The present case study details a six-month-old Chinese male infant who was hospitalized in August 2020 due to persistent symptoms of recurrent fever and prolonged neutropenia lasting over five months. He was the third child, born full-term via cesarean delivery. The non-consanguineous parents reported no family history of genetic disorders, and the patient's immediate family, including two elder brothers, were all healthy with no neutropenia history. Blood tests since birth showed fluctuating neutrophil counts between 0.01 to 0.48×109/L, with generally normal or slightly elevated white blood cells. Infection marker tests were normal, including erythrocyte sedimentation rate, C-reactive protein, and procalcitonin. Screenings for various pathogens like respiratory viruses, mycoplasma, chlamydia, legionella, CMV, EBV, and herpes simplex virus returned negative, as did tests for (1-3)-beta-D-glucan, galactomannan, T-SPOT, HIV, and blood cultures. The immunological assessment showed normal cellular and humoral immunity and complement system function. Autoimmune antibody tests and genetic analysis for myelodysplastic syndrome and leukaemia-related genes revealed no abnormalities. Bone marrow cytology findings indicated active proliferation of nucleated cells, yet a reduction in granulocytic proliferation was observed, along with maturation impediments.
Genetic analysis of the ELANE gene in the patient revealed a nine-base pair deletion in exon three (c.295_303del), leading to the loss of amino acids 99 to 101 (p.F99_V101del) and subsequent inactivation of neutrophil elastase. Notably, this mutation wasn't present in the patient's immediate family. In-depth in vitro experiments using a human precursor neutrophil cell line, with transfected native and mutated ELANE protein, were conducted. Flow cytometric analysis showed significant differences in neutrophil differentiation and maturation between cells with the mutated ELANE gene and wild-type cells, highlighting the mutation's profound impact.
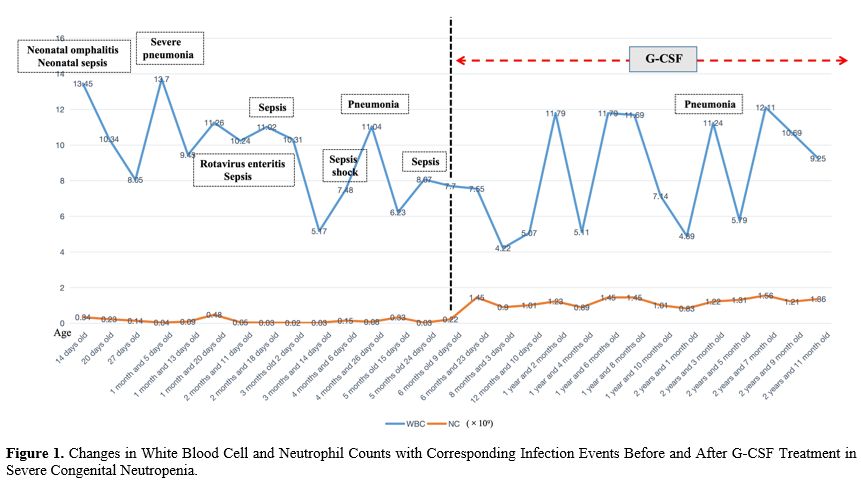
During the hospitalization, the infant received daily subcutaneous injections of 2 micrograms/kg recombinant human granulocyte colony-stimulating factor (G-CSF) along with supportive care, effectively raising his neutrophil count to 0.5-0.9×109/L. In the subsequent three years, outpatient monitoring involved administering 2-5 micrograms/kg/day G-CSF for 3-5 days whenever his neutrophil count dropped below 0.5×109/L, aiming to maintain it above 1.0×109/L. This approach led to a significant decrease in severe infections (see Figure 1).
 |
|
The ELANE gene, situated on the p13.3 region of chromosome 19, consists of five exons and encodes a 267 amino acid protein known as neutrophil elastase. This enzyme is primarily synthesized in immature or promyelocytic cells and is sequestered within the azurophilic granules of neutrophils. It is integral to the immune response, particularly in the degradation of pathogens, playing a pivotal role in the innate immune system.[6] Mutations in the ELANE gene commonly result in CN, are typically heterozygous and adhere to an autosomal dominant inheritance pattern. The precise mechanism leading to neutropenia remains elusive; however, the prevailing hypothesis suggests that these mutations initiate an unfolded protein response coupled with endoplasmic reticulum stress, culminating in neutrophil apoptosis.[7]
In this study, an infant suffering from continuous severe infections and a non-cyclical decrease in peripheral blood neutrophils revealed a previously unreported mutation in the ELANE gene, suggesting severe congenital neutropenia associated with ELANE. Further genetic analysis revealed that the child's parents and brothers have normal wild-type ELANE genes, strongly suggesting that the neutropenia is likely due to a novel somatic mutation rather than familial inheritance. To comprehend the precise ramifications of this novel mutation, we undertook a series of in vitro experiments using a human precursor neutrophil cell line. The observed maturation block in these precursor cells suggests that the c.295_303del mutation could profoundly affect the functionality of the neutrophil elastase protein, thereby interfering with the regular maturation pathway of neutrophils. This observation is consistent with the child's persistently low neutrophil counts, substantiating the hypothesis that the c.295_303del mutation significantly influences neutrophil development and functionality.
Differentiating severe congenital neutropenia from cyclic neutropenia linked to ELANE gene mutations is critical for diagnosis. CyN typically shows recurrent fever and infections with cyclical neutrophil reductions, usually under 0.2×109/L for 3-5 days in a 21-day cycle, often asymptomatic between episodes.[5] However, the case lacked these cyclical patterns in infections and neutrophil counts, and sometimes, the neutrophil count exceeded 0.2×109/L, ruling out CyN and supporting the SCN diagnosis. Treatment with recombinant human granulocyte colony-stimulating factor (G-CSF) significantly improved the patient's condition, as shown by increased neutrophil counts and reduced infection rates. This response underscores G-CSF's vital role in treating ELANE mutation-induced neutropenia and confirms the strategy's effectiveness, offering insights for managing similar genetic forms of neutropenia.