Materials and methods
Demographic and clinical data were obtained, including age, sex, underlying hematological disease, HCT type and conditioning regimen, donor CMV seropositivity, administration of antithymocyte globulin (ATG) or post-HCT cyclophosphamide (PTCy) for graft-versus-host-disease (GVHD) prophylaxis, absolute lymphocyte count at day 50 after allo-HCT, and development of GVHD. Total lymphocyte counts and CMV viral load (CMV VL) at the onset of cs-CMVi were collected from patients who developed cs-CMVi.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the CEMIC Ethics Committee (Approval identification number 1461).
Since this is an observational study, patient informed consent was waived by the Ethics Committee (Data Protection Law 25326, section 7, subsection 2).
Definitions, virologic studies, and CMV management. CMVi was defined as virus isolation or detection of nucleic acid in blood, plasma, or another fluid or tissue specimen. cs-CMVi was defined as CMVi or CMVd requiring antiviral treatment. The end-organ disease is the occurrence of clinical symptoms and signs of organ involvement, with CMV documented in tissue by virus isolation, rapid culture, histopathology, immunohistochemistry, DNA hybridization techniques, or CMV VL.[11,17] uCMVr was defined as CMVi requiring no treatment with antiviral drugs.
The following were considered risk factors for CMVi and CMVd: CMV-seropositive recipient with CMV-seronegative donor, acute GVHD, ex vivo T cell depletion, ATG or alemtuzumab use, prednisone (or equivalent) at a dose of 1 mg or more per kilogram of body weight per day, mismatched or unrelated donor, haploidentical donor, cord blood transplant, lymphopenia with a total lymphocyte count <300/mm3, older age, and PTCy.[2,14,18,19]
Patients were stratified according to the risk of developing CMVd. Those presenting one or more of the following factors were stratified as high risk: ex vivo T cell depletion, ATG or alemtuzumab use, prednisone (or equivalent) at a dose of 1 mg or more per kilogram of body weight per day for acute GVHD grade II-IV, mismatched related or unrelated donor, haploidentical donor, and cord blood transplant.[11] Allo-HCT presenting none of the above factors was considered low risk.
For the diagnosis of CMVi, CMV VL was measured in plasma with real-time polymerase chain reaction (qRT-PCR) assay (LightMix, TIB Molbiol) in LightCycler 2.0 or COBAS 480 from January 2012 to November 2021, with a detection threshold of 20 copies/ml and a quantification threshold of 200 copies/ml. From December 2021 onward, CMV VL detection was done with RealStar altona Diagnostic in COBAS 480 with a detection threshold of 100 IU/ml and a quantification threshold of 500 IU/ml. Weekly monitoring started at the time of engraftment or on day 10, whichever occurred first in the PET group and on day 4 to 10 in the LET group, and continued through day 100 post-HCT or beyond in those patients that remained at risk for CMVi.
LET was started after undetectable CMV VL within the previous 48 hours. It was administered from days 5 to 10 and continued through day 100 post-HCT. Since all patients received cyclosporine for GVHD prophylaxis, 240 mg/day of LET was indicated. Only oral formulation was used.
Patients with a positive qRT-PCR result meeting the institutional threshold for PET or with diagnosed CMVd were started on appropriate antiviral therapy according to institutional guidelines.
Regarding CMV VL, the thresholds to consider antiviral treatment for CMVi in the LET group were ≥200 copies/ml in high-risk patients and ≥500 copies/ml in low-risk patients. On the other hand, in the PET group, thresholds were detectable non-quantifiable PCR in high-risk patients and ≥500 copies/ml in low-risk patients.[20] For those managed with CMV VL measured in IU/ml, thresholds were converted to the equivalent in copies/ml. Detectable CMV VL was confirmed with another sample two days later before starting antiviral treatment.
All patients received prophylaxis with acyclovir 800 mg twice daily or valacyclovir 500 mg/day from admission through at least 1-year post-HCT, sulphamethoxazole-trimethoprim three days a week at least six-month post-HCT and until the end of severe immunosuppression, and antifungal prophylaxis through at least day 75 post-HCT according to IDSA and GITMO guidelines.[21-23] GVHD grading was based on consensus guidelines.[24]
Statistical analysis. Descriptive statistics characterized the study population. For continuous variables, centrality (median) and dispersion (IQR) measures were used according to the distribution of variables. Categorical variables were analyzed using absolute frequency and percentage. Groups were compared using the U Mann-Whitney test for continuous variables and the Fisher exact test or the chi-square test for categorical variables. Kaplan-Meier curves for uCMVi, cs-CMVi, and CMVd were estimated for patients who received primary prophylaxis with LET vs. PET. For all tests, a 95% level of statistical significance was used. Analyses were performed with the SPSS (Statistics for Windows, Version 22.0. Armonk, NY, USA) software packages.
Results
The total study population consisted of 105 patients (28 in the LET group and 77 in the PET group) whose baseline characteristics are described in Table 1. There was a predominance of males, with a median age of 42 years. The most frequent underlying diseases were acute myeloblastic leukemia, acute lymphoblastic leukemia, and myelodysplasia; the disease was active in many patients. Compared to the PET group, patients in the LET group received more allo-HCT from alternative donors (54.5% vs. 82.14%, P=0.012), as well as a reduced-intensity conditioning regimen. In contrast, the PET group more frequently underwent the myeloablative regimen. In both groups, the drugs most commonly used for conditioning regimens were fludarabine (91, 86.67%) and mefalan or busulfan (90, 85.71%). Only patients in the PET group received ATG as part of the conditioning regimen. Regarding GVHD prophylaxis, 101 (96.19%) patients received cyclosporine, with no differences between groups. Likewise, mycophenolate was more frequently administered in the LET group (24, 85.71% vs. 45, 58.44%, P=0.009), as well as PTCy (data shown in Table 1).
 |
|
The median time to granulocyte engraftment in the LET vs. PET group was 18 days (IQR: 17-23) vs. 16 days (IQR: 12-20), respectively, P=0.018. Acute GVHD developed in almost half of the patients with no differences between groups, as did acute GVHD grades II-IV with high doses of corticosteroid requirements. The GVHD target organs involved were the skin in 34 patients (32.38%), gastrointestinal tract in 29 (27.62%), liver in 6 (5.71%), and lung in 1 (0.95%).
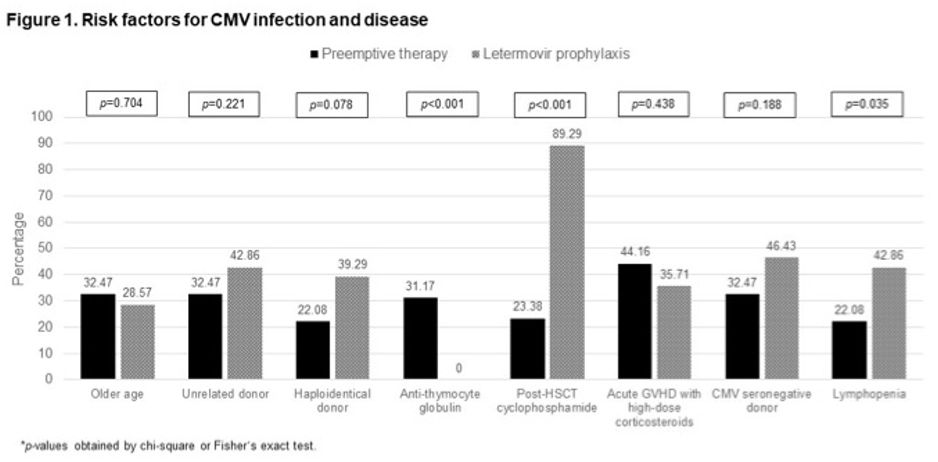
Most patients had several risk factors for CMVi and CMVd, which are outlined in Figure 1. The median number of risk factors for PET vs. LET groups were 3 (IQR: 1-5) vs. 4 (IQR: 3-5), respectively, P=0.72, and more than half of the patients in both groups were classified as high-risk for CMVd.
 |
|
In the LET group, prophylaxis duration was 96 days (IQR: 90-100), with adherence of 100%, and 21 patients evidencing disruption of the gastrointestinal barrier in the pre-engraftment and post-engraftment periods (mucositis in 12, 42.6%, and GVHD in 9, 32.14%). Only one patient discontinued LET for 5 days due to oral mucositis. Two (7.4%) patients presented mild LET-related adverse events (nausea and dysgeusia). Three patients (10.7%) discontinued LET before day 100 post-HCT with no CMVi: the first on day 50 due to refractory acute GVHD grade IV, the second on day 57 due to thrombotic microangiopathy requiring hemodialysis and disseminated adenoviral disease, and the third on day 22 due to underlying disease progression.
Cs-CMVi and CMVd developed in 0 vs. 50 (64.94%), P=<0.0001, and 0 vs. 6 (7.79%), P=0.18, in the LET vs. PET groups, respectively. Five (17.8%) patients in the LET group presented uCMVr, while all episodes in the PET group were cs-CMVi or CMVd. These data are shown in Figure 2. More than one CMVi occurred in 2 (7.14%) patients in the LET group and 16 (20.78%) in the PET group. All the patients with uCMVr had detectable non-quantifiable CMV VL, which became negative in the subsequent weekly control without discontinuation of LET. The patients who developed cs-CMVi had a median CMV VL of 1648 copies/ml (IQR: 478-6240). The median time of occurrence of uCMVr and cs-CMVi after HCT was 43 days (IQR: 22-49) and 40 days (IQR: 26-56), respectively, P=0.84, while the median lymphocyte counts during the episodes were 532/mm3 (IQR: 198-731) and 461/mm3 (220-837), P=0.84.
 |
|
The episodes of cs-CMVi or CMVd were treated with ganciclovir in 32 cases (64%), valganciclovir in 13 (26%), foscarnet in 19 (38%), and cidofovir in 2 (4%). Seventeen (34%) episodes received more than one antiviral drug. The median duration of treatment was 19 days (IQR: 14-33). The 6 patients with CMVd had gastrointestinal (GI) tract involvement, and 2 had CMV VL undetectable at the time of diagnosis. Hospital admission related to cs-CMVi or CMVd in the PET vs. LET group was 47 (61.04%) vs. 0, respectively, P=<0.0001. The 100-day mortality in the LET vs. PET groups was 3 (10.71%) vs. 14 (18.18%), P=0.55, in no case related to CMVi.
Discussion
Several real-world retrospective single-center or multicenter cohort studies have been reported that compared LET primary prophylaxis with controls receiving PET. They could replicate the same results as those obtained in the randomized pivotal phase 3 trial. In one of the largest single-center retrospective studies, Johnsrud et al. compared LET prophylaxis within the first 100 days after allo-HCT in 114 patients at high risk for CMVd with a control group of 637 who received PET. Patients with LET prophylaxis developed no CMVd (0% vs. 5.4%, P=0.006) and required lower hospitalization rates (0.93% vs. 15.23%, P=<0.001).[25] This data agrees with that described in our cohort.
The clinical benefits of LET prophylaxis were evaluated in a systematic review and meta-analysis of all the published real-world studies.[26] They demonstrated a significant decrease in CMVr, cs-CMVi, and CMVd at day 100 and 200 post-HCT, compared to any control group, usually the historical control group. Furthermore, LET significantly reduced the all-cause and non-relapse mortality beyond day 200 post-HCT. Notwithstanding that, considerable heterogeneity in the clinical criteria used to define CMVir and cs-CMVi and related events among these studies could induce a bias in the final results and should, therefore, be assessed.
Unlike most studies that compare LET with the historical control group, ours included a population with clearly defined criteria, and the entire cohort underwent prospective evaluation and follow-up. Other relevant issues need to be outlined. Since the implementation of monitoring with CMV VL and PET strategy, CMVd mainly developed as a gastrointestinal disease worsening GI GVHD. This is a big challenge for diagnoses since the overall incidence of CMVd could be as high as 25%. However, only 42% of the patients with CMV gastroenteritis had preceding evidence of CMV viremia by qRT-PCR VL.[27] In addition, GI CMVd has to be shown as an independent risk factor for reduced overall survival.[28] In agreement with this data, all CMVd in our study were GI; in 2 of 6 patients, CMV VL was negative at the time of diagnosis.
Two studies showed that patients on PET vs. no PET had an increment of readmissions (55% vs. 34%, P=0.0001) and higher antiviral-related adverse events (neutropenia: relative risk [RR] 1.81, 95%CI, 1.48-2.21, and acute kidney injury: RR 2.75, 95%CI, 1.71-4.42).[5,6] Although our study did not evaluate antiviral-related adverse events, we found a higher rate of CMV admissions in the PET group. This data stressed the importance of LET prophylaxis in lowering morbidity in allo-HCT patients.
Unlike Marty's study, we observed that the median time to granulocyte engraftment was longer in the LET group. The slight delay in hematopoietic recovery has been described in haploidentical HCT and those who received PTCy.[29,30] This could explain what was observed in the LET cohort.
Another interesting issue is that no patients in the LET group developed cs-CMVi. In our opinion, this could be due to two reasons. First, compared to Marty's study, in high-risk patients, we chose a higher CMV VL threshold to start PET.[11] Second, our cohort had 100% LET adherence. This is crucial in HIV patients, since virological failure correlates with poor adherence to antiretroviral medications.[31] Given that adherence could not be evaluated in retrospective real-life LET studies, larger prospective studies should be undertaken to address this issue.
Finally, we highlight that all uCMVr in our LET cohort became negative in the subsequent weekly control without discontinuation of LET. These uCMVr were blips defined as the presence of CMV DNA VL at any level in a single plasma specimen, preceded and succeeded by a negative (undetectable) PCR specimen, usually drawn seven days apart.[32] These events were first described in patients without LET prophylaxis and can be frequently observed.[33] Notwithstanding that, this has also been reported in patients under LET.[34] However, as these events usually occur in allo-HCT patients, LET prophylaxis should not be discontinued even in patients at high risk for CMVd until the blip is ruled out.
There are some drawbacks to the present study. 1) The number of patients in each cohort, which limits statistical analysis and hinders assessment of survival in the LET group. Although a more extended follow-up period (beyond day 200 post-HCT) would be more appropriate to evaluate overall mortality, this was not an objective of the study. 2) During the study period, there was a change in the expression of CMV DNA in IU instead of copies/ml, which could lead to a different interpretation of the results. Nevertheless, this was adjusted using a conversion factor. 3) T cell depletion induced by ATG was only observed in the PET group. Thus, this cohort has a higher risk of CMVd. Notwithstanding that, most patients in the LET group received PTCy, which also led to functional and selective T cell depletion by impairment of CD4+ and CD8+ alloreactive T cells.[35] Therefore, patients who received PTCy had lower lymphocyte counts.
The strengths of our research rely on its prospective design, with a high proportion of the cohorts presenting several risk factors for CMVi, as well as increased risk for developing CMVd.
Conclusion
Acknowledgements
Authors’ Contributions
References
- Pande A, Dubberke ER. Cytomegalovirus Infections of
the Stem Cell Transplant Recipient and Hematologic Malignancy Patient.
Infect Dis Clin North Am. 2019; 33 (2): 485-500. doi:
10.1016/j.idc.2019.02.008. https://doi.org/10.1016/j.idc.2019.02.008 PMid:30940460
- Ljungman
P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, Hubacek
P, Navarro D, Cordonnier C, Ward KN. 2017 European Conference on
Infections in Leukaemia group. Guidelines for the management of
cytomegalovirus infection in patients with haematological malignancies
and after stem cell transplantation from the 2017 European Conference
on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19 (8):
e260-e272. doi: 10.1016/S1473-3099(19)30107-0. https://doi.org/10.1016/S1473-3099(19)30107-0 PMid:31153807
- Giménez
E, Torres I, Albert E, Piñana JL, Hernández-Boluda JC, Solano C,
Navarro D. Cytomegalovirus (CMV) infection and risk of mortality in
allogeneic hematopoietic stem cell transplantation (Allo-HSCT): A
systematic review, meta-analysis, and meta-regression analysis. Am J
Transplant. 2019; 19 (9): 2479-2494. doi: 10.1111/ajt.15515. https://doi.org/10.1111/ajt.15515 PMid:31247126
- Yong
MK, Ananda-Rajah M, Cameron PU, Morrissey CO, Spencer A, Ritchie D,
Cheng AC, Lewin SR, Slavin M. Cytomegalovirus Reactivation Is
Associated with Increased Risk of Late-Onset Invasive Fungal Disease
after Allogeneic Hematopoietic Stem Cell Transplantation: A Multicenter
Study in the Current Era of Viral Load Monitoring. Biol Blood Marrow
Transplant. 2017; 23 (11): 1961-1967. doi: 10.1016/j.bbmt.2017.07.025. https://doi.org/10.1016/j.bbmt.2017.07.025 PMid:28797778
- Zavras
P, Su Y, Fang J, Stern A, Gupta N, Tang Y, Raval A, Giralt S, Perales
MA, Jakubowski AA, Papanicolaou GA. Impact of Preemptive Therapy for
Cytomegalovirus on Toxicities after Allogeneic Hematopoietic Cell
Transplantation in Clinical Practice: A Retrospective Single-Center
Cohort Study. Biol Blood Marrow Transplant. 2020; 26 (8): 1482-1491.
doi: 10.1016/j.bbmt.2020.03.019. https://doi.org/10.1016/j.bbmt.2020.03.019 PMid:32315708 PMCid:PMC8220837
- Fang
J, Su Y, Zavras PD, Raval AD, Tang Y, Perales MA, Giralt S, Stern A,
Papanicolaou GA. Impact of Preemptive Therapy for Cytomegalovirus on
Hospitalizations and Cost after Hematopoietic Stem Cell
Transplantation. Biol Blood Marrow Transplant. 2020; 26 (10):
1937-1947. doi: 10.1016/j.bbmt.2020.06.025. https://doi.org/10.1016/j.bbmt.2020.06.025 PMid:32640313 PMCid:PMC8248281
- Meng
XY, Fu HX, Zhu XL, Wang JZ, Liu X, Yan CH, Zhang YY, Mo XD, Wang Y, Han
W, Chen YH, Chen DB, Liu HX, Chang YJ, Xu LP, Liu KY, Huang XJ, Zhang
XH. Comparison of different cytomegalovirus diseases following
haploidentical hematopoietic stem cell transplantation. Ann Hematol.
2020; 99(11):2659-2670. doi: 10.1007/s00277-020-04201-4. https://doi.org/10.1007/s00277-020-04201-4 PMid:32734550
- Haidar
G, Boeckh M, Singh N. Cytomegalovirus Infection in Solid Organ and
Hematopoietic Cell Transplantation: State of the Evidence. J Infect
Dis. 2020; 221(Suppl 1): S23-S31. doi: 10.1093/infdis/jiz454. https://doi.org/10.1093/infdis/jiz454 PMid:32134486 PMCid:PMC7057778
- Green
ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, Sorror ML, Goyal
S, Özkök S, Yi J, Sahoo F, Kimball LE, Jerome KR, Marks MA, Boeckh M.
CMV Viral Load and Mortality after Hematopoietic Cell Transplantation:
A Cohort Study in the Era of Pre-emptive Therapy. Lancet Haematol 2016;
3 (3): e119-e127. doi: 10.1016/S2352-3026(15)00289-6. https://doi.org/10.1016/S2352-3026(15)00289-6 PMid:26947200
- Teira
P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, Green JS,
Saad A, Antin JH, Savani BN, Lazarus HM, Seftel M, Saber W, Marks D,
Aljurf M, Norkin M, Wingard JR, Lindemans CA, Boeckh M, Riches ML,
Auletta JJ. Early cytomegalovirus reactivation remains associated with
increased transplant-related mortality in the current era: a CIBMTR
analysis. Blood. 2016; 127 (20): 2427-2438. doi:
10.1182/blood-2015-11-679639. https://doi.org/10.1182/blood-2015-11-679639 PMid:26884374 PMCid:PMC4874224
- Marty
FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, Haider S,
Ullmann AJ, Katayama Y, Brown J, Mullane KM, Boeckh M, Blumberg EA,
Einsele H, Snydman DR, Kanda Y, DiNubile MJ, Teal VL, Wan H, Murata Y,
Kartsonis NA, Leavitt RY, Badshah C. Letermovir Prophylaxis for
Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med.
2017; 377 (25): 2433-2444. doi: 10.1056/NEJMoa1706640. https://doi.org/10.1056/NEJMoa1706640 PMid:29211658
- Ljungman
P, Schmitt M, Marty FM, Maertens J, Chemaly RF, Kartsonis NA, Butterton
JR, Wan H, Teal VL, Sarratt K, Murata Y, Leavitt RY, Badshah C. A
Mortality Analysis of Letermovir Prophylaxis for Cytomegalovirus (CMV)
in CMV-seropositive Recipients of Allogeneic Hematopoietic Cell
Transplantation. Clin Infect Dis 2020; 70 (8): 1525-1533. doi:
10.1093/cid/ciz490. https://doi.org/10.1093/cid/ciz490 PMid:31179485 PMCid:PMC7146004
- Golan
Y, Tang Y, Mt-Isa S, Wan H, Teal V, Badshah C, Dadwal S. Impact of
Letermovir Use for Cytomegalovirus Prophylaxis on Re-Hospitalization
Following Allogeneic Hematopoietic Stem Cell Transplantation: An
Analysis of a Phase III Randomized Clinical Trial. Pharmacoecon Open.
2021; 5(3):469-473. doi: 10.1007/s41669-021-00264-9. https://doi.org/10.1007/s41669-021-00264-9 PMid:33871830 PMCid:PMC8333192
- Hakki
M, Aitken SL, Danziger-Isakov L, Michaels MG, Carpenter PA, Chemaly RF,
Papanicolaou GA, Boeckh M, Marty FM. American Society for
Transplantation and Cellular Therapy Series: #3-Prevention of
Cytomegalovirus Infection and Disease After Hematopoietic Cell
Transplantation. Transplant Cell Ther. 2021; 27 (9): 707-719. doi:
10.1016/j.jtct.2021.05.001. https://doi.org/10.1016/j.jtct.2021.05.001 PMid:34452721
- Girmenia
C, Lazzarotto T, Bonifazi F, Patriarca F, Irrera G, Ciceri F, Aversa F,
Citterio F, Cillo U, Cozzi E, Gringeri E, Baldanti F, Cavallo R,
Clerici P, Barosi G, Grossi P. Assessment and prevention of
cytomegalovirus infection in allogeneic hematopoietic stem cell
transplant and in solid organ transplant: A multidisciplinary consensus
conference by the Italian GITMO, SITO, and AMCLI societies. Clin
Transplant. 2019; 33 (10): e13666. doi: 10.1111/ctr.13666. https://doi.org/10.1111/ctr.13666 PMid:31310687
- Brissot
E, Alsuliman T, Beauvais D, Bonnin A, Mear JB, Souchet L, Villate A,
Yakoub-Agha I, Bazarbachi A. Prophylaxie antivirale pour le CMV,
l'HSV/VZV et le VHB après allogreffe de cellules souches
hématopoïétiques chez l'adulte : recommandations de la Société
francophone de greffe de mœlle et de thérapie cellulaire (SFGM-TC)
[Antiviral prophylaxis for CMV, HSV/VZV and HBV in allogeneic
hematopoietic cell transplantation in adult patients: Guidelines from
the Francophone Society of Bone Marrow Transplantation and Cellular
Therapy (SFGM-TC)]. Bull Cancer. 2020; 107 (1S): S1-S6. doi:
10.1016/j.bulcan.2019.09.002. https://doi.org/10.1016/j.bulcan.2019.09.002 PMid:31627903
- Ljungman
P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, Pikis A,
Razonable RR, Miller V, Griffiths PD. Disease Definitions Working Group
of the Cytomegalovirus Drug Development Forum. Definitions of
Cytomegalovirus Infection and Disease in Transplant Patients for Use in
Clinical Trials. Clin Infect Dis. 2017; 64 (1): 87-91. doi:
10.1093/cid/ciw668. https://doi.org/10.1093/cid/ciw668 PMid:27682069
- Goldsmith
SR, Abid MB, Auletta JJ, Bashey A, Beitinjaneh A, Castillo P, Chemaly
RF, Chen M, Ciurea S, Dandoy CE, Díaz MÁ, Fuchs E, Ganguly S, Kanakry
CG, Kanakry JA, Kim S, Komanduri KV, Krem MM, Lazarus HM, Liu H,
Ljungman P, Masiarz R, Mulroney C, Nathan S, Nishihori T, Page KM,
Perales MA, Taplitz R, Romee R, Riches M. Posttransplant
cyclophosphamide is associated with increased cytomegalovirus
infection: a CIBMTR analysis. Blood. 2021; 137 (23): 3291-3305. doi:
10.1182/blood.2020009362. https://doi.org/10.1182/blood.2020009362 PMid:33657221 PMCid:PMC8351903
- Einsele
H, Ehninger G, Steidle M, Fischer I, Bihler S, Gerneth F, Vallbracht A,
Schmidt H, Waller HD, Müller CA. Lymphocytopenia as an unfavorable
prognostic factor in patients with cytomegalovirus infection after bone
marrow transplantation. Blood. 1993; 82 (5):1672-1678. PMID: 8395913 https://doi.org/10.1182/blood.V82.5.1672.1672 PMid:8395913
- Boeckh
M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell
transplant recipients. Blood. 2009; 113(23):5711-9. doi:
10.1182/blood-2008-10-143560. https://doi.org/10.1182/blood-2008-10-143560 PMid:19299333 PMCid:PMC2700312
- Girmenia
C, Barosi G, Piciocchi A, Arcese W, Aversa F, Bacigalupo A, Bandini G,
Bosi A, Busca A, Castagnola E, Caselli D, Cesaro S, Ciceri F,
Locasciulli A, Locatelli F, Mikulska M, Pagano L, Prete A, Raiola AM,
Rambaldi A. Primary prophylaxis of invasive fungal diseases in
allogeneic stem cell transplantation: revised recommendations from a
consensus process by Gruppo Italiano Trapianto Midollo Osseo (GITMO).
Biol Blood Marrow Transplant. 2014;20 (8):1080-8. doi:
10.1016/j.bbmt.2014.02.018. https://doi.org/10.1016/j.bbmt.2014.02.018 PMid:24582783
- Freifeld
AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II,
Rolston KV, Young JA, Wingard JR; Infectious Diseases Society of
America. Clinical practice guideline for the use of antimicrobial
agents in neutropenic patients with cancer: 2010 update by the
Infectious Diseases Society of America. Clin Infect Dis 2011; 52:
e56-93. doi: 10.1093/cid/cir073. https://doi.org/10.1093/cid/cir073 PMid:21258094
- Patterson
TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R,
Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach
WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Practice
guidelines for the diagnosis and management of aspergillosis: 2016
update by the Infectious Diseases Society of America. Clin Infect Dis
2016; 63: 433-42. doi: 10.1093/cid/ciw326. https://doi.org/10.1093/cid/ciw326 PMid:27365388 PMCid:PMC4967602
- Przepiorka
D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED.
1994 Consensus Conference on Acute GVHD Grading. Bone Marrow
Transplant. 1995; 15(6):825-8. PMID: 7581076.
- Johnsrud
JJ, Nguyen IT, Domingo W, Narasimhan B, Efron B, Brown JW. Letermovir
Prophylaxis Decreases Burden of Cytomegalovirus (CMV) in Patients at
High Risk for CMV Disease Following Hematopoietic Cell Transplant. Biol
Blood Marrow Transplant. 2020; 26 (10): 1963-1970. doi:
10.1016/j.bbmt.2020.07.002. https://doi.org/10.1016/j.bbmt.2020.07.002 PMid:32653623
- Vyas
A, Raval AD, Kamat S, LaPlante K, Tang Y, Chemaly RF. Real-World
Outcomes Associated With Letermovir Use for Cytomegalovirus Primary
Prophylaxis in Allogeneic Hematopoietic Cell Transplant Recipients: A
Systematic Review and Meta-analysis of Observational Studies. Open
Forum Infect Dis. 2022; 10(1): ofac 687. doi: 10.1093/ofid/ofac687. https://doi.org/10.1093/ofid/ofac687 PMid:36726548 PMCid:PMC9879759
- Cho
BS, Yahng SA, Kim JH, Yoon JH, Shin SH, Lee SE, Choi SM, Lee DG, Eom
KS, Park G, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min
WS, Park CW. Impact of cytomegalovirus gastrointestinal disease on the
clinical outcomes in patients with gastrointestinal graft-versus-host
disease in the era of preemptive therapy. Ann Hematol. 2013;
92(4):497-504. doi: 10.1007/s00277-012-1632-x. https://doi.org/10.1007/s00277-012-1632-x PMid:23180439
- Bhutani
D, Dyson G, Manasa R, Deol A, Ratanatharathorn V, Ayash L, Abidi M, Lum
LG, Al-Kadhimi Z, Uberti JP. Incidence, risk factors, and outcome of
cytomegalovirus viremia and gastroenteritis in patients with
gastrointestinal graft-versus-host disease. Biol Blood Marrow
Transplant 2015; 21(1):159-64. doi: 10.1016/j.bbmt.2014.10.004. https://doi.org/10.1016/j.bbmt.2014.10.004 PMid:25445637 PMCid:PMC4283200
- García-Cadenas
I, Redondo S, Esquirol A, Portos JM, Novelli S, Saavedra S, Moreno C,
Garrido A, Oñate G, López J, Caballero AC, Miqueleiz S, Arguello-Tomas
M, Briones J, Sierra J, Martino R. Successful Outcome in Patients with
Myelofibrosis Undergoing Allogeneic Donor Hematopoietic Cell
Transplantation Using Reduced Doses of Post-Transplantation
Cyclophosphamide: Challenges and Review of the Literature. Transplant
Cell Ther. 2023; 29 (7): 473.e1-473.e6. doi:
10.1016/j.jtct.2023.04.008. https://doi.org/10.1016/j.jtct.2023.04.008 PMid:37086849
- DeZern
AE, Zahurak ML, Symons HJ, Cooke KR, Rosner GL, Gladstone DE, Huff CA,
Swinnen LJ, Imus P, Borrello I, Wagner-Johnston N, Ambinder RF, Luznik
L, Bolaños-Meade J, Fuchs EJ, Jones RJ, Brodsky RA. Haploidentical BMT
for severe aplastic anemia with intensive GVHD prophylaxis including
posttransplant cyclophosphamide. Blood Adv. 2020; 4 (8):1770-1779. doi:
10.1182/bloodadvances.2020001729. https://doi.org/10.1182/bloodadvances.2020001729 PMid:32343796 PMCid:PMC7189283
- SeyedAlinaghi
S, Afsahi AM, Moradi A, Parmoon Z, Habibi P, Mirzapour P, Dashti M,
Ghasemzadeh A, Karimi E, Sanaati F, Hamedi Z, Molla A, Mehraeen E,
Dadras O. Current ART, determinants for virologic failure and
implications for HIV drug resistance: an umbrella review. AIDS Res
Ther. 2023 Oct; 20 (1):74. doi: 10.1186/s12981-023-00572-6. https://doi.org/10.1186/s12981-023-00572-6 PMid:37884997 PMCid:PMC10604802
- Huntley
D, Talaya A, Giménez E, Martínez A, Hernández-Boluda JC, Hernani R,
Torres I, Alberola J, Albert E, Piñana JL, Solano C, Navarro D.
Features of Cytomegalovirus DNAemia Blips in Allogeneic Hematopoietic
Stem Cell Transplant Recipients: Implications for Optimization of
Preemptive Antiviral Therapy Strategies. Biol Blood Marrow Transplant.
2020; 26(5):972-977. doi: 10.1016/j.bbmt.2020.01.015. https://doi.org/10.1016/j.bbmt.2020.01.015 PMid:32007638
- Hill
JA, Mayer BT, Xie H, Leisenring WM, Huang ML, Stevens-Ayers T, Milano
F, Delaney C, Jerome KR, Zerr DM, Nichols G, Boeckh M, Schiffer JT.
Kinetics of Double-Stranded DNA Viremia After Allogeneic Hematopoietic
Cell Transplantation. Clin Infect Dis. 2018; 66(3):368-375. doi:
10.1093/cid/cix804. https://doi.org/10.1093/cid/cix804 PMid:29020348 PMCid:PMC5850428
- Giménez
E, Guerreiro M, Torres I, Aguilar C, Albert E, Hernández-Boluda JC,
Hernani R, Pérez A, Amat P, Piñana JL, Montoro J, Solano C, Navarro D.
Features of cytomegalovirus DNAemia and virus-specific T-cell responses
in allogeneic hematopoietic stem-cell transplant recipients during
prophylaxis with letermovir. Transpl Infect Dis. 2023; 25(2): e14021.
doi: 10.1111/tid.14021. https://doi.org/10.1111/tid.14021 PMid:36748748
- Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Posttransplantation cyclophosphamide prevents graft-versus- host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest 2019; 129:2357-73. doi: 10.1172/JCI124218. https://doi.org/10.1172/JCI124218 PMid:30913039 PMCid:PMC6546453