Armando Tripodi1, Marigrazia Clerici1, Erica Scalambrino1, Pasquale Agosti1,2, Paolo Bucciarelli1 and Flora Peyvandi1,2.
1
Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo
Bianchi Bonomi, Heomophilia and Thrombosis Center, Milan, Italy.
2 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milano, Italy.
Correspondence to: Armando Tripodi, Via Pace 9, 20122 Milano, Italy. Phone: +39 0255035437. Email:
armando.tripodi@unimi.it
Published: March 01, 2024
Received: January 4, 2024
Accepted: February 14, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024027 DOI
10.4084/MJHID.2024.027
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Oral
anticoagulants are widely used to treat or prevent cardiovascular
diseases in millions of patients worldwide. They are the drugs of
choice for stroke prevention and systemic embolism in patients with
non-valvular atrial fibrillation and prosthetic heart valves, as well
as for treatment/prevention of venous thromboembolism. Oral
anticoagulants include vitamin K antagonists (VKAs) and direct oral
anticoagulants (DOACs). The hemostasis laboratory plays a crucial role
in the management of treated patients, spanning from dose adjustment
based on laboratory testing that applies to VKAs to the measurement of
drug concentrations in special situations that apply to DOACs. This
article aims to overview how the hemostasis laboratory can help
clinicians manage patients on oral anticoagulants. Special interest is
devoted to the international normalized ratio, used to manage patients
on VKAs and to the measurement of DOAC concentrations, for which the
role of the laboratory is still not very well defined, and most
interferences of DOACs with some of the most common hemostatic
parameters are not widely appreciated.
|
Introduction
Oral
anticoagulants are widely used for the treatment and prevention of
cardiovascular diseases. Although there is no accurate information, it
can be estimated that around 2% of the general population in Western
countries is currently on oral anticoagulants. They are mainly used for
the prevention of ischemic stroke and systemic embolism in patients
with non-valvular atrial fibrillation and for treatment/prevention of
venous thromboembolism. Oral anticoagulants are also used to prevent
thrombosis in patients with mechanical heart valves and those with
antiphospholipid syndrome. Among the currently used oral
anticoagulants, one may consider the time-honored vitamin K antagonists
(VKAs) and the more recent direct oral anticoagulants (DOACs). The
present article aims to overview the role of the hemostasis laboratory
in the management of patients on oral anticoagulants. The information
reported herein is based on data from the literature and the personal
opinion of the authors.
Vitamin K Antagonists (VKAs)
These
drugs have been (and are still) used as anticoagulants since their
mechanism of action was elucidated. In the early 1920s, massive deaths
from hemorrhage were observed among cattle in the Northern part of the
United States and Canada. Those deaths were soon ascribed to the
ingestion of sweet clover that, during storage and subsequent
fermentation, developed anticoagulant substances called coumarins,
which were responsible for hemorrhage and animal deaths. Years later,
coumarin substances, possessing the same characteristics as those
derived from fermented sweet clover, were synthesized and used first as
rat killers and then as anticoagulants in humans. Meanwhile, the
principle of action of coumarins was fully elucidated, and it is now
known that they act through the inhibition of vitamin K that, in normal
conditions, is the mediator for the post-ribosomal carboxylation of
such procoagulant factors as factor (F) VII, FIX, FX and FII (named
vitamin K-dependent coagulation factors). Curiously, the same mechanism
of action is also operative for two of the most important anticoagulant
factors [i.e., protein C (PC) and protein S (PS)]. The carboxylation of
the vitamin K-dependent coagulation factors is instrumental in making
them adhere to phosphatidyl-serine, a negatively charged phospholipid
expressed at the surface of activated platelets, thus helping to speed
up thrombin generation and fibrin formation at the site of vessel wall
injury. The elucidation of the mechanism of action of coumarins has
been instrumental in the adoption of vitamin K as an antidote for these
drugs, which were later called VKAs. Currently, the two most commonly
used VKAs are warfarin (Coumadin®) and acenocoumarol (Sintrom®). They
differ essentially for the half-life, which is relatively shorter for
acenocoumarol than for warfarin. However, it was soon realized that
VKAs cannot be administered at a fixed dose because their
pharmacokinetic is not favorable, and the effective/safe dose is
unpredictable. As a matter of fact, VKA dosage varies between
individuals and also within the same individual at different time
points. This variation is due to the interference of VKAs with food and
other drugs that are concomitantly taken. VKAs reach their peak
activity on average about one week after administration, and their
effect is reduced markedly only several days after stopping treatment.
The prothrombin time (PT).
This state of affairs led over the years to the use of PT as the test
of choice for dose adjustment. The results of the PT depend, however,
on the thromboplastin used for testing, and it was therefore soon
realized that the application of PT in clinical practice would have
been difficult because local laboratories may use different
thromboplastins and hence may give different results when the PT is
measured for the same patient. This would make dose adjustment of VKAs
inherently difficult.
The international normalized ratio (INR). Starting from the 1980's, a system of thromboplastin calibration was initiated and refined.[1]
It prescribes that local thromboplastins are calibrated against a
common international standard for thromboplastin, prepared and
distributed by the World Health Organization (WHO). The guidelines
issued by WHO[2] require that local thromboplastins
undergo a relatively simple calibration process, whereby PTs (seconds)
for patients stabilized on VKAs and for a group of healthy subjects are
measured with the thromboplastin being calibrated and with the WHO
international standard for thromboplastin. Paired PTs are then plotted
in a double-log scale, and after checking for linearity, an orthogonal
regression line is drawn through the data points. The slope of the
regression line represents the responsiveness of the thromboplastin
being calibrated relatively to the WHO international standard for
thromboplastin (Figure 1).
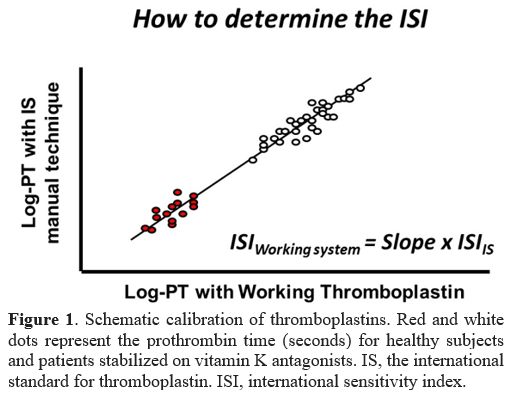 |
- Figure 1. Schematic
calibration of thromboplastins. Red and white dots represent the
prothrombin time (seconds) for healthy subjects and patients stabilized
on vitamin K antagonists. IS, the international standard for
thromboplastin. ISI, international sensitivity index.
|
The
slope of the line is called international sensitivity index (ISI) and
is used to convert PT results in seconds obtained with the local
thromboplastin to those that would have been obtained using the WHO
international standard for thromboplastin. Hence, the INR is the common
scale whereby results of the PT, when used for patients on VKAs, do not
depend on the thromboplastin used for testing. The conversion of PT
into INR is easily obtained by the following equation:
INR = [PTpatients/MNPT]ISI
where the PT is the value in seconds for patients and the MNPT represents the geometric mean PT of 20 or more healthy subjects.
The
development and refinement of the INR have been instrumental in
performing clinical trials aimed at defining the most effective and
safe therapeutic intervals. We now know that patients on VKAs are
considered to be adequately anticoagulated when their INR falls most of
the time between 2.0 and 3.0, a range that minimizes the risk of both
hemorrhage and thrombosis. Patients initiating VKAs should undergo
laboratory screening, including a complete blood cell count and
baseline coagulation tests such as the PT and activated partial
thromboplastin time (aPTT). These are needed to identify patients with
thrombocytopenia or carriers of mild hereditary defects of one or more
coagulation factors that, although not causing bleeding problems in
normal conditions, could put the patient at risk following the addition
of anticoagulants.
Limitations of the INR.
There are limitations of the INR that may impact patients' management.
First, the INR (by definition) harmonizes PT results across
laboratories, but only for patients on VKAs. In fact, the ISI of
thromboplastins is determined using plasma for patients stabilized on
VKAs and is therefore valid only for these patients. When PT is used
for other clinical conditions, it should be expressed as clotting time
(seconds) or simple ratio (patient-to-normal clotting time). Second,
strictly speaking, the INR is valid only in the range of values from
1.5 to 4.5. In fact, plasmas from patients used for calibration are
selected to have an INR comprised in that interval. This means that
there is no assurance that there is dose-response linearity above or
below this range. In practice, an INR above 4.5 has only an indicative
value, and there is no assurance that (for example) an INR of 8.0 is
different from an INR of 10.0. Hence, making a decision on the reversal
of anticoagulation based on INR values higher than 4.5 should be done
with caution and knowledge of the performance of the local reagents.
Third, the INR may be influenced by conditions other than VKAs, such as
partial deficiencies of coagulation factors or the presence of lupus
anticoagulant (LA), which might prolong the PT and hence increase the
INR. Specific studies have been performed to assess the effect of LA on
PT-INR, and it is now known that there is no effect with the majority
of commercial thromboplastins.[3] As a matter of fact,
the PT is insensitive to LA unless thromboplastins are considerably
diluted. Fourth, the INR is not valid at the beginning of treatment
with VKAs, as in this phase, vitamin K-dependent coagulation factors
are depressed at a different rate, depending on their half-life.
However, since there is no other valid method to monitor VKA dosages,
the INR is also used in the initiation phase of treatment. Finally,
most of the commercial thromboplastins are added with chemicals, such
as polybrene or enzymes (heparinase), that make them insensitive to
heparins up to 1.0 UI/mL. This addition is needed to circumvent the
effect that heparins may have on the prolongation of the PT-INR when
anticoagulant therapy for venous thromboembolism is started and
heparins and VKAs are concomitantly administered. On these occasions,
the presence of heparins would make dose-adjustment of VKAs and
attainment of the INR therapeutical interval difficult to achieve.
Direct Oral Anticoagulants (DOACs)
DOACs
are a consolidated reality in the armamentarium available to doctors
for the treatment and antithrombotic prophylaxis of cardiovascular
diseases. It is estimated that at least 70% of patients with
non-valvular atrial fibrillation, who are treated for the prevention of
ischemic stroke, or those treated for venous thromboembolism and its
recurrence, are currently on DOACs, and only the remaining 30% are
treated with VKAs.[4,5] However, there are still
clinical conditions in which DOACs are not adequate. For example, VKAs
are still the drugs of choice in the prevention of thrombosis in
patients with mechanical heart valves and for the prevention of
thromboembolic events in patients with antiphospholipid syndrome.[6]
The reason for the success of DOACs rests mainly in their greater
simplicity and manageability of use since they can be prescribed at a
fixed dose, based on the patient's characteristics, and unlike VKAs, do
not require dosage adjustment based on laboratory testing.
Additionally, there are other direct-acting anticoagulant drugs (e.g.,
anti-FXI) in clinical trials that may have, at least from a theoretical
standpoint, further advantages. It is, therefore, predictable that the
DOACs will replace VKAs in the medium to long term, although the latter
still have a non-negligible role.
Although DOACs do not require
dose adjustment by laboratory testing, it would be wrong to think that
they do not require laboratory support at all.[7,8] In
the following paragraphs, we consider the role of the hemostasis
laboratory in the management of patients on DOACs. Patients initiating
DOACs should undergo laboratory screening, including a complete blood
cell count and baseline coagulation tests such as the PT and aPTT.
These are needed to identify patients with thrombocytopenia or carriers
of mild hereditary defects of one or more coagulation factors that,
although not causing bleeding problems in normal conditions, could put
the patient at risk following the addition of DOACs.[9]
Assessment of the creatinine clearance before initiation of treatment
is paramount since DOACs are excreted through the kidney (Table 1).
The drugs, their characteristics, and mode of action.
DOACs exert their antithrombotic action through the direct inhibitory
effect against an individual coagulation factor. This mode of action is
markedly different from that of VKAs, which exert antithrombotic
function through the impairment of the carboxylation process of vitamin
K-dependent coagulation factors. The function of DOACs also differs
conceptually from that of heparins, which exert their function by
indirectly inhibiting the procoagulant factors through the mediation of
antithrombin.
The mode of action of DOACs determines some of their
important characteristics. For example, DOACs have a much faster
anticoagulant action than VKAs. Peak concentration in plasma and,
therefore, DOAC action is reached about two hours after administration.
At the same time, the trough value is recorded after 12 or 24 hours,
depending on whether the drug is taken twice or once daily (Table 1).
On
the other hand, VKAs reach their peak activity on average about one
week after administration, and their effect is markedly reduced only
several days after stopping treatment. These differences become crucial
in clinical practice. For example, DOACs are more manageable than VKAs,
especially when a temporary discontinuation of treatment is needed for
surgery and/or invasive procedures, which are deemed at potential
bleeding risk. DOACs are eliminated from the circulation through the
kidney and/or liver, with Dabigatran having the highest renal excretion
(Table 1). Because of the above
characteristics, it is recommended to assess for renal and liver
function in individual patients before and during treatment to avoid
possible accumulation of the circulating drug and the consequent
increase in bleeding risk. Renal function is generally assessed by
estimating creatinine clearance (CrCl) with an empirical formula
(Cockcroft-Gault) that considers some biochemical parameters such as
serum creatinine and patient variables such as body weight, age,
height, and gender. In patients with CrCl <30 mL/min, Dabigatran is
contraindicated and anti-FXa drugs should be prescribed with caution
(they are contraindicated for CrCl <15 mL/min). Liver function can
be evaluated by the measurement of liver enzymes.
Currently, there
are four DOACs available: Dabigatran (Pradaxa®) is the only drug with
antithrombin action, while Rivaroxaban (Xarelto®), Apixaban (Eliquis®)
and Edoxaban (Lixiana®) inhibit FXa (Table 1 and Figure 2).
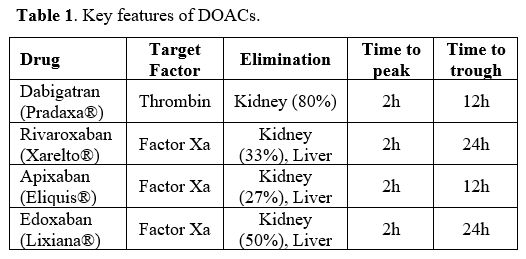 |
Table
1. Key features of DOACs. |
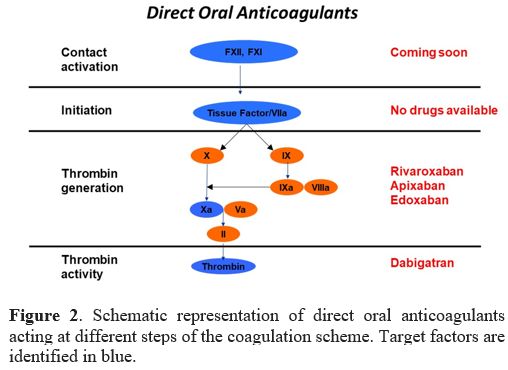 |
Figure
2. Schematic representation of direct oral anticoagulants acting at
different steps of the coagulation scheme. Target factors are
identified in blue.
|
All
drugs are indicated for the prevention of ischemic stroke in patients
with non-valvular atrial fibrillation and for treatment/prevention of
venous thromboembolism, excluding those patients with mechanical heart
valves and those with triple-positive antiphospholipid syndrome
(concomitant positivity for anticardiolipin, anti-β2-GPI, and LA).[6]
Clinical trials performed on large patient series have demonstrated
that the efficacy and safety of DOACs are not inferior (or even
superior) to those of VKAs when used at fixed doses based on patients'
characteristics.
Which role of the hemostasis laboratory in the era of DOACs.
Owing to the favorable pharmacokinetic and dose-response
predictability, DOACs have been designed for fixed-dose use with
particular regard to patient's characteristics. There are at least two
dosages for each medication, whereby the needs of most patients can be
considered. At these doses, the rate of adverse events (hemorrhagic and
thrombotic) that emerged from the studies, although limited, were
measurable and, therefore, the question of whether some sort of dose
adjustment based on laboratory testing may be useful to minimize the
risk is still a matter of debate. For example, a review of clinical and
laboratory data from the pivotal registration trial of Dabigatran has
demonstrated that a dose adjustment in those patients with extreme
plasma concentrations could have spared a number of adverse events.[10] In addition, real-life observational studies have documented wide variability of the plasma concentration for each DOAC[11]
despite being taken at the same dose. The above observations suggest
that the fixed dose is not necessarily applicable to all patients
indiscriminately. Recently, an observational collaborative study
(Measure And See, MAS) showed that a single measure of DOAC
concentrations a few weeks after initiation of therapy may predict
adverse events. In fact, some of the patients who had relatively low
DOAC levels showed a greater propensity to develop thrombotic relapses
more frequently during follow-up.[12]
Although
these observations show that the fixed dose is not applicable to all
patients, regulatory authorities and experts are reluctant to change
this rule in favor of dose adjustment. Most believe that the benefits
likely achieved with dose adjustment would not be offset by the
reduction of adverse events and would further complicate therapy
management in millions of patients.
Given that dose adjustment is
not applicable to all patients and that DOACs will continue to be
prescribed at a fixed dose, the fact remains that plasma concentration
measurement is useful in some circumstances that we consider below. The
personal opinions of the authors and other experts8 suggest dividing
these situations into two categories: those in which DOAC measurement
is recommended and those in which it is considered useful (Table 2).
Cumulatively, the number of subjects to be included in DOAC measurement
would be relatively small and would not entail excessive burdens for
the national health system or problems in the practical management of
patients.
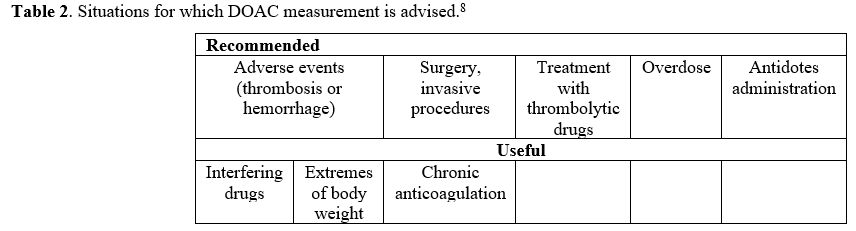 |
Table 2. Situations for which DOAC measurement is advised.[8] |
When DOAC measurement is recommended
Adverse events (thrombotic or hemorrhagic) during therapy.
In patients with adverse events, especially when lack of adherence to
therapy or patient errors can be excluded, it is paramount to establish
the reasons for adverse events in order to exclude that it is due to an
excess (hemorrhage) or a defect (thrombosis) of the drug.
Surgery and/or invasive procedures.
If surgery or invasive procedures deemed at increased bleeding risk are
not programmable, DOAC measurement is needed to assess whether residual
drug concentration in plasma is such to minimize the risk of bleeding.
DOAC measurement may also be required for elective surgery/invasive
procedures, and in such cases, the patient may be instructed to
discontinue therapy 2-3 days before surgery. If renal function is
normal, this protocol of discontinuation, given the short half-life of
DOACs, may be adequate to ensure their complete elimination from
circulation. However, to be on the safe side, it would be necessary to
know (at least) the value of serum creatinine. Very often, this value
is in the patient's records but could date back weeks or months
earlier, and there is no certainty if this value is constant over time,
especially in the elderly, in whom sudden and unpredictable changes may
occur. It would then be necessary to proceed with an emergency serum
creatinine measurement. However, it is still being determined what the
advantage could be compared to the direct measurement of the residual
drug, if one considers also that there is no a clear relationship
between serum creatinine levels and DOAC plasma concentration.[11]
Treatment with thrombolytic agents.
Despite proper treatment, some patients on DOACs for non-valvular
atrial fibrillation may be referred to emergency departments because of
ischemic stroke. In these instances, the therapy of choice is
thrombolytic treatment in an attempt to lyse the thrombus. However,
thrombolytic agents are burdened with a discrete risk of bleeding,
which becomes even more important if there are relatively high
concentrations of DOACs in the circulation. That is why DOAC
measurement is paramount in this circumstance.
Overdose.
In the suspicion of voluntary or accidental overdose, even in the
absence of bleeding, DOAC measurement is needed for obvious reasons.
Administration of antidotes.
There are at least two antidotes for DOACs: Idarucizumab, which
neutralizes Dabigatran, and Andexanet alfa, which neutralizes anti-FX
drugs. The two antidotes have been evaluated in randomized multicenter
trials, which have shown efficacy and safety in patients referred to
emergency departments for active bleeding. However, the respective
study protocols did not prescribe the preventive measurement of DOACs,
and antidotes were administered on the presumption that patients were
bleeding because of an excess of DOACs. Post-hoc measurement on plasma
samples collected during the studies reported that about 1/4 of the
patients had received the antidote in the absence of significant
amounts of circulating DOACs.[13,14] Therefore, based
on these results, it is advisable to measure the DOACs before
administering antidotes in order to optimize their use and treat
patients more appropriately. Observational studies have also reported
that occasionally patients treated with Idarucizumab showed complete
and immediate neutralization of the drug, but after a few hours the
concentration of circulating Dabigatran returned to levels similar to
those measured before administration of the antidote.[15]
This phenomenon is probably explained by the leakage of Dabigatran into
extra-vascular spaces, where it cannot be reached and neutralized by
the antidote because of its relatively high molecular weight. When the
concentration of plasma Dabigatran is reduced by the action of
Idarucizumab, the drug released into the extra-vascular spaces is
recalled to circulation by osmosis, and its concentration increases.
The patient may then need a second dose of antidote. This rebound
phenomenon suggests that, in order to optimize the use of Idarucizumab
and for patient safety, the plasma concentration of Dabigatran should
be measured before and after administration of the antidote.
When DOAC measurement is potentially useful
Interfering drugs.
DOACs interfere with other drugs much less than VKAs, but occasional
potentiation or depotentiation of their activity by other drugs cannot
be excluded. When there is a suspicion of potential interference, the
measurement of DOACs before and after the addition of the additional
drug can provide very useful information.
Patients with extremes of body weight.
The fixed dose is theoretically valid for the type of patients enrolled
in clinical trials. Even though extremes of body weight were not among
the exclusion criteria, these patients were not sufficiently
represented in clinical trials. Therefore, an ad hoc evaluation of the efficacy and safety of DOACs in this type of patient is not possible. Post-hoc
observational studies have yet to solve this problem completely, and
whenever doubts do exist about the optimal dose in overweight or
underweight patients, the measurement of plasma DOAC concentration
could provide useful information.
Chronic anticoagulation.
Given the variability in the plasma concentration of DOACs recorded in
clinical trials and real-life observations, it is conceivable that
individual patients may have plasma levels of DOACs that are her/his
own characteristics. Although ad hoc studies are lacking, it is
reasonable to assume that these concentrations may be constant over
time for the same individual. Therefore, measurement of concentrations
at the achievement of the steady-state of chronic anticoagulation
(e.g., 4 weeks after initiation) and verification of the
constancy/variation of this value in subsequent periods, could give
important information on DOAC plasma levels, whose knowledge could be
useful in particular circumstances. Measurement could also be
occasionally useful in the elderly who do not have the assistance of
caregivers to check for adherence to the therapy.
Which test for which DOAC
Although
PT/aPTT may be more or less prolonged in patients taking DOACs, their
response is variable and depends on the composition of the reagents.
Therefore, PT/aPTT in these patients can give only a partial answer
that could also be misleading in many respects. In fact, these tests
may be prolonged for reasons other than the presence of DOACs (e.g.,
variable concentration/activity of individual coagulation factors).
Hence, the PT/aPTT in patients on DOACs are not advisable. There are
dedicated tests for DOACs that are commercially available and are
relatively simple to perform in general clinical laboratories on
ordinary coagulomaters even in emergencies. These tests can be
calibrated with standards at known and certified concentrations for
each drug, with results expressed in ng/mL.
Dilute thrombin time (dTT).
The traditional thrombin time is over-sensitive to the presence of
Dabigatran, so the test is uncoagulable even when Dabigatran is at
relatively low concentrations. Minor modifications have been made to
this test relating to the dilution of the sample, which allows adequate
sensitivity to Dabigatran and can be used successfully for its
measurement.
Ecarin test.
This test is similar to the dTT but exploits the ability of ecarin
(reptile venom) to activate FII. The thrombin, which is thus generated,
is inhibited by Dabigatran, and the measurement of residual thrombin
(by means of coagulation or chromogenic technique) allows for
measurement of the concentration of the drug to be extrapolated from a
dose-response calibration curve.
Anti-FXa activity tests.
These are the same tests used for the measurement of heparins, which
allow us to evaluate the ability of plasma to inhibit FXa added in
excess to the plasma under test. They are based on FXa-specific
chromogenic substrates and can be successfully used for the measurement
of any of the anti-FX drugs (Rivaroxaban, Apixaban and Edoxaban).
When to perform DOAC measurement
Given
the pharmacokinetics of DOACs, the time elapsing between the last drug
intake and blood sampling is essential for the interpretation of
results. Peak plasma concentration is reached approximately two hours
after taking the drug and trough values after 12 or 24 hours, depending
on whether the drug is administered twice or once daily.
Therapeutic intervals
The
values obtained in the plasma of subjects treated with DOACs are poorly
defined, due to the fact that there are few studies and because of the
wide inter-individual variability, despite the fact that all patients
take the same daily dose. It is, therefore, more useful to refer to the
expected values that are reported in the technical annex of the drugs
or in the literature, examples of which are shown in Table 3.
In cases where DOAC measurement is performed, it is advisable to report
the results as plasma concentrations expressed in ng/mL and the
relative expected values.
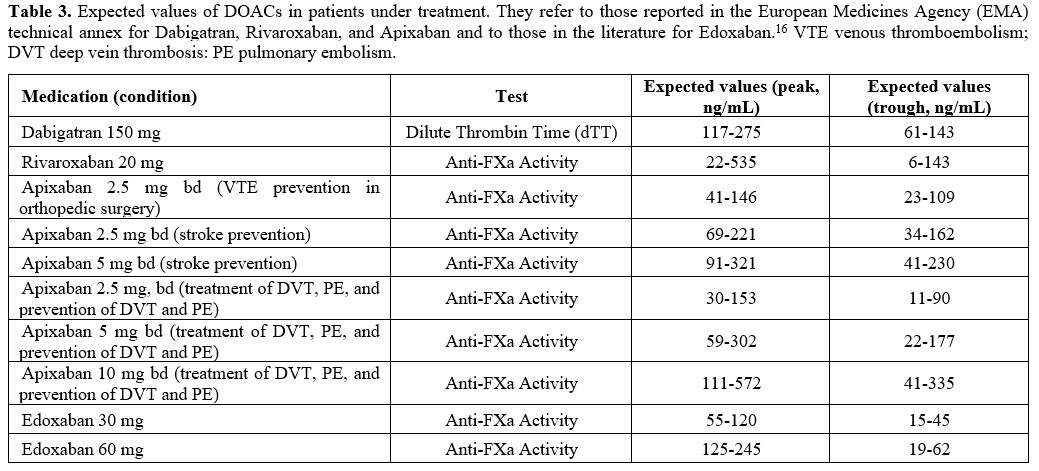 |
Table 3. Expected
values of DOACs in patients under treatment. They refer to those
reported in the European Medicines Agency (EMA) technical annex for
Dabigatran, Rivaroxaban, and Apixaban and to those in the literature
for Edoxaban.[16] VTE venous thromboembolism; DVT deep vein thrombosis: PE pulmonary embolism. |
Interference of DOACs, or VKAs with the most common hemostasis tests
Because
DOACs are anticoagulant drugs, they interfere with the tests commonly
used to evaluate hemostasis. However, some interferences are not so
obvious from a theoretical standpoint and might lead to erroneous and
dangerous conclusions. The consequences of DOAC interference are
briefly discussed in the following paragraphs and reported in Table 4.
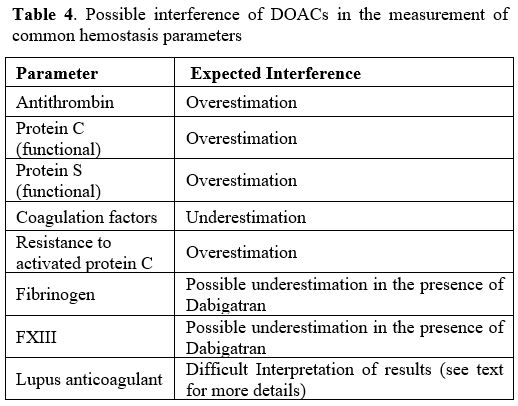 |
Table 4. Possible interference of DOACs in the measurement of common hemostasis parameters |
Antithrombin.
DOACs can significantly interfere with the measurement of antithrombin
activity. For example, if antithrombin measurement is performed using
thrombin as the target enzyme and the drug present in the patient's
plasma is Dabigatran, antithrombin activity is falsely increased
because Dabigatran inhibits the thrombin added in excess to perform the
measurement.Similarly,
if the target enzyme for antithrombin measurement is FXa and the drug
in the patient's plasma is an anti-FXa (Rivaroxaban, Apixaban, or
Edoxaban), the drug will inhibit the excess of FXa added to perform the
measurement, and thus the measured activity of the antithrombin is
falsely increased. In
the cases described, interference can be avoided by using as a target
enzyme for the measurement of antithrombin the one that is not
inhibited by the drug present in the patient's plasma. In other words,
if the drug used for treatment is Dabigatran, the method for measuring
antithrombin should use FXa as the target enzyme. Conversely, if the
drug used for treatment is Rivaroxaban, Apixaban, or Edoxaban, the
method for measuring antithrombin needs thrombin as the target enzyme.Protein C (PC) and protein S (PS).
As mentioned, PC and PS are vitamin K-dependent coagulation factors and
are, therefore, reduced in patients on VKAs. DOACs prolong the
coagulation tests on which functional assays of PC and PS are based.
Therefore, the measured activity values for these proteins may be
overestimated in the presence of DOACs. PC values measured by
chromogenic assays and PS measured by immunoassay are not affected by
the presence of DOACs.Fibrinogen.
Fibrinogen is usually measured by coagulation methods after addition of
thrombin (Claus method) to the patient's plasma. In these cases,
thrombin used as a reagent is inhibited by Dabigatran, leading to an
underestimation of fibrinogen concentration. Interference from
Dabigatran can be minimized by using (as a reagent) thrombin at high
concentrations (e.g., 100 U/mL).Factor XIII (FXIII).
FXIII, when measured as functional activity, may be underestimated if
the patient is treated with Dabigatran. This is due to the fact that
the FXIII must be activated by thrombin prior to measurement.
Therefore, the presence of Dabigatran leads to the inactivation of part
of the thrombin, and this inevitably results in an underestimation of
the FXIII activity.Measurement of individual coagulation factors.
The presence of one of the DOACs may give rise to artificially reduced
levels of individual coagulation factors, which are based on PT or aPTT
in combination with plasmas deficient in the factor to be measured, but
also in case of some chromogenic tests (e.g., FVIII).Lupus anticoagulant (LA). DOACs, but also VKAs, interfere with LA detection.[17,18]
The explanation rests on the fact that both anticoagulants and LA
prolong the clotting time of the aPTT and dRVVT tests used for LA
detection. Therefore, it becomes difficult (if not impossible) to
correctly interpret the results of LA testing when used in
anticoagulated patients. The golden rule prescribes that the search for
LA be performed before initiating anticoagulant therapy or after its
withdrawal for an adequate period. For practical reasons, this is not
always possible, and often, patients' blood samples are sent to the
laboratory with the request to detect LA when the patient has already
been started on anticoagulation. In these cases, the laboratory
approach is dependent on the anticoagulant drug administered.If
the anticoagulant drugs are VKAs, LA diagnosis is complicated because
there is, at the moment, no completely reliable strategy to perform the
LA search other than discontinuation of treatment for the time needed
to clear drugs from circulation. If this is not possible, the most
commonly used strategy is the dilution of patient plasma in normal
plasma (1:1 ratio) in the belief that this dilution leads to a
correction of the clotting time prolongation due to VKAs. This is not
always the case and mostly depends on the composition of the reagent
used for testing; because of this, false-negative or false-positive
values may be expected. In addition, due to dilution (1:2), the potency
of LA is reduced by 50% and, therefore, weak LA may be lost at
diagnosis. Alternatives to make diagnosis of LA for patients on VKAs
are the combined use of such snake venoms as Taipan and Ecarin. They
are able to activate FII directly but have different phospholipids
requirements. Studies have shown that their use may be useful to detect
LA in anticoagulated patients.[19] If
patients are taking DOACs, LA diagnosis should not be performed because
the patient's plasma would almost certainly be (false) positive for LA.
Recent data from the literature show that in a population of
LA-negative patients, more than 80% tested positive when the drug was
Rivaroxaban, and the test used to diagnose LA was dRVVT.[20]In
these cases, however, some alternatives allow LA diagnosis. Activated
carbon chemicals (DOAC-Stop®, DOAC-Remove®) have been developed and are
commercially available, which, when mixed with a patient's plasma,
after short incubation, adsorb DOACs onto their surface. LA can be
measured on the supernatant after centrifugation without significant
interference.[21,22] These substances have been variously studied to evaluate their ability to adsorb DOACs and have demonstrated a good capacity.[20] Studies related to their diagnostic capacity for LA have yielded varying results.In
some cases, activated carbons, in addition to DOACs, also adsorb other
plasma substances on their surface, which could modify the procoagulant
strength of the plasma, making the diagnosis of LA complicated.
However, at present, activated charcoals are the only valid means of
performing LA diagnostics in DOAC patients. They do not affect VKAs.
Conclusions
Clinical
registration studies were designed to evaluate whether a fixed dose of
DOACs, without dose adjustment based on laboratory testing, was
effective in treating and preventing thrombotic recurrence while
ensuring adequate hemostasis to avoid bleeding events. This design was
strongly taken into consideration because a positive result would have
been a significant advantage of the DOACs compared to VKAs, which need
dosage adjustment (based on the INR). History has shown that the fixed
dose intuition was valid and DOACs are now used with this regime,
although real-life observations show that some patients could benefit
from dose-adjustment. However, this does not mean that the plasma
concentration of DOACs should never be measured, and there are, in
fact, conditions and patients for whom the measurement of DOACs would
be of extreme value to clinicians. Therefore, it is the responsibility
of clinical laboratories to set up tests that are commercially
available and relatively simple to perform at a time that allows them
to be performed even in an emergency.
Conflicts of interest
AT
received speakers’ fees from Stago, Roche, BioMarin, Werfen. AP
received honoraria for participating as a speaker at educational
meetings organized by Sanofi.
Financial support
This work was partially supported by the Italian Ministry of Health. Ricerca corrente 2023
References
- Poller L. International Normalized Ratios (INR): the first 20 years. J Thromb Haemost 2004;2:849-860. https://doi.org/10.1111/j.1538-7836.2004.00775.x PMid:15140114
- Chantarangkul
V, Tripodi A, van den Besselaar AMHP. Guidelines for thromboplastins
and plasma used to control oral anticoagulant therapy with vitamin K
antagonists. WHO Technical Report Series 2013; No.979:273-305.
- Tripodi
A, Chantarangkul V, Clerici M, Negri B, Galli M, Mannucci PM.
Laboratory control of oral anticoagulant treatment by the INR system in
patients with the antiphospholipid syndrome and lupus anticoagulant.
Results of a collaborative study involving nine commercial
thromboplastins. Br J Haematol 2001;115:672-8. https://doi.org/10.1046/j.1365-2141.2001.03178.x PMid:11736953
- Beier
L, Lu S, França LR, Marler S, Lip GYH, Huisman MV, et al. Evolution of
antithrombotic therapy for patients with atrial fibrillation: The
prospective global GLORIA-AF registry program. PLoS One. 2022; 6;
17:E0274237. https://doi.org/10.1371/journal.pone.0274237 PMid:36201473 PMCid:PMC9536607
- Huisman
MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, et al;
GLORIA-AF Investigators. The Changing Landscape for Stroke Prevention
in AF: Findings From the GLORIA-AF Registry Phase 2. J Am Coll Cardiol
2017;69:777-785.
- PRAC
recommendations on signals. Adopted at the 8-11 April 2019 PRAC
meeting. EMA Pharmacovigilance Risk Assessment Committee (PRAC) https://www.ema.europa.eu/en/documents/prac-recommendation/prac-recommendations-signals-adopted-8-11-april-2019-prac-meeting_en.pdf. Accessed November 2023
- Baglin
T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring Oral
Direct Inhibitors (ODIs) of thrombin and factor Xa: A recommendation
from the Subcommittee on Control of Anticoagulation of the Scientific
and Standardisation Committee of the International Society on
Thrombosis and Haemostasis. J Thromb Haemost 2013. https://doi.org/10.1111/jth.12149 PMid:23347120
- Tripodi
A, Ageno W, Ciaccio M, Legnani C, Lippi G, Manotti C, Marcucci R, Moia
M, Morelli B, Poli D, Steffan A, Testa S. Position Paper on laboratory
testing for patients on direct oral anticoagulants. A Consensus
Document from the SISET, FCSA, SIBioC and SIPMeL. Blood Transfusion
2018;16:462-470.
- Sayar Z, Speed V, Patel JP, Patel RK, Arya R. The perils of inhibiting deficient factors. J Thromb Haemost 2018. https://doi.org/10.1111/jth.14195 PMid:29883037
- Reilly
PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, Ezekowitz
MD, Nehmiz G, Wang S, Wallentin L; RE-LY Investigators. The effect of
dabigatran plasma concentrations and patient characteristics on the
frequency of ischemic stroke and major bleeding in atrial fibrillation
patients: the RE-LY Trial (Randomized Evaluation of Long-Term
Anticoagulation Therapy). J Am Coll Cardiol 2014;63:321-8. https://doi.org/10.1016/j.jacc.2013.07.104 PMid:24076487
- Testa
S, Tripodi A, Legnani C, Pengo V, Abbate R, Dellanoce C, Carraro P,
Salomone L, Paniccia R, Paoletti O, Poli D, Palareti G;
START-Laboratory Register. Plasma levels of direct oral anticoagulants
in real life patients with atrial fibrillation: Results observed in
four anticoagulation clinics. Thromb Res. 2016;137:178-183.https://doi.org/10.1016/j.thromres.2015.12.001 PMid:26672898
- Testa
S, Palareti G, Legnani C, Dellanoce C, Cini M, Paoletti O, Ciampa A,
Antonucci E, Poli D, Morandini R, Tala M, Chiarugi P, Santoro RC,
Iannone AM, De Candia E, Pignatelli P, Faioni EM, Chistolini A, del
Pilar Esteban M, Marietta M, Tripodi A, Tosetto A, for the MAS Study
group. Low levels of direct oral anticoagulants at steady state in
atrial fibrillation patients who later develop thrombotic
complications; the prospective MAS (measure and see) study. Blood
Advance 2024. In press. https://doi.org/10.1101/2023.12.08.23299746
- Pollack
CV Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA,
Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Levy JH,
Sellke FW, Stangier J, Steiner T, Wang B, Kam CW, Weitz JI.
Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511-20. https://doi.org/10.1056/NEJMoa1502000 PMid:26095746
- Siegal
DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, Mathur VS,
Castillo J, Bronson MD, Leeds JM, Mar FA, Gold A, Crowther MA.
Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl
J Med 2015;373:2413-24. https://doi.org/10.1056/NEJMoa1510991 PMid:26559317
- Simon
A, Domanovits H, Ay C, Sengoelge G, Levy JH, Spiel AO. The recommended
dose of idarucizumab may not always be sufficient for sustained
reversal of Dabigatran. J Thromb Haemost 2017;15:1317-1321. https://doi.org/10.1111/jth.13706 PMid:28426914
- Douxfils
J, Adcock DM, Bates SM, Favaloro EJ, Gouin-Thibault I, Guillermo C,
Kawai Y, Lindhoff-Last E, Kitchen S, Gosselin RC. 2021 Update of the
International Council for Standardization in Haematology
Recommendations for Laboratory Measurement of Direct Oral
Anticoagulants. Thromb Haemost 2021;121:1008-1020. https://doi.org/10.1055/a-1450-8178 PMid:33742436
- Tripodi
A, Cohen H, Devreese KMJ. Lupus anticoagulant detection in
anticoagulated patients. Guidance from the Scientific and
Standardization Committee for lupus anticoagulant/antiphospholipid
antibodies of the International Society on Thrombosis and Haemostasis.
J Thromb Haemost. 2020;18:1569-1575. https://doi.org/10.1111/jth.14846 PMid:32619349
- Tripodi
A, Scalambrino E, Clerici M, Peyvandi F. Laboratory Diagnosis of
Antiphospholipid Syndrome in Anticoagulated Patients. Biomedicines
2023;11:1760. doi: 10.3390/biomedicines11061760. https://doi.org/10.3390/biomedicines11061760 PMid:37371855 PMCid:PMC10296059
- Moore
GW, Jones PO, Platton S, Hussain N, White D, Thomas W, Rigano J,
Pouplard C, Gray E, Devreese KMJ. International multicenter,
multiplatform study to validate Taipan snake venom time as a lupus
anticoagulant screening test with ecarin time as the confirmatory test:
Communication from the ISTH SSC Subcommittee on Lupus
Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost
2021;19:3177-3192. https://doi.org/10.1111/jth.15438 PMid:34192404
- Tripodi
A, Scalambrino E, Chantarangkul V, Paoletti O, Clerici M, Novembrino C,
Boscolo-Anzoletti M, Peyvandi F, Testa S. Impact of a commercially
available DOAC absorbent on two integrated procedures for lupus
anticoagulant detection. Thromb Res 2021;204:32-39. https://doi.org/10.1016/j.thromres.2021.06.001 PMid:34126321
- Exner
T, Michalopoulos N, Pearce J, Xavier R, Ahuja M Simple method for
removing DOACs from plasma samples. Thromb Res 2018;163:117-122. https://doi.org/10.1016/j.thromres.2018.01.047 PMid:29407622
- Jourdi
G, Delrue M, Stepanian A, Valaize J, Foulon-Pinto G, Demagny J,
Duchemin J, Nedelec-Gac F, Darnige L, Curis E, Delavenne X, Gaussem P,
Siguret V, Gouin-Thibault I. Potential usefulness of activated charcoal
(DOAC Remove) for dRVVT testing in patients receiving Direct Oral
Anticoagulants. Thromb Res 2019;184:86-91. https://doi.org/10.1016/j.thromres.2019.11.001 PMid:31710863




