veyradier A, Vanhoorelbeke K. Open ADAMTS13,
induced by antibodies, is a biomarker for subclinical immune-mediated
thrombotic thrombocytopenic purpura. Blood. 2020 Jul 16;136(3):353-361 https://doi.org/10.1182/blood.2019004221 PMid:32356859
- Scully
M, Knöbl P, Kentouche K, Rice L, Windyga J, Schneppenheim R, Kremer
Hovinga JA, Kajiwara M, Fujimura Y, Maggiore C, Doralt J, Hibbard C,
Martell L, Ewenstein B. Recombinant ADAMTS-13: first-in-human
pharmacokinetics and safety in congenital thrombotic thrombocytopenic
purpura. Blood. 2017 Nov 9;130(19):2055-2063. https://doi.org/10.1182/blood-2017-06-788026 PMid:28912376 PMCid:PMC5680611

