Eugenio Galli1, Filippo Frioni2, Tanja Malara1, Enrico Attardi3, Silvia Bellesi1, Stefan Hohaus1,2, Simona Sica1,2, Federica Sorà1,2 and Patrizia Chiusolo1,2.
1
Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed
Ematologia, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma
2 Sezione di Ematologia, Dipartimento di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Roma
3 Dipartimento di Oncoematologia, Fondazione PTV Policlinico Tor Vergata, Roma.
Correspondence to:
Eugenio Galli, MD, PhD. Dipartimento di Diagnostica per Immagini,
Radioterapia Oncologica, ed Ematologia, Fondazione Policlinico
Universitario A. Gemelli IRCCS. Largo A. Gemelli 8 00184 Roma (RM)
Italy. Tel: +390630154180. E-mail:
eugenio.galli@policlinicogemelli.it
Published: March 01, 2024
Received: January 09, 2024
Accepted: February 12, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024029 DOI
10.4084/MJHID.2024.029
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Despite
advancements in Chimeric Antigen Receptor T-cell (CAR-T) therapy and
its notable successes, relapses and non-relapse mortality (NRM) still
significantly affect prognosis. These factors contribute to the ongoing
complexities in achieving favorable outcomes for patients undergoing
CAR-T treatment. Secondary leukemias represent a potential complication
that may manifest subsequent to CAR-T treatment. Overall, secondary
leukemia may account for mutations in the FLT3 gene. These mutations
can induce uncontrolled proliferation of blood cells, thereby fostering
the development of aggressive and refractory forms of leukemia.
The
onset of clonal hematopoiesis and persistent cytopenias, both preceding
and following CAR-T therapy, has been variably reported. Additionally,
there is emerging evidence highlighting a heightened incidence of
secondary myeloid malignancies subsequent to CAR-T treatment. Our team
encountered a case involving a 33-year-old male diagnosed with diffuse
large B cell lymphoma (DLBCL), who subsequently developed
therapy-related acute myeloid leukemia (t-AML) featuring a FLT3-ITD
mutation, which occurred following treatment with multiple lines of
therapy and CD19-directed CAR-T.
To the best of our knowledge,
there have been no reported cases of FLT3-mutated t-AML following CAR-T
therapy up to the present moment.
Previous History and CHIP.
The patient was diagnosed with indolent lymphoma at the age of 27
years, with mediastinal bulky disease and without bone marrow
involvement, with subsequent evolution in aggressive B cell lymphoma
requiring intensive treatment. Overall, the patient received 6 cycles
of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine,
prednisone), 2 courses of IEV (ifosfamide, epirubicin, and etoposide)
consolidated with FEAM-conditioned (fotemustine, cytarabine, etoposide,
melphalan) autologous hematopoietic stem cell transplantation and 30 Gy
radiating treatment, 3 cycles of R-DHAP (rituximab, dexamethasone,
cytarabine, and cisplatin), lenalidomide, 6 courses of R-GEMOX
(rituximab, gemcitabine, and oxaliplatin) and 6 cycles of pixantrone.
Unfortunately, the response to treatment was poor, with a maximal
treatment-free remission of only 20 months. The patient was finally
proposed for treatment with chimeric antigen T-cell receptor cells
(CAR-T) and received CD28-costimulated CAR-T infusion with a high
burden bulky refractory disease.
At the time of CAR-T therapy, a
bone marrow examination was performed: no signs of dysplasia or
lymphoma were described, and cytogenetic analysis documented a normal
46XY karyotype. At that time, Next Generation Sequencing (NGS) tests
revealed a clonal hematopoiesis (CH), with CBLp.(Asp460del),
DNMT3Ap.(Arg882His), and JAK3p.(Arg582Trp); all showing a VAF >4%
(between 5 and 49%) (Figure 1A, Appendix A and B).
As no cytopenia was present in peripheral blood counts, the patient was
characterized as hosting a clonal hematopoiesis of indeterminate
potential (CHIP).
CAR-T Treatment.
During treatment with CAR-T, the following complications occurred:
cytokine release syndrome (CRS) of grade 2, neurotoxicity of grade 2,
deep vein thrombosis with pulmonary embolism, and consumptive
coagulopathy. Supportive therapy and specific treatment included four
doses of tocilizumab, dexamethasone, transfusions of red blood cells
(RBC) and fresh frozen plasma.
One and up to two months after
CAR-T cells, the patient was experiencing a good hematological recovery
with no grade 3-4 cytopenias and partial remission of lymphoma at the
PET-CT scan.
Phase I: Hypoplasia and Macrophage Activation.
Three months after CAR-T, increasing neutropenia was observed, with
oscillations of absolute neutrophils count (ANC) and platelets count.
At that time, CAR-T cells were still detectable in PB (2 CAR+
cells/microL). Four months after CAR-T, the patient presented at the
Emergency Department with severe grade 4 trilinear cytopenia. Major
viral infections were excluded, and a bone marrow examination revealed
severe hypocellularity with some signs of erythrophagocytosis (Figure 1B-C-D). From that moment on, the patient became dependent on RBC transfusions.
Phase II. Acute Myeloid Leukemia with Clonal Evolution.
Two months later, six months after CAR-T infusion, cytopenia persisted,
and a bone marrow biopsy was therefore repeated. The lymphoma was
persistently in a stable partial response. This time, findings were
consistent with therapy-related acute myeloid leukemia (t-AML), with
30% of blasts at the cytomorphological examination; the blasts were
mostly medium to large with basophilic cytoplasm. Trilinear dysplasia
with the presence of micro megakaryocytes, as well as several images of
erythrophagocytosis, was also described (Figure 1E-F-G). Histological
examination confirmed abundant cellularity, dyserythropoiesis with
megaloblastic aspects and megakaryocyte dysplasia, with microforms and
nuclei lobulation defects. Blasts were CD34+ CD117+ HLA-DR+ CD13+
CD33+, with partial aberrant expression of CD7. The cytogenetic
analysis showed a partial mosaic karyotype with 40% of cells with
monosomy 7 and the presence of a small supernumerary chromosome derived
from chromosome 7. Molecular research for FLT3-ITD, NPM1, and core
binding factors tests were negative. Macrophage hyperplasia with
aspects of hemophagocytosis and MF-3 reticulin fibrosis were also
described, together with a modest interstitial T lymphoid component.
Still, there was no evidence of lymphomatous infiltration. NGS revealed
a more than doubled DNMT3A clone and the appearance of RUNX1 clone with
VAF 9% (Figure 1A, Appendix B).
At
that point, the patient was treated with two cycles of demethylating
agents and BCL2 inhibitors, which were complicated by sepsis and
cardiac failure. Those toxicities have been interpreted as a cumulative
result of all previous treatments. The patient was then discharged and
received palliative care for seven more months.
Phase III: Hyperleukocytosis and FLT3-ITD Gain.
Finally, 15 months after CAR-T infusion and 8 months after diagnosis of
AML, a new access at the emergency department documented
hyperleukocytosis (WBC 160x10^9/L), and a new-onset mutation of
FLT3-ITD was found. NGS analysis was not substantially modified, with
the exception of the disappearance of the RUNX1 clone and a further
increase of the DNMT3A clone (Figure 1A, Appendix B).
The patient was treated with leukocyte apheresis and hydroxyurea but finally died from a subsequent infectious complication.
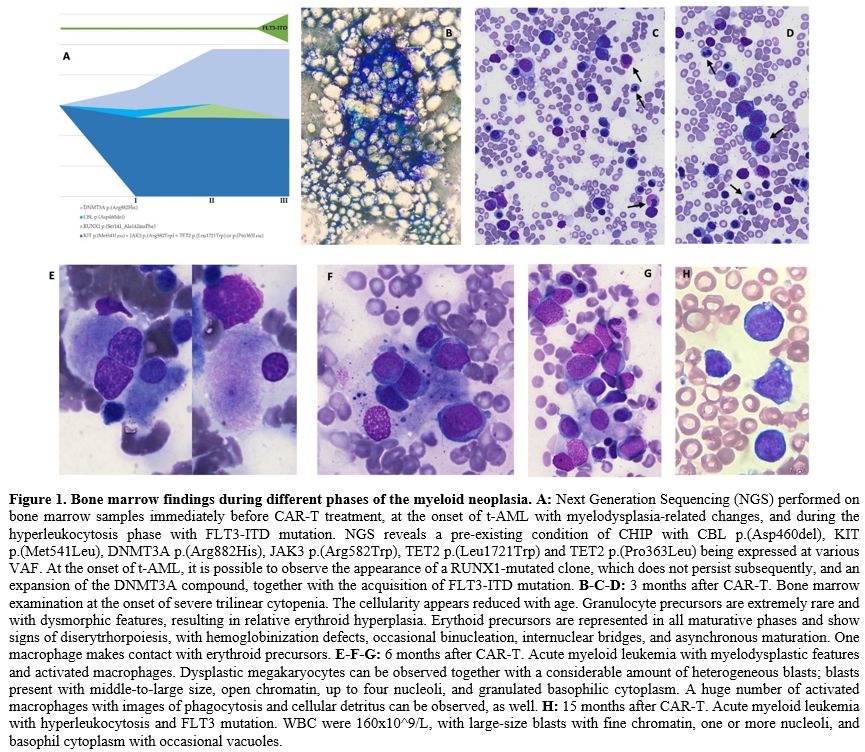 |
Figure 1. Bone marrow findings during different phases of the myeloid neoplasia. A:
Next Generation Sequencing (NGS) performed on bone marrow samples
immediately before CAR-T treatment, at the onset of t-AML with
myelodysplasia-related changes, and during the hyperleukocytosis phase
with FLT3-ITD mutation. NGS reveals a pre-existing condition of CHIP
with CBL p.(Asp460del), KIT p.(Met541Leu), DNMT3A p.(Arg882His), JAK3
p.(Arg582Trp), TET2 p.(Leu1721Trp) and TET2 p.(Pro363Leu) being
expressed at various VAF. At the onset of t-AML, it is possible to
observe the appearance of a RUNX1-mutated clone, which does not persist
subsequently, and an expansion of the DNMT3A compound, together with
the acquisition of FLT3-ITD mutation. B-C-D:
3 months after CAR-T. Bone marrow examination at the onset of severe
trilinear cytopenia. The cellularity appears reduced with age.
Granulocyte precursors are extremely rare and with dysmorphic features,
resulting in relative erythroid hyperplasia. Erythoid precursors are
represented in all maturative phases and show signs of
diserytrhorpoiesis, with hemoglobinization defects, occasional
binucleation, internuclear bridges, and asynchronous maturation. One
macrophage makes contact with erythroid precursors. E-F-G:
6 months after CAR-T. Acute myeloid leukemia with myelodysplastic
features and activated macrophages. Dysplastic megakaryocytes can be
observed together with a considerable amount of heterogeneous blasts;
blasts present with middle-to-large size, open chromatin, up to four
nucleoli, and granulated basophilic cytoplasm. A huge number of
activated macrophages with images of phagocytosis and cellular detritus
can be observed, as well. H:
15 months after CAR-T. Acute myeloid leukemia with hyperleukocytosis
and FLT3 mutation. WBC were 160x10^9/L, with large-size blasts with
fine chromatin, one or more nucleoli, and basophil cytoplasm with
occasional vacuoles. |
Discussion
The incidence of CH before CAR-T treatment has been reported in up to 48% of cases.[1]
In some papers, the presence of CH has been associated with better
lymphoma outcomes and worse CRS and neurological toxicities,[2]
although the data on efficacy have not always been confirmed.
Patients
with lymphoma undergoing autologous stem cell transplantation exhibit
Clonal Hematopoiesis of Indeterminate Potential (CHIP) with at least
one mutation in approximately 30% of cases. In this population, the
presence of clonal hematopoiesis predicts the development of
therapy-related myeloid neoplasms (t-MDS/AML).
Individuals with
CHIP share an increased risk of advancing to hematological malignancies
compared to those without mutated clones. This elevated risk seems to
correlate with the VAF of the mutated genes. TP53 can significantly
influence the development of therapy-related myeloid neoplasms (t-MN)
during the clonal evolution of t-MN, while others, such as ASXL1, may
contribute to the onset of leukemia. The risk is approximately 11 to 13
times higher in individuals with clonal hematopoiesis, with an overall
transformation rate of about 1% per year.[3] The
cumulative incidence of therapy-related myeloid neoplasms (t-MN) for
patients with or without CHIP has been reported as 7.4% versus 1.7% at
5 years and 14.1% versus 4.3% at 10 years, respectively.[4] Certain specific alterations, such as TET2, may be more indicative of drug-induced toxicities.[5]
Secondary myeloid neoplasms have been described in 1-13% of cases after CAR-T cells, according to different reports.[1,6,7]
When a retroactive analysis was possible, t-AMLs after CAR-T have been proven as derived from a previous clone in some cases,[1,7,8] despite there being no prospective data to determine if t-AML tends to arise from a previous CH.
So
far, the number of previous lines, together with increased
lymphoma-related survival- may be reasonably considered as leading
risks for the development of t-AML.
The addition of
topoisomerase II inhibitors to alkylating agents has been associated
with a shorter latency in the onset of t-AML (mean 6 months).[9]
Our patient was exposed to an impressive number of alkylating agents
and other chemotherapies and developed t-AML seven months after the
last chemotherapy and three years after radiating treatment,
immediately after prolonged aplasia. Alkhateeb and colleagues report a
very short delay between CAR-T infusion and the onset of t-MN (around
9.1 months).[7]
The hypothesis that an aplastic
milieu may favor leukemogenesis or clonal escape in the setting of
CAR-T treatment is evocative. However, it has never been properly
explored and may need further evidence. On the other side, some t-MN
seem not to derive from previous CH, as reported by Alkhateeb.[7]
Detection
of FLT3-ITD mutations has been described in 12-18% therapy-related AML,
possibly with lower incidence when compared to de-novo AML (12 vs 24%).[10]
To
the best of our knowledge, this is the first case in which acquired
FLT3-ITD mutations have been reported in a t-AML following CAR-T. In
this case, we have not identified any FLT3 mutated clone before CAR-T
treatment, despite there not being enough evidence to hypothesize a
causative correlation between FLT3 and CAR-T.
The presence of CH
before CAR-T is a challenging topic in terms of efficacy of treatment,
impact on hematological recovery, and onset of subsequent myeloid
neoplasms. More integrated and prospective data are needed to frame the
risk of potential candidates for CAR-T treatment.
Declarations
The
authors acknowledge the support of "Centro di Ricerca sulle Cellule
Staminali Emopoietiche e le Terapie Cellulari "Università Cattolica del
Sacro Cuore, Roma”.
We want to extend our sincere gratitude to dr
Monica Rossi and dr Ilaria Pansini for their invaluable contribution to
the coordination and processing of the biological material. Our
gratitude also to prof. Maria Teresa Voso and dr Maria Colangelo for
their generous availability and willingness to share crucial
information and materials essential for this article.
The patient had consented to the use of anonymized data.
For data availability, please contact the corresponding author.
References
- Miller PG, Sperling AS, Brea EJ, et al. Clonal
hematopoiesis in patients receiving chimeric antigen receptor T-cell
therapy. Blood Adv. 2021;5(15):2982-2986. https://doi.org/10.1182/bloodadvances.2021004554 PMid:34342642 PMCid:PMC8361461
- Teipel
R, Kroschinsky F, Kramer M, et al. Prevalence and variation of CHIP in
patients with aggressive lymphomas undergoing CD19-directed CAR T-cell
treatment. Blood Adv. 2022;6(6):1941-1946. https://doi.org/10.1182/bloodadvances.2021005747 PMid:35008107 PMCid:PMC8941459
- Fabiani
E, Cristiano A, Hajrullaj H, Falconi G, Leone G, Voso MT.
Therapy-Related Myeloid Neoplasms: Predisposition and Clonal Evolution.
Mediterr J Hematol Infect Dis. 2023;15(1). doi:10.4084/MJHID.2023.064 https://doi.org/10.4084/MJHID.2023.064 PMid:38028397 PMCid:PMC10631709
- Gibson
CJ, Lindsley RC, Tchekmedyian V, et al. Clonal hematopoiesis associated
with adverse outcomes after autologous stem-cell transplantation for
lymphoma. Journal of Clinical Oncology. 2017;35(14):1598-1605. https://doi.org/10.1200/JCO.2016.71.6712 PMid:28068180 PMCid:PMC5455707
- Testa
U, Castelli G, Pelosi E. Clonal Hematopoiesis: Role in Hematologic and
Non-Hematologic Malignancies. Mediterr J Hematol Infect Dis.
2022;14(1). https://doi.org/10.4084/MJHID.2022.069 PMid:36119457 PMCid:PMC9448266
- Strati
P, Varma A, Adkins S, et al. Hematopoietic recovery and immune
reconstitution after axicabtagene ciloleucel in patients with large
B-cell lymphoma. Haematologica. 2021;106(10):2667-2672. https://doi.org/10.3324/haematol.2020.254045 PMid:32732355 PMCid:PMC8485681
- Alkhateeb
HB, Mohty R, Greipp P, et al. Therapy-related myeloid neoplasms
following chimeric antigen receptor T-cell therapy for Non-Hodgkin
Lymphoma. Blood Cancer Journal 2022 12:7. 2022;12(7):1-5. https://doi.org/10.1038/s41408-022-00707-4 PMid:35882844 PMCid:PMC9325766
- Cordeiro
A, Bezerra ED, Hirayama A V., et al. Late Events after Treatment with
CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood
Marrow Transplant. 2020;26(1):26-33. https://doi.org/10.1016/j.bbmt.2019.08.003 PMid:31419568 PMCid:PMC6953906
- Fianchi
L, Pagano L, Piciocchi A, et al. Characteristics and outcome of
therapy-related myeloid neoplasms: Report from the Italian network on
secondary leukemias. Am J Hematol. 2015;90(5):E80-E85. https://doi.org/10.1002/ajh.23966 PMid:25653205
- Kayser
S, Döhner K, Krauter J, et al. The impact of therapy-related acute
myeloid leukemia (AML) on outcome in 2853 adult patients with newly
diagnosed AML. Blood. 2011;117(7):2137-2145. https://doi.org/10.1182/blood-2010-08-301713 PMid:21127174
Appendix A
The
NGS panel included the following genes: ASXL1, CALR, CBL, CBLB, CEBPA,
KIT, CSF3R, CUX1, DNMT3A, EZH2, IDH1, IDH2, IKZF1, JAK2, JAK3, KRAS,
MPL, NRAS, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1, ZRSR2
Appendix B
NGS
was performed before CAR-T, at the onset of t-AML and at the
hyperleukocytosis phase. VAF of single clones are showed in the table.
KIT p.(Met541Leu), TET2 p.(Leu1721Trp), and TET2 p.(Pro363Leu) are
considered as polymorphisms.

