Ugo Testa1, Francesco D’Alò2,3, Elvira Pelosi1, Germana Castelli1 and Giuseppe Leone3.
1 Istituto Superiore di Sanità, Roma.
2
Dipartimento di Diagnostica per Immagini,
Radioterapia Oncologica ed Ematologia,
Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma,
Italy. Sezione di Ematologia.
3 Dipartimento di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Roma, Italy
Published: January 01, 2024
Received: November 28, 2023
Accepted: December 15, 2023
Mediterr J Hematol Infect Dis 2024, 16(1): e2024012 DOI
10.4084/MJHID.2024.012
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Follicular
lymphoma is the second most diagnosed lymphoma in Western Europe.
Significant advancements have considerably improved the survival of FL
patients. However, 10-20% of these patients are refractory to standard
treatments, and most of them will relapse. The treatment of follicular
lymphoma patients with multiply relapsed or refractory disease
represents an area of high-unmet needing new treatments with stronger
efficacy. Chimeric antigen receptor (CAR)-T cell therapy targeting
B-cell antigens, such as CD19 or CD20, is emerging as an efficacious
treatment for R/R follicular lymphoma patients, particularly for those
with early relapse and refractory to alkylating agents and to anti-CD20
monoclonal antibodies, resulting in a high rate of durable responses in
a high proportion of patients.
|
Introduction
Follicular
lymphoma (FL) is an indolent B-lymphoproliferative disease of
transformed follicular center B cells, characterized by diffuse
lymphadenopathy, bone marrow involvement, and splenomegaly. FL is the
second most diagnosed lymphoma in Western Europe. At the molecular
level, FL is characterized by the presence of a chromosomal
translocation, t(4;18), resulting in the overexpression of the
anti-apoptotic protein BCL-2; additional recurrent genetic alterations
consist in mutations of chromatin modifying genes KMT2D, CREBBP and EZH2.
Significant
advancements have been in the treatment of FL in the last two decades.
Survival of FL patients has improved significantly due to the
development of efficacious front-line treatments involving anti-CD20
antibodies combined with chemotherapy or lenalidomide. Furthermore, the
treatment of FL patients with relapsed/refractory disease evolved
during the last years, with the introduction of new classes of drugs,
including immunomodulatory agents, phosphoinositide 3-kinase
inhibitors, and epigenetic modulators, in addition to the standard
treatments (cytotoxic agents, anti-CD20 antibodies and allogeneic
hematopoietic stem cell transplantation).[1] However,
the treatment of patients with multiple relapsed or refractory FL
represents an area of high unmet need for which newer treatments with
stronger efficacy or novel mechanisms of action are required. In this
context, two novel T-cell engager therapies, namely bispecific
antibodies and chimeric antigen receptor (CAR)-T cell therapy, have
been recently introduced in the treatment landscape of FL patients with
R/R disease.
CD19 CAR-T Cells Used in the Treatment of FL Patients
Initial studies.
Initial studies carried out using CD19-targeted CAR-T cells have
involved treating a few non-Hodgkin lymphoma (NHL) patients and have
shown high-rate responses, including complete responses.[2-4]
The
Fred Hutchinson Cancer Research Center reported the results on a few FL
patients treated with CD19 CAR-T on a 1:1 ratio of CD4/CD8+ T-cells and
the co-stimulatory molecule 4-1BB.[5] The phase I/II
clinical trial using these cells reported a high rate of ORR and CR in
patients with B-cell lymphomas, including FLs.[6] In
fact, Hirayama et al. reported a clinical study on 21 patients with R/R
FL (8 patients) and with transformed FL (13 patients); after
lymphodepletion with cyclophosphamide and fludarabine, the patients
were infused with 2x106 CD19 CAR-T
cells per kilogram.[6] The CR rates were 88% for R/R FL patients and 46%
for patients with histological transformation (tFL). All patients who
achieved a CR remained in remission at a median follow-up of 24
months.[6] For tFL patients who achieved a CR, at a median follow-up of
38 months, the median PFS was 11.2 months.[6] No severe toxicity events related to CRS or neurologic events were observed.[6]
Tisagenleucel.
The University of Pennsylvania reported initial results in FL patients
using CTL 019 anti-CD19 CAR-CD3ζ-4-1BB lentiviral gene vector transfer
(this vector will become tisagenleucel) in T cells of 15 R/R FL
patients who have received multiple lines of therapy.[7] An update of these patients reported a five-year PFS of 43% in these patients.[8]
CTL
019 represented the basis for developing the Tisagenleucel (Tisa-Cel)
industrial product. Tisa-Cel is an autologous anti-CD19 CAR-T cell
therapy with clinically demonstrated efficacy in patients with various
B-cell malignancies. An initial pivotal study showed that 71% of R/R FL
patients treated with Tisa-Cel achieved a CR. Based on this evidence,
the phase II ELARA trial evaluated the safety and efficacy of Tisa-Cel
in 97 R/R FL patients with two or more lines of prior treatments or
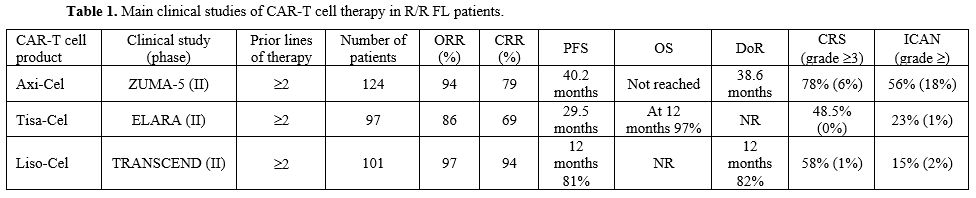
relapsing after autologous HSCT[9] (Table 1). ORR was 86%, with a CRR of 69%.[9]
Although cytokine release syndrome (CRS) and neurological events were
frequently observed, these events were of mild entity, with only 0-3%
of patients exhibiting grade ≥3 toxicity events.[9]
 |
- Table 1. Main clinical studies of CAR-T cell therapy in R/R FL patients.
|
Since
ELARA is a single-arm trial, a subsequent study performed a comparative
effectiveness analysis to compare historical control data from a
matched retrospective cohort of patients with R/R FL treated with
standard care.[10] This analysis showed a better
efficacy of Tisa-Cel with respect to standard care: ORR was 86% for
Tisa-Cel compared to 64% for standard treatment; at 12 months, PFS was
70.5% for Tisa-Cel and 52% for standard care; 12-month OS was 97% for
Tisa-Cel compared to 72% for usual care.[10]
Fowler
et al. reported an analysis of healthcare resource utilization and
hospitalization costs for the patients with R/R FL undergoing CAR-T
cell therapy with Tisa-Cel, comparing inpatients (88% of total) to
outpatients (12% of total).[11] The results of this
analysis showed that the therapeutic efficacy of Tisa-Cel between these
two groups of patients was similar; these findings support the view
that Tisa-Cel can be safely administered to some R/R FL patients in the
outpatient setting, thus reducing healthcare resource utilization and
hospitalization costs.[11]
Axicabtagene Ciloleucel.
Axicabtagene Ciloleucxel (Axi-Cel), previously known as KTE-C19, is
based on three components: an extracellular domain with the svFc domain
targeting CD19, a transmembrane or hinge domain and an intracellular
signaling domain composed by a CD3zeta activation subdomain coupled
with the stimulatory molecule CD28 (CD19-CD28-CD3ζ).
ZUMA-5 is a
single-arm, multicenter, phase II trial that included 124 patients with
R/R FL, mostly at stage IV (85%), with bulky disease (52%), and
frequently pretreated with more than three lines of therapy (62%) or
with progression of disease within 24 months of receiving
chemoimmunotherapy (55%) or who failed to a previous ASCT (24%)[12] (Table 1).
After conditioning lymphodepletion chemotherapy (cyclophosphamide and
fludarabine), the patients received a single infusion of Axi-Cel (2x106
CAR-T cells per Kg).[12] Among 84 FL patients who were eligible for the
primary analysis, the ORR was 94%, with 79% of patients achieving a CR.[12]
According to the updated 3-year follow-up analysis of ZUMA-5, at a
median follow-up of 40.5 months, the median duration of response (DOR)
PFS and OS were 38.6, 40.2 and not reached, respectively. Long-term PFS
rates were also high in patients with high tumor burden and >3 lines
of prior therapy.[13]
A final report of the
ZUMA-5 trial with a three-year follow-up was recently published. A
ottal of 127 FL patients were evaluated, with an ORR of 94%, 79% of CR
and 19% of PR; the median DOR was 38.6 months, with an estimate of 57%
at 36 months; the median duration of CR was not reached, with 62% of
CRs at 36 months; the median duration of PR was of only 4.9 months.[14] Importantly, all 13 patients retreated with Axi-Cel responded to the treatment, with a DOR of 5 months.[14] Median PFS was 40.2 months, with a 36-months PFS rate of 54%.[14]
Two events of disease progression and 10 deaths occurred >24 months
after CAR-T treatment. The estimated cumulative PFS rate was 32%.[14]
The median OS was not reached, with an estimated OF at 36 months of
76%; the 36-month cumulative incidence of lymphoma-specific death was
13%.[14] It is important to note that patients in
ZUMA-5 who had recent exposure to bendamustine (within 6 months) had
shorter PFS after Axi-Cel, a phenomenon seemingly related to the
immunosuppressive effects of bendamustine.[14] Longer
follow-up will be required to assess the curative potential of Axi-Cel
in FL patients. A phase 3 randomized trial was launched to evaluate the
benefit of Axi-Cel compared to standard-of-care therapy for R/R FL
patients (ZUMA-22; NCT 05371093).
A subsequent study compared the
outcomes from ZUMA-5 with the International Scholar-5 cohort involving
R/R FL patients treated with a third or higher line of standard
therapy.[14] This comparative analysis showed a
consistent improvement in outcomes related to Axi-Cel administration:
the ORR and CRR were 50% and 30%, respectively, in Scholar-5 and 94%
and 79%, respectively in ZUMA-5; the median OS and PFS in Scholar-5
were 59.8 months and 12.7 months, respectively, compared to not reached
in ZUMA-5.[15] Thus, compared with available
therapies, Axi-Cel showed a consistent clinical improvement in treating
R/R FL patients after 3 or 4 lines of treatment.
A real-world
study analyzed early outcomes in 151 R/R FL patients undergoing
treatment with Axi-Cel: ORR and CRR were 93% and 84%, respectively;
estimated PFS and OS at 6 months were 88% and 96%, respectively;
grade ≥3 CRS and ICANS occurred in 2% and 13% of patients, respectively.[16] These findings supported the broad use of Axi-Cel for treating R/R FL.
Lisocabtagene maraleucel.
Lisocabtagene maraleucel (Liso-Cel) is an autologous, CD19-directed,
CAR-T cell product. Liso-Cel was approved by the FDA for second-line
treatment of large B cell lymphoma.
The open-label, single-arm,
multicenter phase II study enrolled patients with R/R FL who were at
least 18 years of age, who had an ECOG performance status of 0 or 1,
and who had previously received 2 or 3 lines of therapy and ≥2 prior combination systemic therapy, including an anti-CD20 antibody and an alkylator[17] (Table 1). Patients were treated with one single infusion of Liso-Cel (100x106
CAR-T cells) after lymphodepleting chemotherapy. 101 patients were
suitable for analysis of efficacy. ORR was 97%, with a CR rate of 94%, 1
and 2-month DOR and PFS were 82% and 81%, respectively. The grade ≥3 CRS and neurologic events were very rare.[17]
Comparative Analysis of the Results Observed in ZUMA-5 and ELARA Trials
Mothy
and coworkers have performed a comparative analysis of the results
observed in the ZUMA-5 and ELARA trials. The ORR, CRR, and PFS were
slightly better in ZUMA-5 (Axi-Cel) than in the ELARA (Tisa-Cel) trial:
92% vs. 86%, 76% vs. 69%, and 39.6 months vs. 29.5 months,
respectively.[18] However, the ELARA study included
more patients with advanced, bulky, and refractory disease compared to
ZUMA-5 trial: ≥3 FLIPI 59.8% in ELARA and 44% in ZUMA-5; POD24 59.8% in
ELARA and 55% in ZUMA-5; median number of prior therapies 4 in ELARA
and 3 in ZUMA-5; prior autologous HSCT 36.4% in ELARA and 24% in
ZUMA-%; patients with refractory disease 77.3% in ELARA and 68% in
ZUMA-5.[18]
CD20 CAR-T Targeting in FL Patients
Some
recent studies based on the treatment of a few R/R FL patients have
provided evidence about the efficacy of CAR-T cells targeting CD20 or
CD20 in combination with CD19.
Shadman and coworkers reported the
development of MB-106, a third-generation CD20-targeted CAR-T with both
4-1BB and CD28 co-stimulatory domains.[19] A first
pilot clinical study explored the safety and efficacy of MB-106 in 11
R/R B-cell NHL, including 3 FL patients: 2/3 FL patients displayed a
CR, while the third patient showed a PD.[19]
The
Mustang Bio Inc. developed an industrial procedure for the generation
of MB-106 CAR construct. A recent report was recently presented at the
17th International Conference on
Malignant Lymphoma, Lugano 2023, involving 20 R/R FL patients treated
with MB-106 in a single institution.[20] The median
age of these patients was 63 years and 75% of these patients had POD24
(progressive disease within 24 months), 20% had prior history of
histological transformation and 5% had prior treatment with a CD19
CAR-T; median prior lines of treatment was 4.[20] ORR
was 95% and CRR was 80%; patients who received the highest MB-106 doses
had ORR of 100% and CRR of 91%; the patient who received prior CD19
CAR-T cell therapy achieved a CR.[20] No grade ≥3 CRS or neurologic events were observed in these 20 patients.
Other studies have explored bispecific CAR-T targeting both CD20 and CD19 in patients with R/R B-cell NHLs.[20-21]
These studies included a limited number of R/R FL patients and provided
initial evidence about a consistent clinical efficacy of these
bispecific CAR-T.[21-22]
A recent study reported
a novel CD19/CD20 bispecific CAR-T construct transduced into autologous
naïve (TN) and memory cells (MEM); CAR-T cells engineered using this
CAR-T construct were able to induce a high rate of responses in 10 R/R
N/R NHL patients at low dosages, not inducing toxicity-related events.[23]
The 3 R/R FL patients, all in the category of POD24 high-risk patients,
included in this study, achieved a CR. These observations showed that
CART19/20 TN/MEM cells are safe and effective in patients with R/R NHL,
and particularly in FL patients, with durable responses achieved at low
dosage levels.[23]
Conclusions and perspective
In
the third-line setting, the treatment opportunities for R/R FL patients
are highly heterogeneous, reflecting the lack of a standard therapy.
Standard treatments involve immunochemotherapy, anti-CD20 monotherapy
or combined with lenalidomide, PI3K inhibitors and HSCT; usually, high
response rates to these therapies are observed, but of short duration.[24]
Particularly limited responses are observed in patients with high FLIPI
(follicular lymphoma international prognostic index) and refractory to
alkylators.[24]
CAR-T cell therapies represent
an additional tool to the armamentarium of R/R FL patients, achieving
high rates of responses in heavily pretreated patients and with
acceptable side effects. The results so far obtained support the view
that in the third-line setting, particularly for patients refractory to
alkylating agents and anti-CD20 monoclonal antibody and early relapse
patients, CAR-T cell therapy can be proposed as a part of the standard
care armamentarium, considering its high efficacy and its capacity to
induce long-lasting remissions. However, some problems remain for the
widespread utilization of CAR-T. They are fundamentally the high cost,
the not prompt availability, and the side effects.
Two points
need to be explored in future studies. First, longer follow-up is
required to assess the durability of responses and overall survival
induced by CAR-T cell therapy more carefully. Second, prospective
studies are needed to compare CAR-T versus available therapeutic
options, standard or experimental.
Actually, a recent trial
utilizing Mosunetuzumab, a CD20 × CD3 T-cell-engaging bispecific
monoclonal antibody that redirects T cells to eliminate malignant B
cells, induces the belief that this approach also merits consideration
and further studies, having a similar efficacy.[25]
The
response rates in the current study are more similar to those observed
in studies evaluating chimeric antigen receptor (CAR) T-cell therapies
in patients with relapsed or refractory follicular lymphoma and two or
more lines of therapy, in which high objective response rates (86–94%)
and complete response rates (60–79%) were reported, along with durable
remissions at relatively short follow-up.[23-25]
With
a median follow-up of 18∙3 months, responses in the current study of
mosunetuzumab were also durable and maintained for 18 months or longer
in 70∙2% of complete responders and 56∙9% of all responders. Both CAR
T-cell therapies and bispecific antibodies are likely to have essential
roles in the future management of relapsed or refractory follicular
lymphoma. Of note, however, mosunetuzumab is an off-the-shelf
immunotherapy that avoids many of the logistical challenges associated
with current CAR T-cell therapies, including the need for
leukapheresis, lymphodepleting chemotherapy, and centralized
manufacturing with an extended lead time (median 17–29 days).
Furthermore, the typical side effects of T-CAR cells, the Cytokine
release syndrome, and neurological adverse events seem very rare during
the therapy with mosunetuzumab. Neutropenia was the most common
hematological adverse event, with no febrile neutropenia and manageable
with growth factor support. No grade 5 (ie, fatal) adverse events due
to infection were reported.
References
- Qualls D, Salles G. Prospects in the management of
patients with follicular lymphoma beyond first-line therapy.
Haematologica 2022; 107: 19-34. https://doi.org/10.3324/haematol.2021.278717 PMid:34985231 PMCid:PMC8719064
- Kochenderfer
JN, Wilson WH, Janik JE. Eradication of B-lineage cells and regression
of lymphoma in a patient treated with autologous T cells genetically
engineered to recognize CD19. Blood 2010; 116: 4099-4102. https://doi.org/10.1182/blood-2010-04-281931 PMid:20668228 PMCid:PMC2993617
- Kochenderfer
JN, Dudley ME, Kassim SH. Chemotherapy-refractory diffuse large B-cell
lymphoma and indolent B-cell malignancies can be effectively treated
with autologous T cells expressing an anti-CD19 chimeric antigen
receptor. J Clin Oncol 2015; 33: 540-549. https://doi.org/10.1200/JCO.2014.56.2025 PMid:25154820 PMCid:PMC4322257
- Cappell
KM, Sherry RM, Yang JC. Long-term follow-up of anti-CD19 chimeric
antigen receptor T-cell therapy. J Clin Oncol 2020; 38: 3805-3815. https://doi.org/10.1200/JCO.20.01467 PMid:33021872 PMCid:PMC7655016
- Turtle
CJ, Hanafi LA, Berger C. CD19 CAR-T cells of defined CD4:CD8
composition in adult B cell ALL patients. J Clin Invest 2016; 126:
2123-2138. https://doi.org/10.1172/JCI85309 PMid:27111235 PMCid:PMC4887159
- Hirayama
AV, Gauthier J, Hay KA. High rate of durable complete remission in
follicular lymphoma fater CD19 CAR-T cell immunotherapy. Blood 2019;
134: 636-640. https://doi.org/10.1182/blood.2019000905 PMid:31648294 PMCid:PMC6695558
- Schuster
SJ, Svodoba J, Chong EA, Nasta SD, Mato AR, Anak O, Brogdon JL,
Prutanu-Malinci I, Bhoj V, Landsburg D, et al. Chimeric antigen
receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;
377: 2545-2554. https://doi.org/10.1056/NEJMoa1708566 PMid:29226764 PMCid:PMC5788566
- Chong
EA, Ruella M, Schuster SJ. Lymphoma Prigram Investigators at the
University of P. Five-year outcomes for refractory B-cell lymphomas
with CAR T cell therapy. N Engl J Med 2021; 384: 673-674. https://doi.org/10.1056/NEJMc2030164 PMid:33596362
- Fowler
NH, Dickinson M, Dreyling M, Martinez-Lopez J, Koldstad A, Butler J,
Ghosh M, Popplewell L, Chavez JC, Bachy E, et al. Tisagenleucel in
adult relapsed or refractory follicular lymphoma: the phase 2 ELARA
trial. Nat Med 2022; 28: 325-332. https://doi.org/10.1038/s41591-021-01622-0 PMid:34921238
- Salles
G, Schoster SJ, Dreyling M, Fischer L, Kuruvilla J, Patten P, van
Treschow B, Smith SM, Jimenez-Ubieto A, Davis KL, et al. Efficacy
comparison of tisagenleucel vs usual care in patients with relapsed or
refractory follicular lymphoma. Blood Adv 2022; 6: 5835-5843. https://doi.org/10.1182/bloodadvances.2022008150 PMid:35973192 PMCid:PMC9649992
- Fowler
NH, Dickinson M, Ghosh M, Thiebelmont C, Dreyling M, Schuster SJ.
Assessment of health care resource utilization and hospitalization
costs in patients with relapsed or refractory follicular lymphoma
undergoing CAR-T cell therapy with Tisagenleucel: results from the
ELARA study. Transplant Cell Ther 2023; 29: 60e1-60e4. https://doi.org/10.1016/j.jtct.2022.09.022 PMid:36182104
- Jacobson
CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, Munshi PN,
Casulo C, Maloney DG, de Vos S, et al. Axicabtagene ciloleucel in
relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a
single-arm, multicentre, phase 2 trial. Lancet Oncol 2022; 23: 91-103. https://doi.org/10.1016/S1470-2045(21)00591-X PMid:34895487
- Neelapu
SS, Chavez J, Sehgal AR, Epperla N, Ulrickson M, Bachy E, Munshi PN,
Casulo C, Maloney DG, de Vos S, et al. 3-Year Follow-up Analysis of
ZUMA-5: A Phase 2 Study of Axicabtagene Ciloleucel (Axi-Cel) in
Patients with Relapsed/Refractory (R/R) Indolent Non-Hodgkin Lymphoma
(iNHL). Blood 2022 140; Supplement 1: 10380-10383. https://doi.org/10.1182/blood-2022-156120
- Neelapu
SS, Chavez J, Sehgal AR, Epperla N, Ulrickson M, Bachy E, Munshi PN,
Casulo C, Maloney DG, de Vos S, et al. Three-year follow-up analysis of
axicabtagene ciloleucel in relapsed/refractory indolent non-Hodgkin
lymphoma (ZUMA-5). Blood 2023; in press. https://doi.org/10.1182/blood.2023021243 PMid:37879047
- Ghione
P, Palomba ML, Patel AR, Bobillo S, Deighton K, Jacobson CA, Nahas M,
Hatswell AJ, Scott Young A, Kanters S, et al. Comparative effectiveness
of ZUMA-5 (axi-cel) vs SCHOLAR-5 external control in
relapsed/refractory follicular lymphoma. Blood 2022; 140: 851-856. https://doi.org/10.1182/blood.2021014375 PMid:35679476 PMCid:PMC9412012
- Jacobson
CA, Hemmer MT, Hu ZH, Frank MJ, Popplewell L, Ahmed N, Lin Y, Best T,
Beygi S, Miao HH, et al. Real-world outcomes of axicabtagene ciloleucel
for relapsed or refractory (R/R) follicular lymphoma (FL). Journal of
Clinical Oncology 2023 41:16_suppl, 7509-7509 https://doi.org/10.1200/JCO.2023.41.16_suppl.7509
- Morschhauser
S, Dahiya S, Palomba ML, Garcia-Sancho AM, Reguera Ortega JL, Kuruvilla
J, Jager U, Cartron G, Izutsu K, Dreyling M, et al. TRANSCEND FL: phase
2 study results of lisocabtagene maraleucel (LISO-CEL) in patients
(PTS) with relapsed/refractory (R/R) follicular lymphoma. Haematol
Oncol 2023; 41 (S2): 877-880. https://doi.org/10.1002/hon.3196_LBA4
- Mothy
R, Kharfan-Dabaja MA, Chavez J. Axicabtagene Ciloleucel in the
management of follicular lymphoma: current perspectives on clinical
utility, patient selection and reported outcomes. Cancer Manag Research
2023; 15: 367-375. https://doi.org/10.2147/CMAR.S368588 PMid:37155519 PMCid:PMC10122857
- Shadman
M, Yeung C, Redman MW, Lee SY, Lee DH, Ramachandran A, Ra S, Marzbani
E, Graf SA, Warren EH, et al. Third generation CD20 targetd CAR T-cell
therapy (MB-106) for treatment of patients with relapsed/refractory
B-cell non-Hodgkin lymphoma. Blodd 2020; 132 (suppl.1): abst. 1443. https://doi.org/10.1182/blood-2020-136440
- Shadman
M, Yeung C, Redman M, Lee SY, Lee DH, Qian DH, Ra S, Ujjani CS, Dezube
BJ, Warren EH, et al. High efficacy and favorable safety of 3rd
generation CD20 CAR-T (MB-106) for outpatient treatment of follicular
lymphoma (FL) - results of a single-institution trial. Hematological
Oncology 2023; 41 (suppl. S2): 87-88. https://doi.org/10.1002/hon.3163_49
- Shah
NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor W, Krueger
W, Worden AA, Kadan MJ, Yim S, et al. Biuspecific anti-CD20, anti-CD19
CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation
and expansion trial. Nat Med 2020; 26: 1569-1575. https://doi.org/10.1038/s41591-020-1081-3
- Tong
C, Zhang Y, Liu Y, Ji X, Zhang W, Guo Y, Han X, Ti D, Dai H, Wang C, et
al. Optimized tandem CD19/CD20 CAR-engineered T cells in
refractory/relapsed B-cell lymphoma. Blood 2020; 136: 1632-1644. https://doi.org/10.1182/blood.2020005278 PMid:32556247 PMCid:PMC7596761
- Larson
SM, Walthers C, Ji B, Gahfouri SN, Naparstek J, Trent J, Chen JM,
Roshandell M, Harris C, Khericha M, et al. CD19/CD20 bispecific
chimeric antigen receptor (CAR) in naïve/memory T cells for the
treatment of relapsed or refractory non-Hodgkin lymphoma. Cancer Discov
2023; 13: 580-597. https://doi.org/10.1158/2159-8290.CD-22-0964 PMid:36416874 PMCid:PMC9992104
- Casulo
C, Larson MC, Lunde JJ, Habermann TM, Lossos IS, Wang Y, Natsoupil LJ,
Strouse C, Chihara D, Martin P, et al. Treatment patterns and outcomes
of patients with relapsed/refractory follicular lymphoma receiving
three or more line of systemic therapy: results from a lymphoma
epidemiology of outcomes consortium observational study. Lancet Oncol
2022; 9: e289-e300. https://doi.org/10.1016/S2352-3026(22)00033-3 PMid:35358443
- Budde
LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, Kuruvilla J,
Canales M, Dietrich S, Fay K, Ku M, Nastoupil L, Cheah CY, Wei MC, Yin
S, Li CC, Huang H, Kwan A, Penuel E, Bartlett NL. Safety and efficacy
of mosunetuzumab, a bispecific antibody, in patients with relapsed or
refractory follicular lymphoma: a single-arm, multicentre, phase 2
study. Lancet Oncol. 2022 Aug;23(8):1055-1065. doi:
10.1016/S1470-2045(22)00335-7. https://doi.org/10.1016/S1470-2045(22)00335-7 PMid:35803286
