Alessandro Laganà1, Giovanni Manfredi Assanto1, Chiara Masucci1, Mauro Passucci1, Livia Donzelli1, Alessandra Serrao2, Erminia Baldacci1, Cristina Santoro1 and Antonio Chistolini1.
1 Hematology, Department of Translational and Precision Medicine, Sapienza University of Rome, Italy
2 Haematology and Stem Cell Transplant Unit, A. O. San Camillo Forlanini (L.R.), Rome, Italy.
Correspondence to:
Antonio Chistolini. Hematology Department of Translational and
Precision Medicine Sapienza University of Rome Italy. Via Benevento 6,
00161 Rome, Italy. Tel. +390649974778, Fax +390644241984. E-mail:
antonio.chistolini@uniroma1.it
Published: March 01, 2024
Received: November 21, 2023
Accepted: February 08, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024020 DOI
10.4084/MJHID.2024.020
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Direct oral anticoagulants (DOACs) are widely used for the treatment
and secondary prophylaxis of venous thromboembolism (VTE). Nowadays,
DOACs represent the gold standard for long-term anticoagulation, with
low-intensity DOACs administration becoming increasingly used worldwide
in such scenario. Albeit low-intensity apixaban and rivaroxaban are
approved for clinical usage as secondary VTE prophylaxis, there are few
literature data regarding their efficacy and safety with a long
follow-up.
Objectives: The
aim of our study was to evaluate the efficacy and safety of low-dose
DOACs for VTE secondary prophylaxis in patients at high risk of VTE
recurrence.
Methods: We
retrospectively evaluated patients who required long-term anticoagulant
secondary prophylaxis to prevent recurrent VTE, treated with apixaban
2.5 mg BID or rivaroxaban 10 mg daily with a follow-up ≥ 12 months.
Results: The
examined patients were 323. The median low-dose DOAC administration
time was 25.40 months (IQR 13.93-45.90). Twelve (3.7%) VTE recurrences
were observed; 21 bleeding events were registered (6.5%), including one
episode of Major bleeding (MB) (0.3%), 8 Clinically relevant nonmajor
bleeding (CRNMB) (2.5%) and 12 minor bleeding (3.7%). No statistically
significant difference in the rate of VTE recurrence and/or bleeding
events emerged between the rivaroxaban and apixaban groups. Patients
included in the study for multiple episodes of VTE presented a
significantly higher risk of a new VTE recurrence during low-intensity
DOAC.
Conclusions: Our
data suggest that low-dose DOACs may be effective and safe in secondary
VTE prophylaxis in patients at high risk of VTE recurrence; however,
attention might be needed in their choice in such a scenario for
patients who experienced multiple episodes of VTE.
|
Introduction
Venous
thromboembolism (VTE), which includes deep vein thrombosis (DVT) and
pulmonary embolism (PE), is the third most common cause of death
worldwide.[1] Its estimated incidence is around 1 to 2 per 1,000 people annually in the general population.[2]
Direct
oral anticoagulants (DOACs) represent a more convenient and often safer
treatment option for VTE prevention or treatment compared to vitamin-K
antagonists or heparins.[3-7] Nowadays, DOACs
represent the gold standard for long-term anticoagulation and, as well,
are used for stroke prevention in patients with not valvular atrial
fibrillation (NVAF)[8] and extended-duration treatment (secondary prevention) of VTE.[9-11]
In particular, DOACs are the cornerstone for VTE secondary prophylaxis,
and nowadays, there is an increasing tendency to perform such
prophylaxis with low-dose DOAC administration. To date, only apixaban
and rivaroxaban are approved for clinical usage for low-intensity DOAC
secondary prophylaxis. The AMPLIFY-EXT trial[9] led to the FDA approval
for low-intensity apixaban for extended-duration treatment. As for rivaroxaban, the EINSTEIN-CHOICE trial[11] assessed
once-daily Rivaroxaban (at doses of 20 mg or 10 mg) vs 100 mg of
aspirin doses for VTE extended-duration treatment. The risk of a
recurrent event was significantly lower with Rivaroxaban at either a 20
mg or 10 mg dose than with aspirin, without a significant increase in
bleeding rates, with consequent FDA approval for low-intensity
Rivaroxaban in this scenario.
As secondary prophylaxis, DOAC
treatment may be administered lifelong to some patients; however, few
data are available concerning the efficacy and safety of low-intensity
DOAC extended-duration treatment in a long follow-up.
Herein, we
report our single-center experience with low-intensity DOACs as
secondary VTE prophylaxis in non-oncological patients with a high risk
of VTE recurrence. In particular, the study was focused on evaluating
the efficacy and safety of low-intensity DOACs in patients with a
follow-up≥ 12 months under this treatment regimen (median follow-up of
25.4 months).
Methods
Study design.
In this single-center, non-randomized, observational, retrospective
study, we evaluated the efficacy and safety of low-intensity apixaban
or rivaroxaban used in the context of VTE extended-duration secondary
prophylaxis. In particular, we collected data related to all our
patients receiving a low-dose DOAC, focusing on the evaluation of cases
with a follow-up ≥ 12 months. The study was conducted in compliance
with Institutional Review Board/Human Subjects Research Committee
requirements. All patients signed the informed consent to the treatment
and the use of their clinical data for scientific purposes.
Patients
(≥ 18 years) were eligible for the study if they had an objectively
confirmed, provoked, or unprovoked proximal deep-vein thrombosis,
pulmonary embolism, or both. The extended prophylaxis with a low dose
of apixaban or rivaroxaban was started because patients were considered
at high risk of VTE recurrence because of unprovoked VTE, recurrence of
VTE, residual vein obstruction (RVO), presence of a permanent inferior
vena cava filter, VTE with major thrombophilia, defined by congenital
deficiency of antithrombin (AT), Protein C or S (PC or PS), homozygous
Factor V (FV) Leiden or Factor II (FII) G20210A or combined
heterozygous FV Leiden and FII G20210A.
Exclusion criteria were:
subjects with active cancer (solid or hematological); cardiac
mechanical valve; active bleeding or high risk for bleeding
contraindicating DOAC treatment; active and clinically significant
liver disease (Child-Pugh B or C); ALT or AST>2 times the upper
limit of normal; total bilirubin >1.5 times the upper limit of
normal (unless an alternative causative factor is identified [e.g.,
Gilbert's syndrome]); platelet count < 50x109/L;
serum creatinine >2.5 mg/dL (221 umol/L) or calculated creatinine
clearance < 30 mL/min; subjects meeting the criteria for
antiphospholipid syndrome (APS).
The acute VTE phase was treated
(for at least 3 months) with low molecular weight heparin (LMWH),
vitamin K antagonists (AVKs), or DOACs, depending on recommendations in
force at the time of VTE event and on the clinician's choice. The
extended secondary prophylaxis was started with low-intensity apixaban
2.5 mg BID or rivaroxaban 10 mg daily: the choice of the specific used
DOAC was based on the clinician's choice.
Assessments.
Patients underwent clinical assessment every 3-6 months during the
treatment period. Patients were monitored for symptoms suggestive of
recurrent VTE or bleeding. The assessment was on an outpatient basis
with control of blood tests (including at least full blood count,
liver, and kidney function) and imaging controls [computerized
tomography (CT) or ultrasound (US)] when clinically appropriate.
Outcome Measures.
The primary endpoint was the symptomatic recurrence of VTE, which has
been employed in trials reporting extended treatment of VTE.[12] Recurrent VTE included fatal and nonfatal PE and DVT.
The
primary safety outcome was major bleeding (MB). The secondary safety
outcome was the composite of major or clinically relevant nonmajor
bleeding (CRNMB). Major bleeding was defined as overt bleeding that was
associated with a decrease in the hemoglobin level of 2 g per deciliter
or more, led to transfusion of 2 or more units of red cells, occurred
in a critical site, or contributed to death. CRNMB was defined as overt
bleeding that did not meet the criteria for major bleeding, but that
was associated with the need for medical intervention, unscheduled
contact with a physician, or discomfort or impairment of activities of
daily living.[9]
We evaluated a general
event-free-survival (EFS) considering as event a bleeding or thrombotic
episode, a thrombotic event-free-survival (tEFS), and hemorrhagic
event-free-survival (bEFS) during low-dose therapy.
Statistical analysis.
Descriptive statistics, such as the frequency (n), arithmetic mean,
median, range, and standard deviation (SD), are presented for normally
distributed variables. Differences in terms of bleeding and thrombotic
events between groups were evaluated with univariate logistic
regression. The chi-square test was used for categorical variables, and
the T student or Mann-Whitney U-test was used for continuous variables.
The odds ratio (OR) for each independent variable was determined with a
confidence interval (CI) of 95%. Kaplan-Meier curves and survival
tables were also employed to assess the difference in terms of
thrombotic and bleeding adverse event-free survival (t-EFS or b-EFS),
considered as the time range between the start of low dose DOACs and
the occurrence of AE. Log-rank Mantel-Cox on Kaplan-Meyer curves was
used to assess statistical significance. A p-value of 0.05 was
considered significant. A receiver operating characteristic (ROC) curve
was performed on continuous variables to determine a cut-off predictive
of increased adverse event incidence.
Results
We
selected a total of 447 patients that were receiving low-dose DOAC
treatment. Our analysis has been focused on the 323 non-oncologic
patients who were reaching a follow-up ≥ 12 months. Patients’
characteristics are shown in Table 1.
 |
- Table
1. Low-dose DOACs Patients characteristics with a follow-up ≥12 months.
|
Median
age was 56.5 years (IQR 44.60-70.59), 191 (59.1 %) were male, and 132
(40.9%) were female. Two-hundred-ten (65.0%) patients presented with
isolated proximal DVT, 16 (5.0%) with isolated PE, and 97 (30.0%) with
both proximal DVT and PE. During the VTE acute phase, 97 (30%) patients
were treated with AVKs or LMWH, and 226 (70.0%) were treated with full
doses of DOACs. All patients were considered at high risk of VTE
recurrence and switched to extended treatment low-intensity DOACs for
recurrent VTE 145 (44.9%), unprovoked thrombosis event 74 (22.9%),
thromboembolic events in patients affected by major hereditary
thrombophilia 55 (17.0%), RVO after acute phase VTE treatment 38
(11.8%) and permanent Inferior Vena Cava Filter in situ 11 patients
(3.4%). The 55 patients with major thrombophilia were represented by 5
patients (9.1%) with AT deficiency, 5 (9.1%) with PC deficiency, 18
(32.7%) with PS deficiency, 3 (5.5%) with homozygous F II G20210A, 13
(23.6%) with homozygous FVL, and 11 (20%) patients with combined
heterozygous FVL and F II G20210A.
During low dose, 135 patients
(41.8%) received rivaroxaban and 188 (58.2%) apixaban. The median
low-dose DOAC administration time was 25.40 months (IQR 13.93-45.90).
During
low dose, 12 (3.7%) VTE recurrence events were observed after a median
low-dose treatment of 27.68 months (IQR 17.0-47.3). These patients were
switched on to full-dose DOAC therapy. The low-dose DOAC thrombotic
event-free-survival (tEFS) was 99.4% at 1 year, 98.1% at 2 years, and
96.9% at 5 years, as shown in Figure 1A.
Four patients died, but no death was VTE-related: 3 patients died
because of old age, and one patient's death was caused by SARS-CoV-2
pneumonia. No statistically significant difference in the rate of VTE
recurrence emerged between the rivaroxaban and apixaban groups (1/135
vs. 11/188), as shown in Figure 2
(99.3% vs. 94.1% - p=0.176). We analyzed tEFS according to the reason
for low dose DOAC start, and patients with multiple episodes of VTE
presented a significantly higher risk of a new VTE recurrence during
low-intensity DOAC (p=0.03 – Figure 3)
(11/12 events verified in this subgroup). In this subgroup, the mean
number of previous thrombosis was 2.3 (range 2-5), with unprovoked VTE
representing 64% of the episodes, while 22% were provoked episodes; the
presence of a provoking factor was not clear in the other cases. For
the 11 patients developing VTE with low-dose DOACs, all 11 (100%)
thrombotic events were represented by unprovoked VTEs.
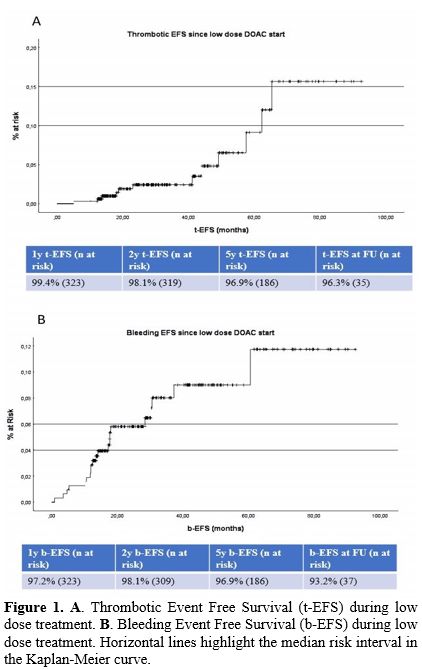 |
Figure
1. A. Thrombotic Event Free Survival (t-EFS) during low dose treatment. B.
Bleeding Event Free Survival (b-EFS) during low dose treatment.
Horizontal lines highlight the median risk interval in the Kaplan-Meier
curve. |
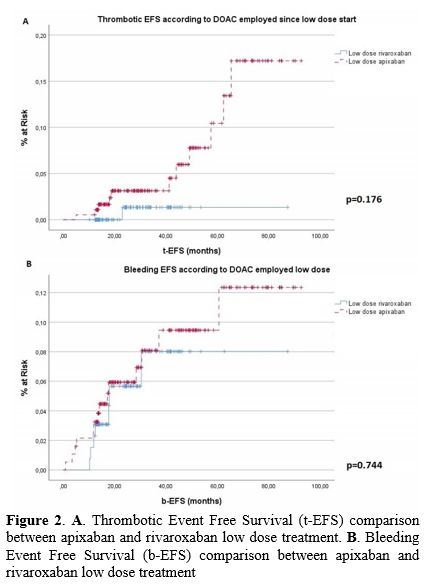 |
Figure
2. A. Thrombotic Event Free Survival (t-EFS) comparison between apixaban and rivaroxaban low dose treatment. B. Bleeding Event Free Survival (b-EFS) comparison between apixaban and rivaroxaban low dose treatment |
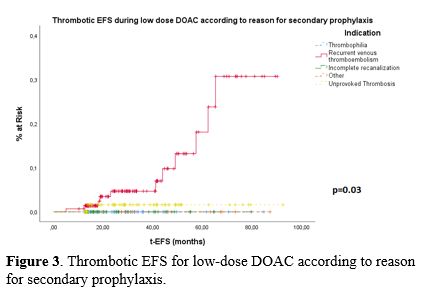 |
Figure 3. Thrombotic EFS for low-dose DOAC according to reason for secondary prophylaxis.
|
During low dose, one
MB (after 11.7 months of low-dose apixaban treatment) was observed
(0.3%), represented by rectal bleeding requiring one blood transfusion
and apixaban withdrawal for 5 days. In total 21 patients (6.5%) had a
bleeding event during low dose after a median treatment period of 13.6
months (IQR 10.6-17.9): one MB (0.3%), 8 CRNMB (2.5%) and 12 minor
bleeding (3.7%). Three CRNMB (0.9%) were registered within the first
year; the other 5 CRNMB (1.5%) after more than one year. Two patients
presented with a CRNMB required low-dose withdrawal up to a maximum of
3 days. The low-dose DOAC bleeding event-free-survival (bEFS) was 97.2%
at 1 year, 98.1% at 2 years, and 96.9% at 5 years, as shown in Figure 1B.
No statistically significant difference in the rate of bleeding events
emerged between the rivaroxaban and apixaban groups (7/135 vs. 14/188),
as shown in Figure 2 (94.7%
vs. 92.6% - p=0.744). A significant correlation was found between
previous estrogenic-progestin therapy and b-EFS (94.5% vs 66.7% -
p=0.0001).
During follow-up, 33 patients, after a median low-dose
treatment of 20.35 months (IQR 14.02 – 29.45), interrupted low-dose
DOAC secondary prophylaxis due to complete venous recanalization and,
therefore, not being considered at high risk of VTE recurrence anymore.
None of these 33 patients experienced a VTE recurrence after low-dose
DOAC withdrawal.
Discussion
The
current study analyzed data collected in our center regarding long-term
treatment of patients with a VTE episode managed in real-life
conditions with low-dose DOACs.
International guidelines
recommend extending anticoagulation for secondary prevention to
patients at high risk of VTE recurrence;[13]
nevertheless, selecting which patients may need long-term anticoagulant
secondary prophylaxis is a complex clinical decision. Such guidelines,
in patients selected for extended-phase anticoagulation, suggest the
use of reduced-dose over full-dose of apixaban or rivaroxaban.[13] Since low-intensity DOAC FDA approval (2013 for apixaban 2.5 mg BID and 2017 for rivaroxaban 10 mg),[9,11]
these doses for apixaban and rivaroxaban have been extensively used
worldwide. Compared to their extensive usage in clinical practice,
literature data regarding low-intensity DOAC efficacy and safety in
real life are quite scant. Hence, our objective was to provide data on
long-term low-intensity DOAC efficacy and safety, which are lacking in
pivotal studies such as the AMPLIFY-EXT trial[9] and the EINSTEIN-CHOICE trial.[11]
In fact, secondary prophylaxis was given for up to 12 months in both
trials. In our study, we report a median extended administration of 2
years, which is, to our knowledge, the longest period reported.
In the AMPLIFY-EXT trial,[9]
recurrent VTE or death related to VTE occurred in 14 patients (1.7%)
receiving 2.5/5 mg of apixaban; MBs were registered in 0.15% of
patients, as well, in the EINSTEIN-CHOICE trial;[11]
patients receiving 10 mg rivaroxaban had similar VTE recurrence rate to
those receiving 20 mg (1.2% vs 1.5%), moreover, MB rate was
superimposable (0.4% vs 0.5%).
Our data compare favorably with
these findings: we report a VTE recurrence rate of 0.6% at 1 year and
1.9% at 2 years, confirming the efficacy of low doses on a longer time
frame. As well, the bleeding event rate was low, with only one MB and
2.5% of CRNMB rate, confirming the efficacy of the reduced schedule.
A
systematic review and meta-analysis of studies on patients presenting
with an unprovoked VTE reported a rate of VTE recurrence of 10.1% at
one year after anticoagulation therapy discontinuation.[14]
In
2022, our group evaluated patients requiring long-term anticoagulation
for recurrent VTE, receiving DOACs as per VTE secondary prophylaxis.[15]
In this retrospective, single-center study, 209 patients were included.
DOACs were administered for a median time of 20 months, including VTE
acute phase treatment; 157 patients continued DOACs at full dosage
(75%), 52 (25%) switched to apixaban 2.5 mg BID or rivaroxaban 10 mg
daily after a median time of 13 months. VTE recurrence occurred in
7/209 (3.1%), with an average time of occurrence of 18 months. Bleeding
events occurred in 25/209 (12%) patients with an average time of
occurrence of 9 months. Among the patients treated with low-intensity
DOACs, the VTE recurrence rate was 5.7%; the hemorrhagic event rate was
11.5%. In the present paper, expanding the sample size, follow-up, and
including different patients in this experience, we observed a better
outcome.
Recently, Palareti et al.[16] (WHITE
study) evaluated clinicians’ decisions and clinical events occurring
during at least one year of follow-up during maintenance treatment
following diagnosis of a first-ever DVT and PE event. In this
international, prospective, observational study, the type, dose, and
duration of patient treatment were left to the attending physician’s
discretion. Hence, many different drugs were used as secondary
prophylaxis, with DOACs representing the most frequently prescribed
drugs for extended treatment despite not reporting DOACs' type and
dosing. Outcome assessment was performed on 715 patients on follow-up
who were compliant with the treatment prescribed by the participant
clinical centers. Overall, across a median follow-up of 17.47 months, a
total of 40 venous thrombotic recurrences (5.6%) were registered.
Bleeding complications were represented by 5 MB (0.7%) and 5 CRNMB
(0.7%) (MB + CRNMB – 1.4%). A sub-analysis of the 310 evaluable
patients who received DOACs as secondary prophylaxis was performed.
After a median follow-up of 16.49 months, 17 VTE recurrences (5.5%), 0
MB (0.0%), and 3 CRNMB (1.0%) were registered. We report a similar
cohort with longer follow-up, including patients treated with low doses
exclusively, and also, in this case, our data on thrombotic and
bleeding outcomes are comparable.
In our real-life study, the
efficacy and safety of DOACs in the secondary prophylaxis of VTE seem
to be comparable or even with a better outcome than studies published
on DOAC's long-term administration. In particular, the overall 3.7%
incidence of VTE recurrence seems lower than the VTE recurrence rate
registered in other studies, regardless of dosing. The bleeding outcome
is comparable. A correlation was observed between b-EFS and
estrogen-progestin therapy, probably due to increased gynecological
bleeding risk in women requiring such treatment.
Therefore, given
our long median follow-up (25.4 months), considering that just patients
receiving low-dose DOACs as secondary prophylaxis were included, we can
suggest that apixaban 2.5 mg BID or rivaroxaban 10 mg daily seem to be
effective in reducing the risk of VTE recurrence in high-risk patients
compared to anticoagulation therapy discontinuation and seem to be not
inferior to full-dose DOACs in such scenario. We want to highlight that
the patients included in the study for multiple episodes of VTE
presented a significantly higher risk of a new VTE recurrence during
low-intensity DOAC, warning about the use of such a schedule in this
subset of patients, in which probably thrombotic predisposition is
sustained by not well-defined mechanisms which limit DOACs' efficacy.
The bleeding incidence in our study was 6.5%, including 12 minor
bleeding (3.7%). These data are comparable to the general bleeding rate
reported in the literature with low-intensity DOACs, pinpointing that
low-dose DOAC administration may reduce bleeding adverse events in
patients requiring secondary VTE prophylaxis.
Nevertheless,
our study presents some limitations. The first limitation is
represented by its retrospective nature; data were collected in a
single center; moreover, it was an observational study, and all the
treatment decisions were left to the attending physician. For all these
reasons, the interpretation of our findings requires caution and
further studies. A prospective study with extended low-dose DOACs could
be designed with defined practical guidelines and randomized DOACs to
overcome such limitations.
Conclusions
Based
on this retrospective observation, administration of low-intensity
DOACs (apixaban 2.5 mg BID or rivaroxaban 10 mg daily) in long-term
secondary VTE prophylaxis for high-risk patients is effective and safe
in the real-world setting. Further studies should investigate the
choice of a low-dose DOAC secondary prophylaxis for patients presenting
with multiple episodes of VTE. In this scenario, a careful clinical
evaluation between full-dose and low-dose DOAC secondary prophylaxis
might be useful.
Further prospective multicentric management
studies are needed to identify the best use, efficacy, and safety
profile of DOACs for secondary prevention in real-life conditions in
non-oncological patients at high risk for VTE recurrence after a VTE
event.
Author Contributions
AL
wrote the manuscript. GMA + CM + AC revised and corrected the
manuscript. AL + GMA + AC provided and interpreted case data, GMA
performed the statistical analysis, and AC conceived and edited the
manuscript. All authors have read and agreed to the published version
of the manuscript.
Data Availability Statement
The
data that support the findings of this study are available in the text
and from the corresponding author, AL + AC, upon reasonable request.
Informed Consent
Written informed consent was collected according to local practice.
References
- Klemen, N.D.; Feingold, P.L.; Hashimoto, B.; Wang,
M.; Kleyman, S.; Brackett, A.; Gross, C.P.; Pei, K.Y. Mortality risk
associated with venous thromboembolism: A systematic review and
Bayesian meta-analysis. Lancet Haematol. 2020, 7, e583-e593 https://doi.org/10.1016/S2352-3026(20)30211-8 PMid:32735837
- Cushman
M. Epidemiology and risk factors for venous thrombosis. Semin Hematol.
2007 Apr;44(2):62-9. doi: 10.1053/j.seminhematol.2007.02.004. https://doi.org/10.1053/j.seminhematol.2007.02.004 PMid:17433897 PMCid:PMC2020806
- Schulman
S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus
warfarin in the treatment of acute venous thromboembolism. N Engl J
Med. 2009;361(24):2342-2352 https://doi.org/10.1056/NEJMoa0906598 PMid:19966341
- Agnelli
G, Buller HR, Cohen A, et al; AMPLIFY Investigators. Oral apixaban for
the treatment of acute venous thromboembolism. N Engl J Med.
2013;369(9):799-808. https://doi.org/10.1056/NEJMoa1302507 PMid:23808982
- Bauersachs
R, Berkowitz SD, Brenner B, et al; EINSTEIN Investigators. Oral
rivaroxaban for symptomatic venous thromboembolism. N Engl J Med.
2010;363(26):2499-2510. https://doi.org/10.1056/NEJMoa1007903 PMid:21128814
- Büller
HR, Décousus H, Grosso MA, et al; Hokusai-VTE Investigators. Edoxaban
versus warfarin for the treatment of symptomatic venous
thromboembolism. N Engl J Med. 2013;369(15):1406-1415 https://doi.org/10.1056/NEJMoa1306638 PMid:23991658
- Ortel
TL, Neumann I, Ageno W, et al. American Society of Hematology 2020
guidelines for management of venous thromboembolism: treatment of deep
vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693-4738
https://doi.org/10.1182/bloodadvances.2020001830 PMid:33007077 PMCid:PMC7556153
- Hindricks
G, Potpara T, Dagres N, et al; ESC Scientific Document Group. 2020 ESC
guidelines for the diagnosis and management of atrial fibrillation
developed in collaboration with the European Association for
Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and
management of atrial fibrillation of the European Society of Cardiology
(ESC) developed with the special contribution of the European Heart
Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42 (5):373-498 https://doi.org/10.1093/eurheartj/ehaa612 PMid:32860505
- Agnelli
G, Buller HR, Cohen A, et al; AMPLIFY-EXT Investigators. Apixaban for
extended treatment of venous thromboembolism. N Engl J Med.
2013;368(8):699-708. https://doi.org/10.1056/NEJMoa1207541 PMid:23216615
- Schulman
S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE
Trial Investigators. Extended use of dabigatran, warfarin, or placebo
in venous thromboembolism. N Engl J Med. 2013;368(8):709-718. https://doi.org/10.1056/NEJMoa1113697 PMid:23425163
- Weitz
JI, Lensing AWA, Prins MH, et al; EINSTEIN CHOICE Investigators.
Rivaroxaban or aspirin for extended treatment of venous
thromboembolism. N Engl J Med. 2017;376(13):1211-1222. https://doi.org/10.1056/NEJMoa1700518 PMid:28316279
- Note
for guidance on clinical investigation of medicinal products for the
treatment of venous thromboembolic disease. London: European Agency for
the Evaluation of Medicinal Products, December 1999. (Document no.
CPMP/EWP/563/98.)
- Stevens
SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ,
Huisman MV, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch
JRE, Wells PS, Moores LK. Antithrombotic Therapy for VTE Disease:
Second Update of the CHEST Guideline and Expert Panel Report. Chest.
2021 Dec;160(6):e545-e608. https://doi.org/10.1016/j.chest.2021.07.055 PMid:34352278
- Khan
F, Rahman A, Carrier M, Kearon C, Weitz JI, Schulman S, et al.;
MARVELOUS Collaborators. Long term risk of symptomatic recurrent venous
thromboembolism after discontinuation of anticoagulant treatment for
first unprovoked venous thromboembolism event: systematic review and
meta-analysis. BMJ 2019;366:l4363. https://doi.org/10.1136/bmj.l4363 PMid:31340984 PMCid:PMC6651066
- Serrao
A, Assanto GM, Mormile R, Brescini M, Santoro C, Chistolini A.
Secondary prophylaxis of venous thromboembolism with direct oral
anticoagulants: comparison between patients with major congenital
thrombophilia versus non-thrombophilic patients. Intern Emerg Med. 2022
Jun;17(4):1081-1085. https://doi.org/10.1007/s11739-021-02917-3 PMid:35018544
- Palareti
G, Barinov V, Urbanek T, Cini M, Li YJ, Bouslama K, Matuška J, Mansilha
A, Madaric J, Sokurenko GY, Andreozzi GM; WHITE study group.
Recurrences and bleeding during extended treatment of patients with
venous thromboembolism: results of the international, prospective,
observational WHITE study. Int Angiol. 2023 Feb;42(1):37-44. https://doi.org/10.23736/S0392-9590.22.04970-7



