Nicola Sgherza1, Anna Mestice1, Angela
Maria Vittoria Larocca2 and Pellegrino Musto1,3.
1
Hematology and Stem Cell Transplantation Unit, AOUC Policlinico, Bari,
Italy.
2 Hygiene Unit, AOUC Policlinico, Bari, Italy.
3 Department of Precision and Regenerative Medicine and
Ionian Area, "Aldo Moro" University School of Medicine, Bari, Italy.
Correspondence to:
Prof. Pellegrino Musto, Hematology and Bone Marrow Transplantation
Unit, AOUC Policlinico and Department of Precision and Regenerative
Medicine and Ionian Area, “Aldo Moro” University School of Medicine,
Bari, Italy. E-mail:
pellegrino.musto@uniba.it
Published: March 01, 2024
Received: January 07, 2024
Accepted: February 16, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024022 DOI
10.4084/MJHID.2024.022
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To
the editor
Lymphopenia
(particularly low CD19+ B-lymphocyte count) and current treatment with
either anti-CD38 or anti-BCMA monoclonal antibodies (MoAbs) have been
reported to significantly correlate with poor antibody response to
conventional doses of anti-SARS-CoV-2 vaccines in patients with
multiple myeloma (MM).[1-3] Notably, “booster” doses
have been shown to enhance the humoral response of these patients.[4-7]
We recently reported the greatly improved clinical outcome of
breakthrough SARS-CoV-2 infection in MM patients who had received three
or more doses of anti-SARS-CoV-2 vaccines during the different phases
of the pandemic COVID-19 including the most recent viral variants of
concern (VOCs);[8,9] most of the tested patients had
developed an adequate antibody response (anti-spike IgG) to the virus.[9]
Due to the scarcity of data about the role of different lymphocyte
subsets in this specific population of patients, in the present study,
we aimed to evaluate a possible relationship between antibody response
after SARS-CoV-2 infection in “booster” vaccinated (at least 3 doses)
MM patients and main circulating lymphocyte subpopulations. We also
investigated the possible correlation between antibody titer and
current treatments, including anti-CD38 MoAbs (daratumumab and
isatuximab), in the same patient population. Sixty-two MM patients with
breakthrough SARS-CoV-2 infection (men, 58.1%; median age, 65.5 years)
followed at our Institution were included in this study between January
2022 and April 2023, when prevailing VOCs were Omicron BA.1, BA.2 and
BA.5. Their main baseline characteristics of are listed in Table 1.
All patients had been previously vaccinated against SARS-CoV-2
infection with at least three doses. Acquisition of informed consent
and collection of serum samples were performed at the first outpatient
visit after a median number of 22 days (range: 9-162) from SARS-CoV-2
infection. Determination of anti-spike IgG antibodies was performed
using the SARS-CoV-2 IgG II Quant ABBOTT assay, an automated, two-step
immunoassay (Chemiluminescent Microparticle ImmunoAssay technology) for
the qualitative and quantitative determination of immunoglobulin class
G (IgG) antibodies, including neutralizing antibodies to the receptor
binding domain of the S1 subunit of the spike protein of SARS-CoV-2 in
human serum and plasma. It utilizes a four Parameter Logistic Curve fit
data reduction method (4PLC, Y-weighted) to generate a calibration and
results. The chemiluminescent reaction is measured as a relative light
unit (RLU). There is a direct relationship between the amount of IgG
antibodies to SARS-CoV-2 in the sample and the RLU detected by the
system optic. Results were reported as arbitrary units (AU), with a
positivity cut-off of ≥ 50 AU/mL as an arbitrary threshold for
“adequate” response. Flow cytometric analyses were performed for
assessment of the patients' lymphocyte status. Briefly, 50 μl of EDTA
whole blood was stained with 20 μl of BD Multitest™
CD3/CD16+CD56/CD45/CD4/ CD19/CD8 (FITC-labeled CD3, clone SK7;
PE-labeled CD16, clone B73.1, and CD56, clone NCAM 16.2; PerCP-labeled
CD45, clone 2D1 (HLe-1); PE-Cy7-labeled CD4, clone SK3; APC-labeled
CD19, clone SJ25C1; and APC-Cy7-labeled CD8, clone SK1) in BD Trucount
tubes after 15 minutes in the dark at room temperature 450 µL of
lysing solution were added to the tube. After 10-15 minutes, samples
were analyzed on the BD FACSCanto II flow cytometer. Absolute counts of
T cell subsets, B cells, and NK cells were determined by the software
BD FACSCanto™. Correlation between different lymphocyte subpopulations
and anti-SARS-CoV-2 antibody titers was investigated using Spearman’s
Rho criterion, while comparisons between groups were performed by the
Mann–Whitney U test. Statistical analyses were carried out using Jamovi
(version 2.4.7) and GraphPad Prism (version 8.3.0). The favorable
clinical outcome of breakthrough SARS-CoV-2 infection in this cohort of
patients has been previously reported;[9] in
particular, only 4
hospitalizations (6.4%) were observed, but none in an intensive cure
unit. After a median number of 22 days (range 9-162) from positive
swabs for SARS-CoV-2, almost all patients (60/62, 96.8%) achieved a
titer greater than 50 AU/mL. Only two patients showed a lower titer
after 5 and 3 vaccine doses, respectively: a 79-year-old female,
receiving isatuximab, pomalidomide, and dexamethasone as 4th
line therapy, and an 82-year-old female, receiving elotuzumab,
pomalidomide, and dexamethasone as 3rd
line treatment. At the time of
SARS-CoV-2 infection, these patients were respectively in partial
response and very good partial response, according to the International
Myeloma Working Group (IMWG) criteria. Notably, both these patients
showed a low count of CD19+ B-lymphocytes and the concomitant use of
pomalidomide. Regarding the antibody response according to the absolute
count of CD19+ B-Lymphocytes, the presence of a direct correlation
between the two variables (Spearman: 0.417; p= 0.007) (Figure 1A), and a significant
difference according to the median value used as a cut-off level (Figure 2A)
were observed. By contrast, assessing the impact of the absolute count
of other lymphocyte populations on the development of antibody titer,
no correlation was found (CD4+: Spearman: -0.010, p = 0.950; CD8+:
Spearman: -0.108, p = 0.506; CD16+CD56+: Spearman: 0.098, p = 0.547) (Figure 1, B-D).
Likewise, no statistically significant differences in terms of antibody
titer emerged comparing patients with lower versus higher median CD4,
CD8, and CD16/CD56 positive lymphocyte absolute values (Figure 2, B-D).
Finally, evaluating the antibody response according to current
treatment with anti-CD38 Mo-abs, no statistically significant
correlation was identified between 31 patients undergoing these
treatments and 31 patients who did not (Figure
2E).
Overall, our study suggests that, in patients who have previously
received three or more doses of anti-SARS-CoV-2 vaccines, the absolute
number of CD19+ B cells may marginally reduce the production of
specific antibodies after breakthrough SARS-CoV-2 infections without
significantly decreasing; however, the percentage of patients with
potentially “protective” titers. In this setting, the absolute number
of T and NK populations, as well as the use of anti-CD38 antibodies for
the treatment of MM, did not show significant effects on humoral
response to viral infection. Curiously, the only two patients with
suboptimal humoral response after breakthrough SARS-CoV-2 infection
were both receiving pomalidomide; the possible detrimental effect of
this drug on antibody production would warrant further investigation.
However, evaluating the antibody response according to current
treatment with IMIDs, no statistically significant correlation was
identified between 48 patients undergoing these treatments and 14
patients who did not (Figure 2F).
The study has several limitations, particularly the limited number of
patients tested, the lack of information about serological response and
lymphocyte counts before infection, the heterogeneous timing of blood
collection, the different types of vaccine employed, and the lack of a
control group. Furthermore, the important role of specific functional
aspects of T and NK-cell responses to breakthrough SARS-CoV-2 infection
in fully vaccinated MM patients[10-12] was not
investigated. Notwithstanding, our observation is in line with the
generally favorable clinical outcome of COVID-19 we observed in these
patients and would seem to reflect the independence of clinical and
serological response upon quantitative amounts of different lymphocyte
sub-populations present at the time of viral infection, including
patients receiving anti-CD38 therapies after booster vaccinations and
infected by novel SARS-CoV-2 VOCs that represent the current
epidemiological scenario.
 |
Table
1. Clinical and laboratory characteristics of MM patients with
breakthrough SARS-CoV-2 Infection after at least three anti-SARS-CoV-2
vaccine doses. |
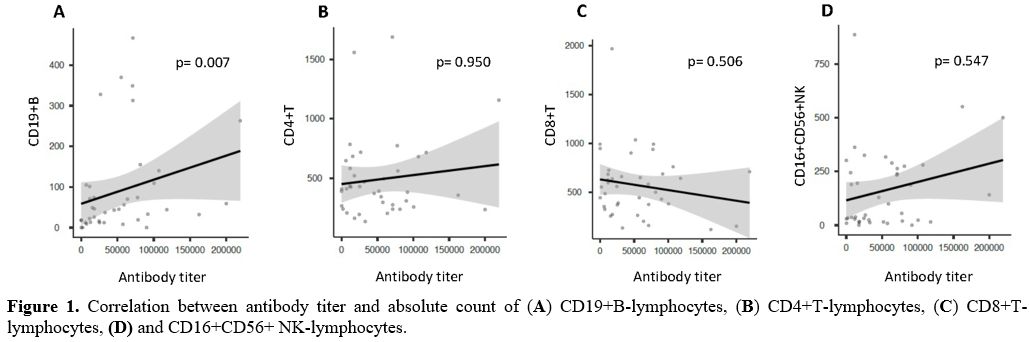 |
Figure 1.
Correlation between antibody titer and absolute count of (A) CD19+B-lymphocytes, (B) CD4+T-lymphocytes, (C) CD8+T-lymphocytes, (D) and CD16+CD56+
NK-lymphocytes. |
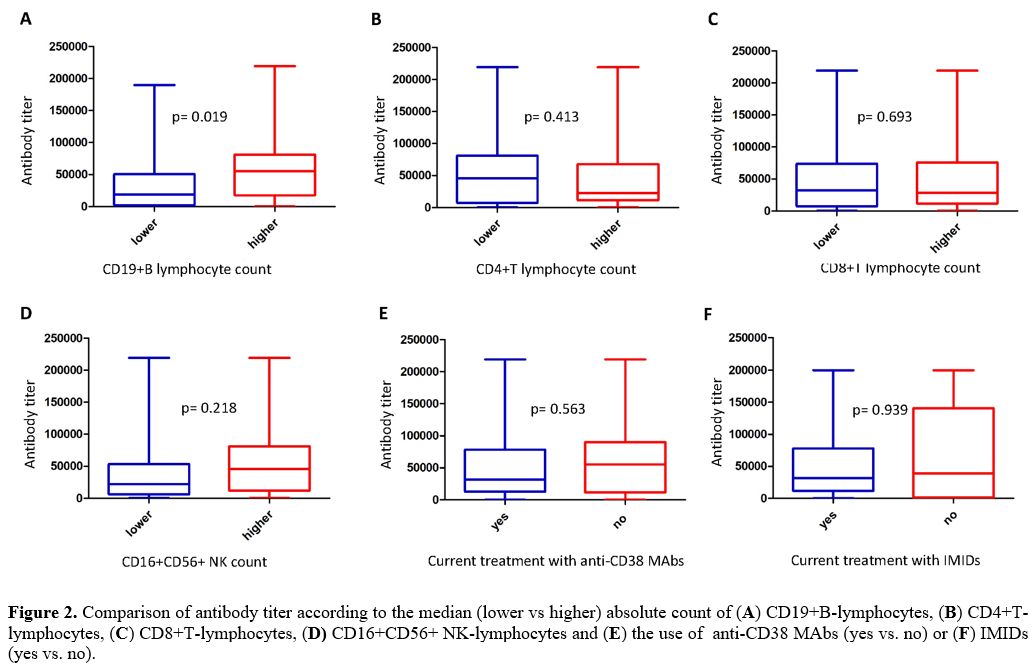 |
Figure 2.
Comparison of antibody titer according to the median (lower vs higher)
absolute count of (A)
CD19+B-lymphocytes, (B)
CD4+T-lymphocytes, (C)
CD8+T-lymphocytes, (D)
CD16+CD56+ NK-lymphocytes and (E)
the use of anti-CD38 MAbs (yes vs. no) or (F) IMIDs (yes vs. no).
|
Authorship
Contributions
Nicola
Sgherza and Pellegrino Musto conceived and led the project. Nicola
Sgherza conducted database building, extraction and coding. Nicola
Sgherza and Pellegrino Musto queried and analyzed the data, wrote the
main manuscript text, and created figures and tables. All authors made
a substantial intellectual contribution to the study, interpreted the
data, discussed the results, and reviewed, edited, and approved the
final version of the manuscript.
References
- Ghandili S, Schönlein M, Lütgehetmann M, Schulze
Zur Wiesch J, Becher H, Bokemeyer C, Sinn M, Weisel KC, Leypoldt LB.
Post-Vaccination Anti-SARS-CoV-2-Antibody Response in Patients with
Multiple Myeloma Correlates with Low CD19+ B-Lymphocyte Count and
Anti-CD38 Treatment. Cancers (Basel). 2021 Jul 28;13(15):3800. https://doi.org/10.3390/cancers13153800
PMid:34359701 PMCid:PMC8345197
- Van
Oekelen O, Gleason CR, Agte S, Srivastava K, Beach KF, Aleman A, Kappes
K; PVI/Seronet team; Mouhieddine TH, Wang B, Chari A, Cordon-Cardo C,
Krammer F, Jagannath S, Simon V, Wajnberg A, Parekh S. Highly variable
SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA
vaccination in patients with multiple myeloma. Cancer Cell. 2021 Aug
9;39(8):1028-1030. https://doi.org/10.1016/j.ccell.2021.06.014
PMid:34242572 PMCid:PMC8238657
- Terpos
E, Gavriatopoulou M, Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S,
Malandrakis P, Fotiou D, Papanagnou ED, Migkou M, Theodorakakou F,
Roussou M, Eleutherakis-Papaiakovou E, Kanellias N, Trougakos IP,
Kastritis E, Dimopoulos MA. The neutralizing antibody response
post-COVID-19 vaccination in patients with myeloma is highly dependent
on the type of anti-myeloma treatment. Blood Cancer J. 2021 Aug
2;11(8):138. https://doi.org/10.1038/s41408-021-00530-3
PMid:34341335 PMCid:PMC8327056
- Faustini
SE, Hall A, Brown S, Roberts S, Hill H, Stamataki Z; (PITCH)
consortium; Jenner MW, Owen RG, Pratt G, Cook G, Richter A, Drayson MT,
Kaiser MF, Heaney JLJ. Immune responses to COVID-19 booster
vaccinations in intensively anti-CD38 antibody-treated patients with
ultra-high-risk multiple myeloma: results from the Myeloma UK (MUK)
nine OPTIMUM trial. Br J Haematol. 2023 Jun;201(5):845-850. https://doi.org/10.1111/bjh.18714
PMid:36895158
- Terpos
E, Gavriatopoulou M, Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S,
Malandrakis P, Papanagnou ED, Migkou M, Kanellias N, Kastritis E,
Trougakos IP, Dimopoulos MA. Booster BNT162b2 optimizes SARS-CoV-2
humoral response in patients with myeloma: the negative effect of
anti-BCMA therapy. Blood. 2022 Mar 3;139(9):1409-1412. https://doi.org/10.1182/blood.2021014989
PMid:34986251 PMCid:PMC8736278
- Aleman
A, Van Oekelen O, Upadhyaya B, Beach K, Kogan Zajdman A, Alshammary H,
Serebryakova K, Agte S, Kappes K, Gleason CR, Srivastava K;
PVI/MM/Seronet Study Group; Almo S, Cordon-Cardo C, Krammer F, Merad M,
Jagannath S, Wajnberg A, Simon V, Parekh S. Augmentation of humoral and
cellular immune responses after third-dose SARS-CoV-2 vaccination and
viral neutralization in myeloma patients. Cancer Cell. 2022 May
9;40(5):441-443. https://doi.org/10.1016/j.ccell.2022.03.013
PMid:35390296; PMCid:PMC8983835
- Šušol
O, Hájková B, Zelená H, Hájek R. Third dose of COVID-19 vaccine
restores immune response in patients with haematological malignancies
after loss of protective antibody titres. Br J Haematol. 2022
May;197(3):302-305. https://doi.org/10.1111/bjh.18073
PMid:35076937
- Musto
P, Salmanton-García J, Sgherza N, Bergantim R, Farina F, Glenthøj A,
Cengiz Seval G, Weinbergerová B, Bonuomo V, Bilgin YM, van Doesum J,
Jaksic O, Víšek B, Falces-Romero I, Marchetti M, Dávila-Valls J,
Martín-Pérez S, Nucci M, López-García A, Itri F, Buquicchio C, Verga L,
Piukovics K, Navrátil M, Collins GP, Jiménez M, Fracchiolla NS,
Labrador J, Prezioso L, Rossi E, Čolović N, Meers S, Kulasekararaj A,
Cuccaro A, Blennow O, Valković T, Sili U, Ledoux MP, Batinić J,
Passamonti F, Machado M, Duarte RF, Poulsen CB, Méndez GA, Espigado I,
Demirkan F, Čerňan M, Cattaneo C, Petzer V, Magliano G, Garcia-Vidal C,
El-Ashwah S, Gomes-Da-Silva M, Vena A, Ormazabal-Vélez I, van Praet J,
Dargenio M, De-Ramón C, Del Principe MI, Marques-De-Almeida J, Wolf D,
Szotkowski T, Obr A, Çolak GM, Nordlander A, Izuzquiza M, Cabirta A,
Zambrotta GPM, Cordoba R, Žák P, Ammatuna E, Mayer J, Ilhan O,
García-Sanz R, Quattrone M, Arellano E, Nunes-Rodrigues R, Emarah Z,
Aiello TF, Hanakova M, Ráčil Z, Bavastro M, Limongelli A, Rahimli L,
Marchesi F, Cornely OA, Pagano L. Survival in multiple myeloma and
SARS-COV-2 infection through the COVID-19 pandemic: Results from the
epicovideha registry. Hematol Oncol. 2023 Dec 4. https://doi.org/10.1002/hon.3240
PMid:38050405
- Sgherza
N, Curci P, Rizzi R, Battisti O, Perfetto A, Weigl S, Larocca AMV,
Chironna M, Tafuri S, Musto P. Clinical outcome of breakthrough
COVID-19 in multiple myeloma patients after three or more
anti-SARS-CoV-2 vaccine doses: a single center analysis of 64 cases.
Ann Hematol. 2024 Jan;103(1):351-355. https://doi.org/10.1007/s00277-023-05484-z
PMid:37782371
- Aleman
A, Upadhyaya B, Tuballes K, Kappes K, Gleason CR, Beach K, Agte S,
Srivastava K; PVI/Seronet Study Group; Van Oekelen O, Barcessat V,
Bhardwaj N, Kim-Schulze S, Gnjatic S, Brown B, Cordon-Cardo C, Krammer
F, Merad M, Jagannath S, Wajnberg A, Simon V, Parekh S. Variable
cellular responses to SARS-CoV-2 in fully vaccinated patients with
multiple myeloma. Cancer Cell. 2021 Nov 8;39(11):1442-1444. https://doi.org/10.1016/j.ccell.2021.09.015
PMid:34706273 PMCid:PMC8523488
- Azeem
MI, Nooka AK, Shanmugasundaram U, Cheedarla N, Potdar S, Manalo RJ,
Moreno A, Switchenko JM, Cheedarla S, Doxie DB, Radzievski R, Ellis ML,
Manning KE, Wali B, Valanparambil RM, Maples KT, Baymon E, Kaufman JL,
Hofmeister CC, Joseph NS, Lonial S, Roback JD, Sette A, Ahmed R, Suthar
MS, Neish AS, Dhodapkar MV, Dhodapkar KM. Impaired SARS-CoV-2 Variant
Neutralization and CD8+ T-cell Responses Following 3 Doses of mRNA
Vaccines in Myeloma: Correlation with Breakthrough Infections. Blood
Cancer Discov. 2023 Mar 1;4(2):106-117. https://doi.org/10.1158/2643-3230.BCD-22-0173
PMid:36511813 PMCid:PMC9975771
- Enssle
JC, Campe J, Moter A, Voit I, Gessner A, Yu W, Wolf S, Steffen B, Serve
H, Bremm M, Huenecke S, Lohoff M, Vehreschild M, Rabenau HF, Widera M,
Ciesek S, Oellerich T, Imkeller K, Rieger MA, von Metzler I, Ullrich E.
Cytokine-responsive T- and NK-cells portray SARS-CoV-2
vaccine-responders and infection in multiple myeloma patients.
Leukemia. 2023 Dec 4. https://doi.org/10.1038/s41375-023-02070-0
PMid:38049509 PMCid:PMC10776400


