Michele
Bibas.
Department
of Clinical Research, Hematology. National Institute for Infectious
Diseases “Lazzaro Spallanzani” I.R.C.S.S. Via Portuense 292 00148 Rome
Italy.
Correspondence to:
Michele Bibas. Department of Clinical Research, Hematology. National
Institute for Infectious Diseases “Lazzaro Spallanzani” I.R.C.S.S. Via
Portuense 292 00148 Rome Italy. E-mail:
Michele.bibas@inmi.it
Published: March 01, 2024
Received: January 25, 2024
Accepted: February 14, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024015 DOI
10.4084/MJHID.2024.015
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
objective of this two-part review is to present a current and
comprehensive understanding of the diagnosis and management of
plasmablastic lymphoma. The first part, which was published previously,
focused on the study of epidemiology, etiology, clinicopathological
characteristics, differential diagnosis, prognostic variables, and the
impact of plasmablastic lymphoma on specific populations. This second
part addresses the difficult topic of the treatment of plasmablastic
lymphoma, specifically examining both the conventional, consolidated
approach and the novel therapeutic strategy.
|
Article Highlights
- Several
factors that could impact the result include achieving complete
remission, performance status (PS), clinical stage, MYC status, and the
International Prognostic Index (IPI).
- Physically
fit patients might benefit from a more intensive treatment protocol,
such as DA-EPOCH, instead of the conventional CHOP regimen.
Combinations with Bortezomib, Daratumumab, or Lenalidomide may be
considered.
- Patients
who achieve complete remission following the initial treatment (CR1)
may be eligible for additional intensification through autologous bone
marrow transplantation (ABMT).
- The
effectiveness of rituximab is reduced since tumor cells do not have
CD20 expression, although it may be employed in certain rare CD20+
cases.
- CNS prophylaxis can be considered in a case-by-case basis.
- It
is highly recommended that patients who are HIV-positive and receiving
chemotherapy utilize combination antiretroviral treatment (cART).
Consider the possibility of simultaneous toxicity.
- Individuals with persistent or recurring illnesses that do not respond to treatment should consider palliative care.
- Consolidation and palliative care applications can benefit from radiation therapy as an effective approach.
- New
drugs like Bortezomib, Daratumumab, Lenalidomide, Brentuximab vedotin,
PD1/PDL1 blocking agents, and Selinexor can be used to treat cases that
have relapsed or are not responding to treatment.
- The
range of median progression-free survival (PFS) is between 6 and 11
months, whereas the most recent reported median overall survival (OS)
is from 14 to 57 months.
- Individuals with limited stages of the disease, especially among pediatric populations, have achieved long-term survival.
Introduction
The identification and characterization of plasmablastic lymphoma (PBL) date originally from 1992,[1] after that, it was recognized as a distinct form of oral lymphoma occurring in individuals who are HIV-positive.[2]
This particular subtype of large B-cell lymphoma (LBCL) is now
categorized as a distinct entity, with extranodal disease being the
prevailing characteristic.[3-6]
Despite earlier
studies indicating a strong association, it has been observed that
around 50% to 60% of cases are associated with HIV. 3–5 PBL accounts
for just 2% of lymphomas in individuals who are HIV-positive.[3-6]
The disease has the potential to impact individuals with compromised
immune systems as well as those with robust immune systems.
Histological
examination reveals the presence of several neoplastic plasmablasts and
immunoblasts. The identified cells exhibit a significant proliferation
index and possess a plasma cell immunophenotype, characterized by the
presence of plasma cell markers and a limited or missing expression of
B cell markers.
Notably, transcription factors linked to
plasmacytic differentiation, including CD38, CD138, MUM1, Blimp1, and
XBP1, are present. The absence of CD20 and PAX5 has been very
frequently observed. PBL frequently has a connection to the
overexpression of the MYC gene, which can result from translocations,
amplifications, or constitutive STAT3 activation. This is in contrast
to plasma cell neoplasms.[3-7] Moreover, around 80% of
PBL cases were found to have a simultaneous Epstein-Barr virus (EBV)
infection. This feature can be regarded as a discerning element
differentiating PBL from plasmablastic myeloma. Hence, distinguishing
between plasmablastic myeloma, lymphomas, and PBL can pose a
significant difficulty. Translational research encounters several
challenges, such as the limited occurrence of PBL and the absence of an
established treatment strategy due to a scarcity of thorough clinical
data.[3-8]
Historically, PBL has been associated
with a negative outlook, as early estimates of median overall survival
(OS) ranged from 8 to 15 months.[3-6] There have been more survival estimates reported in recent literature that display significant diversity.
A
population-based SEER survival analysis examined 248 patients who had
treatment between 2010 and 2016. The analysis revealed that the median
overall survival was 47 months.[9]
Further, a
total of 1,800 patients were very recently assessed using SEER and the
NCDB. An exceptional median overall survival of 58.6 months was seen in
the treated patients.[10]
Although there have
been some improvements recently, CD38-directed monoclonal antibody
therapy, proteasome inhibitors, and immunomodulatory therapeutic
regimens, along with intensive polychemotherapy, are still rarely
available for older and weaker patients. Those limitations highlight
the requirement for therapeutic strategies that are precisely
personalized to fulfill individual requirements.
Treatment
Establishing
a therapeutic standard is quite problematic due to the rarity of the
disease and the lack of controlled trials available for comparing
different treatments. In this second part of the review, our intent is
to provide a comprehensive and detailed description of the achievements
of the diverse consolidated therapies, as well as those that are
currently being developed. We will organize the information into
pertinent chapters for clarity.
Standard Polychemotherapy
Patients
with untreated PBL exhibit a median overall survival of 3 months in
individuals who are HIV-positive and 4 months in those who are
HIV-negative.[3-6]
Patients diagnosed with PBL
have been subjected to a wide range of treatment options, covering
localized disease management by radiation as well as the administration
of diverse chemotherapy combinations. Individuals who are diagnosed
with limited-stage disease tend to have a more favorable prognosis, and
in certain instances, aggressive treatment measures may not be
recommended. Disease control may be achieved through the utilization of
a combination therapy involving doxorubicin-based chemotherapy and
radiation therapy.[3-6] Of note, the majority of PBL
patients must be considered high-risk patients and treated with
polychemotherapy. Polychemotherapy has yielded complete remissions
(CRs) in nearly 50% of patients with disseminated disease. The response
and relapse rates among different first-line regimens are presented in Table 1.
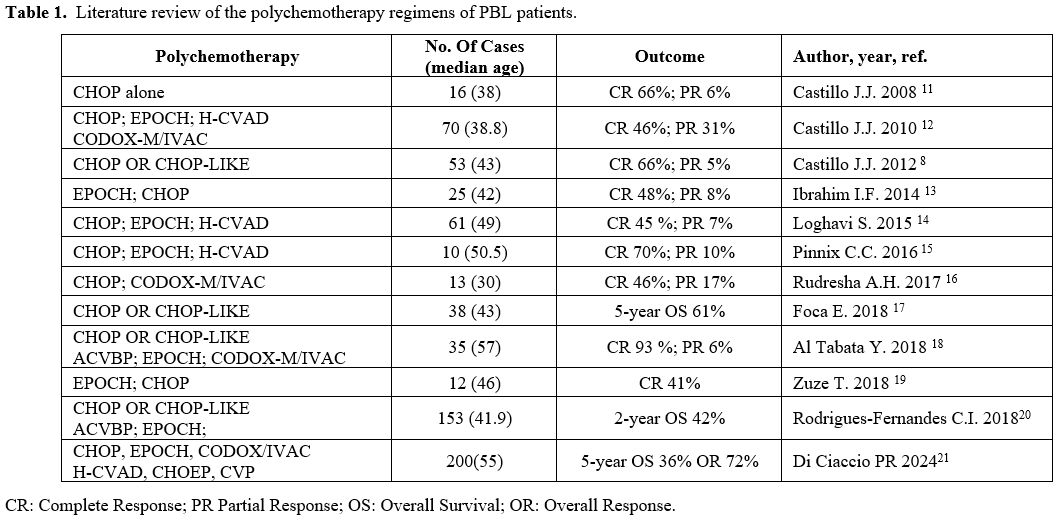 |
- Table
1. Literature review of the polychemotherapy regimens of PBL patients.
|
However, a
significant proportion of patients, approximately 70%, inevitably die
of progressive disease. At present, there is a lack of established care
standards that delineate the most effective therapy method. Throughout
history, the use of CHOP has been widely adopted as the primary
therapeutic approach for PBL, with a particular emphasis on its
utilization in nations with lower economic resources. According to the
latest National Comprehensive Cancer Network (NCCN) guidelines on
B-cell lymphomas (version 1.2024, January 18, 2024),[22] it has been determined that CHOP is not adequate as a first-line therapy.
NCCN
supports using more intensive treatment plans for PBL and suggests
dose-adjusted (DA)-EPOCH as an alternative way to treat the disease.
DA-EPOCH includes etoposide, vincristine, doxorubicin,
cyclophosphamide, and prednisone given in bolus doses. Some other
treatment plans that have been suggested are CODOX-M/IVAC (modified)
and HyperCVAD (which includes cyclophosphamide, vincristine,
doxorubicin, dexamethasone, and high doses of methotrexate and
cytarabine). Furthermore, these guidelines suggest the potential use of
high-dose therapy with autologous stem cell rescue during the first
complete remission for a particular subgroup of patients at high risk.
A high-risk factor includes an International Prognostic Index (IPI)
score above 2 and changes to the MYC gene or deletion of the TP53 gene.
It is important to acknowledge that individuals who are HIV-negative
and diagnosed with plasmablastic lymphoma are commonly recognized as
having a condition associated with an elevated risk. Typically, those
who are HIV-negative and diagnosed with plasmablastic lymphoma are
generally characterized as having a more threatening disease. On the
other hand, there is a more favorable outlook for individuals who are
HIV-positive and diagnosed with PBL when they successfully attain
complete remission with the administration of chemotherapy.
Antiretroviral therapy is crucial for improving the management of PBL
HIV+, and the attainment of complete remission (CR) has been shown to
enhance the short-term prognosis of PBL.[3-6]
Bortezomib
Bortezomib
is currently approved for treating adult patients with multiple myeloma
and adult patients with mantle cell lymphoma. The small molecule
bortezomib is a reversible proteasome inhibitor that works on the 26S
proteasomes. It stops many signaling pathways by targeting a single
molecular target, the proteasome. Bortezomib's anti-neoplastic effect
likely involves several distinct mechanisms, such as inhibition of cell
growth and survival pathways, induction of apoptosis, and inhibition of
the expression of genes that regulate cellular adhesion, migration, and
angiogenesis. Thus, the mechanisms by which bortezomib elicits its
anti-tumor activity may differ between tumor types, as could the
importance of each affected pathway in inhibiting tumor growth.
Bortezomib is thought to work against multiple myeloma by stopping NF-B
from working and stopping the breakdown of phosphorylated IB. Because
of this, it seems like a good way to treat PBL patients, whose
biological and phenotypic traits are somewhere between those of
ABC-DLBCL and MM. Several reports have reported its activity in
lymphoma, specifically in non-Germinal Center B-cell lymphomas such as
DLBCL and mantle cell lymphoma.[23-26] Thus,
bortezomib is one of the most frequently used drugs in the treatment of
PBL. It has been utilized as a single agent and in conjunction with
chemotherapy. The objective of our extensive literature review was to
determine if patients with PBL would experience more benefits from the
inclusion of bortezomib in their polychemotherapy (Table 2).
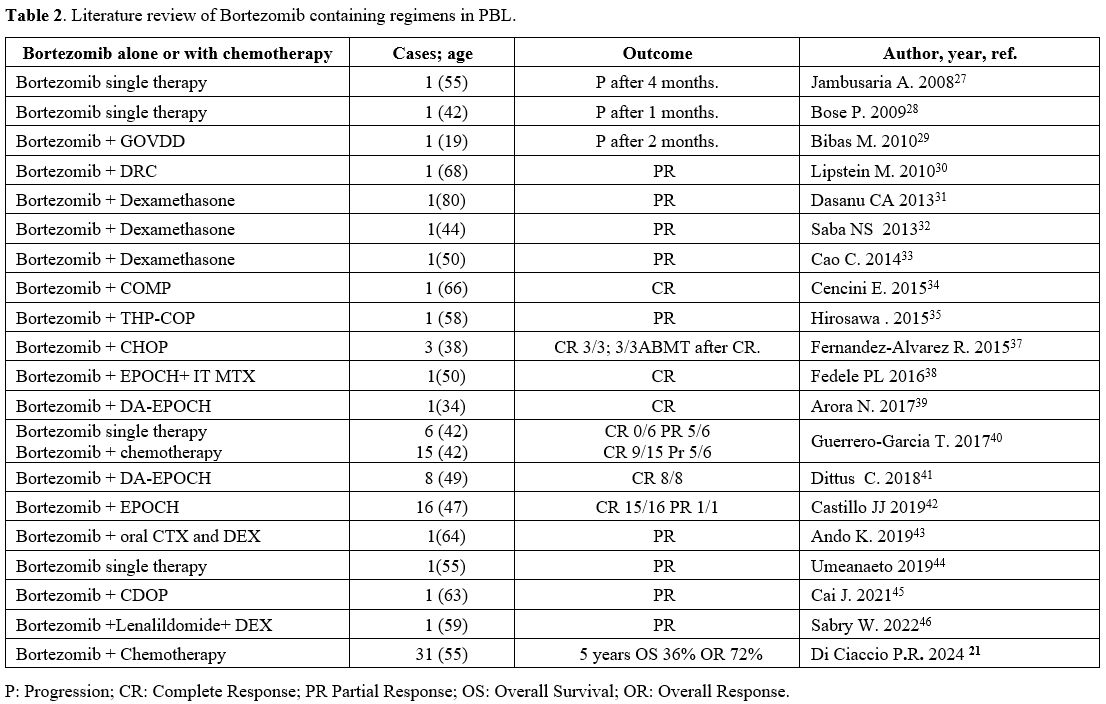 |
- Table 2. Literature review of Bortezomib containing regimens in PBL.
|
Five
out of six patients treated with bortezomib alone in a small series
achieved a partial response—two as first-line therapy and three as
salvage therapy.[40] Bortezomib has been used in
combination with CHOP as frontline therapy in three HIV-associated PBL
patients, all of whom achieved a CR, and two of whom are still alive 14
and 22 months after completing V-CHOP, respectively.[37]
According to a positron emission tomography scan, Castillo et al.
reported three patients without relapse at 12, 18, and 24 months.[36] Recently, Dittus and Castillo reported 8 and 16 patients, respectively, with CR rates of 87.5% and 94%.[41,42]
In the latter series, two patients received an ASCT for consolidation.
Castillo reported a 5-year OS of 63%, while Dittus reported a 2-year
PFS and OS of 50%.[41,42] Bortezomib has also been
used with THP-COP (pirarubicin, cyclophosphamide, vincristine, and
prednisone), ESHAP (etoposide, high-dose prednisolone, high-dose
cytarabine, and platinum), ICE (ifosfamide, carboplatin, and
etoposide), bendamustine, rituximab, and DT.[35] A
recent systematic review found 21 patients with PBL, 11 of whom had HIV
and 10 of whom did not. Eleven of them were given bortezomib as the
first line of treatment, and the other 10 were given it after a
relapse, either by itself or with other standard cytostatic drugs. The
ORR for bortezomib-containing regimens was 100% in the frontline
setting and 90% in the relapse setting. In addition, the 2-year overall
survival rate for patients who received initial treatment was 55%,
while the median OS for relapsed patients was 14 months.[40]
Finally,
upon evaluating the existing literature, it was found that patients
with PBL benefitted from including bortezomib in their treatment.
However, we acknowledge that we are currently experiencing a shortage
of randomized studies comparing chemotherapy regimens with and without
bortezomib.
Lenalidomide
Lenalidomide
is an oral immunomodulator with direct antitumor activity and
immunologic effects, such as stopping tumor cell growth and
angiogenesis and increasing the killing power of T- and natural killer
(NK) cells in lab experiments. In vitro and in vivo studies revealed
antitumor, antiproliferative, and increased NK cell number and activity
against B-cell malignant lymphoma in general and against DLBCL, FL, and
MCL cells. In preclinical models of activated B-cell (ABC)-subtype
DLBCL, lenalidomide-induced cytotoxicity required the presence of
cereblon to downregulate interferon regulatory factor-4 and B-cell
receptor-NFB and boost interferon production. Of note, low cereblon
expression is a potential lenalidomide resistance mechanism.[47]
According
to a thorough review of the literature, lenalidomide has only been used
in a few cases of PBL, and each case is very different from the others (Table 3).
Cases of refractory PBL treated with lenalidomide as a single agent
were reported to have a favorable response, albeit a brief one.
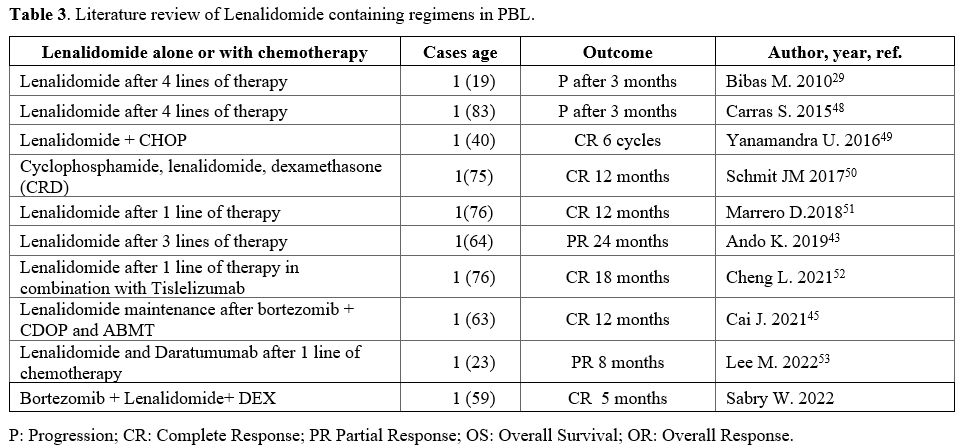 |
- Table 3. Literature review of Lenalidomide containing regimens in PBL.
|
It also demonstrated efficacy when combined with CHOP, or cyclophosphamide-dexamethasone.[49]
Ando et al. utilized bortezomib to treat chemotherapy-resistant PBL
patients, which resulted in a clinical response but was discontinued
due to peripheral neuropathy.[43] The patient was
then treated with a combination of lenalidomide and dexamethasone for
more than two years, with a partial response that persisted.[43]
A
person with PBL who was not responding to mini-CHOP as a first-line
treatment went into complete remission after taking tislelizumab, a
checkpoint inhibitor, and lenalidomide.[52]
Another patient with relapsed PBL with parotid involvement was treated with a combination of lenalidomide and bortezomib.[51]
This patient received only two cycles of the protocol before it was
discontinued due to bortezomib-induced pancreatitis. However, a PET CT
scan performed after the two cycles revealed no evidence of disease,
and the patient remained in complete remission for at least a year
following the initiation of salvage therapy.
Brentuximab Vedotin (BV)
CD30
is a 120-kilodalton transmembrane cytokine receptor, part of the tumor
necrosis factor receptor family 4. It is found on the lymphoid cells of
almost all HL and ALCL patients. Expression of CD30 is restricted to
activated lymphocytes and eosinophils, typically found in lymphoid
tissues but not in peripheral blood cells. Thus, CD30 has been
identified as a desirable therapeutic target. Brentuximab vedotin (BV)
is a chimeric IgG1 anti-CD30 antibody-drug conjugated by a
protease-cleavable linker to the microtubule-disrupting agent
monomethyl auristatin E, which has demonstrated significant anti-tumor
activity in both HL and ALCL.[54,55]
The main
way that BV works is by delivering monomethyl auristatin E to tumor
cells that express CD30. In addition to antibody-dependent cellular
phagocytosis, immunogenic cell death, and the bystander effect, other
mechanisms of tumor cell death may contribute to the clinical activity
of this drug. The availability of BV has become a significant advance
in the treatment of patients with relapsed and resistant HL. In
addition, the significant clinical activity observed and the good
tolerability of BV have allowed for widespread investigation and use of
BV in a variety of lymphoma patients, and several groups are testing
BV-based therapies in the management of newly diagnosed patients with
HL and ALCL, with promising preliminary results.[54,55] Positive expression of CD30 has been detected in 30–50% of PBL cases, making CD30 a viable target for PBL.[3-10]
It is reported that the use of brentuximab vedotin resulted in an
impressive reduction in tumor size but also a fatal outcome due to
tumor lysis syndrome and comorbidities.[56] The
patient's tumor, which had undergone extensive prior treatment and was
unresponsive to numerous chemotherapy regimens, had a positive response
when treated with brentuximab vedotin as a standalone therapy and
ionizing radiation. This information shows that more research should be
done on brentuximab vedotin for CD30-positive PBL, either as a single
treatment or in combination with standard chemotherapy.
Selinexor
The
overexpression of XPO1 (exportin 1), one of eight nucleocytoplasmic
shuttling proteins that help move proteins from the nucleus to the
cytoplasm, is linked to a poor prognosis in DLBCL.[57]
XPO1 mediates the functional inactivation of multiple tumor suppressor
proteins (such as p53, p73, IkB, and FOXO) and facilitates the
increased translation of oncoproteins relevant to B-cell biology and
DLBCL.[58,59] By forcing these proteins to stay in
the nucleus, blocking XPO1 in DLBCL may restore the tumor-suppressing
and growth-regulating effects of several tumor-suppressor proteins.
This may also reverse chemotherapy resistance.[60]
Selinexor, an oral selective inhibitor of XPO1-mediated nuclear export,
induces the expected nuclear accumulation and activation of tumor
suppressor proteins and decreases the levels of Bcl2, Bcl-XL, and c-Myc
oncoproteins. Based on the safety and effectiveness data from the STORM
study, the US Food and Drug Administration approved the use of low-dose
dexamethasone and selinexor (80 mg twice weekly) together for people
with advanced refractory multiple myeloma.[61] In a
phase 1 study that showed selinexor's preliminary activity in several
types of blood cancer, such as myeloma and DLBCL, the single drug
selinexor showed an overall response rate (ORR) of 32% in 13 of 41
patients who had already received a lot of treatment for DLBCL, and a
complete response rate of 10% in 4 of those patients. Based on that
study, the recommended dose was 35 mg/m2 (60 mg) twice weekly.[62]
The FDA has approved selinexor to treat diffuse large B-cell lymphoma
(RR DLBCL) and relapsed or refractory multiple myeloma (RR MM). It is
very effective as a type of treatment.[63,64]
Regarding PBL, a case of a profound response to selinexor in
HIV-negative, EBV-negative, heavily pretreated young PBL patients has
been reported recently. 60 mg of Selinexor were administered on days 1,
8, and 15, followed by a GDP (gemcitabine, cisplatin, and
dexamethasone) regimen every three weeks. A rapid partial response (PR)
to selinexor was observed within two weeks of treatment. Selinexor was
found to be tolerable and safe. This patient reported mild
hemocytopenia as the most common adverse reaction, without nausea,
vomiting, or hyponatremia.[65]
Daratumumab
CD38
is a 48-kDa transmembrane glycoprotein that can be observed on the
surface of many hematopoietic cells, such as multiple myeloma cells. It
provides several functions, including receptor-mediated adhesion,
signaling, and regulation of cyclase and hydrolase activity.[66,67]
Daratumumab
is a human IgG1 monoclonal antibody that binds with high affinity to a
unique CD38 epitope expressed on malignant cells and possesses direct
and indirect antitumor activity and multiple mechanisms of action.
Immune-mediated actions include complement-dependent cytotoxicity
(CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and
antibody-dependent cellular phagocytosis (ADCP); and immunomodulatory
functions that target and deplete CD38-positive regulator immune
suppressor cells, resulting in T-cell expansion and activation in
patients with a response.[68,69]
Currently, daratumumab is under investigation for many types of hematological malignancies.
CD38
expression has been linked to a number of these diseases: in addition
to multiple myeloma, consistent expression has been observed in the
malignant cells of CLL, and it has also been reported in Waldenstrom
macroglobulinemia, mantle cell lymphoma, acute lymphoblastic leukemia,
acute myeloid leukemia, NK cell leukemia, and NK/T-cell lymphoma.[70-74]
Daratumumab
induces the death of CD38-expressing tumor cells through multiple
mechanisms, including complement-mediated cytotoxicity (CDC) and
antibody-dependent cell-mediated cytotoxicity (ADCC) effects,
antibody-dependent cellular phagocytosis (ADCP), apoptosis, and, to a
lesser extent, inhibition of the enzymatic activity of CD38.[75-79]
Plasmablastic
lymphomas are often CD38+ and share some biological and phenotypic
features with multiple myeloma. Because of this, it seems reasonable to
think that daratumumab could be used as proof of concept of activity in
people with PBL who fail their first line of conventional chemotherapy
or who are not eligible for autologous stem cell transplantation.
Table 4 summarizes studies of the use of daratumumab in PBL.
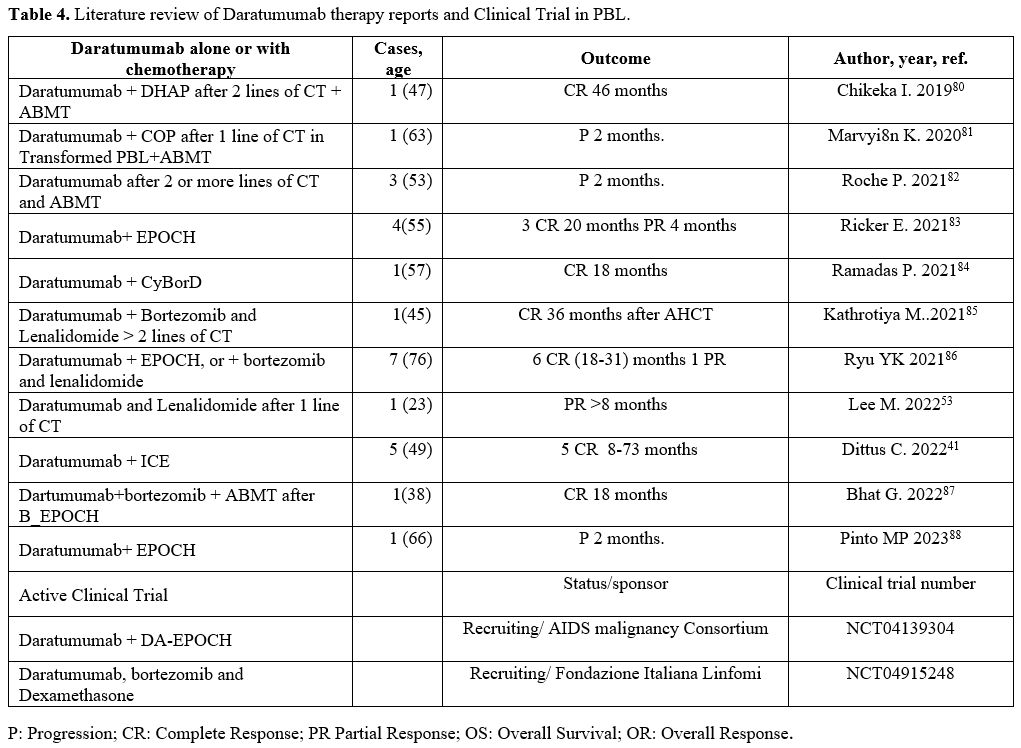 |
- Table 4. Literature review of Daratumumab therapy reports and Clinical Trial in PBL.
|
Ryu assessed the effectiveness and safety of daratumumab-based treatment plans in seven patients with advanced-stage PBL.[86]
Out of these patients, 6 were considered evaluable, 4 had classic PBL,
and 1 did not satisfy the precise criteria for PBL as established by
the World Health Organization (WHO).[86] The initial
assessment revealed that the median age of the patients included was 48
years. Additionally, all seven patients had disease locations outside
the lymph nodes, 4 patients had ECOG scores of 3 or 4 at diagnosis, and
5 patients proved positive for CD38. Five patients underwent six cycles
of daratumumab in conjunction with DA-EPOCH, and one patient underwent
weekly daratumumab in addition to the addition of bortezomib. All
patients who could be evaluated obtained a complete response (CR); four
individuals were in remission after completing treatment. As of the
data cutoff, the median duration of response (DOR) for the patients who
could be evaluated was 16.8 months, and all of them were still showing
a response. Patients diagnosed with classic PBL had a median duration
of response (DOR) of 23.7 months, whereas patients who did not fulfill
the stringent criteria for PBL had a median DOR of 3 months. The median
overall survival (OS) and progression-free survival (PFS) were
indeterminate in patients with classic PBL, except for one patient who
died due to illicit drug use, asthma exacerbation, and respiratory
arrest. In contrast, the median OS and PFS were 7 months and 6 months
in patients who did not meet the strict criteria for PBL. Patients who
failed to satisfy the stringent specifications for PBL experienced
relapse and succumbed quickly after the completion of treatment. The
24-month overall survival rate for all patients who could be evaluated,
regardless of whether they died from causes unrelated to treatment or
disease, was 57%. Out of seven patients, six experienced severe adverse
effects. There were no recorded fatalities associated with the
treatment. Following the administration of daratumumab, two patients
experienced infusion-related events, including a rash and itching in
one patient and bradycardia in another.
In another small study,
Daratumumab was also combined with the standard NHL salvage regimen,
ICE. Five PBL patients were described.[41] Three
patients were administered daratumumab in combination with EPOCH as
their first-line treatment, while one patient received daratumumab
together with lenalidomide, dexamethasone, and doxorubicin after
experiencing a relapse. Three of the four patients maintained a state
of remission for a minimum of 15 months after obtaining a complete
response (CR).[41]
The AIDS Malignancy
Consortium is now carrying out a limited, prospective investigation on
the use of daratumumab-EPOCH in the first-line treatment of PBL
(NCT04139304).
This study aims to assess the efficacy of
daratumumab in combination with dose-adjusted etoposide, prednisone,
vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride
(DA-EPOCH) in the treatment of patients who have recently been
diagnosed with stage I-IV plasmablastic lymphoma. The main goal is to
determine the effectiveness of including daratumumab in the DA-EPOCH
regimen by examining the proportion of patients with PBL who
successfully complete at least three cycles of treatment according to
the established protocol.
Daratumumab is administered to patients
intravenously (IV) on days 1 (± 3 days), 8 (± 2 days), and 15 (± 2
days) during cycles 1-3, and on day 1 of cycles 4-6. Patients are given
etoposide, doxorubicin hydrochloride, and vincristine sulfate
intravenously for a continuous period of 96 hours on days 1-4. In
addition, they orally provide prednisone for a period of 5 days,
beginning on the first day, and intravenously administer
cyclophosphamide for a length of 1 hour on the fifth day. The treatment
is given at intervals of 21 days for a maximum of 6 cycles, unless
there is a clear indication of disease recurrence or the patient
experiences unacceptable adverse reactions.
Presently, another
ongoing clinical study is assessing the efficacy of Daratumumab in
conjunction with Bortezomib and dexamethasone for patients with
refractory or recurrent PBL (NCT04915248).
This study is a phase II clinical trial conducted at many centers. It is an open-label, single-arm trial.
A
total of 28 patients are expected to commence therapy over a period of
18 months, with recruitment taking place in 19 Italian FIL centers. The
study's primary endpoint will be examined approximately 12 months after
the final patient is enrolled, independent of the patient's response to
the treatment outlined in the protocol. The study is expected to last
around 2.5 years. Patients will be recruited based on their specific
diagnosis and local assessment of CD38 expression at a level of 5%. The
screening phase of the study involves conducting baseline assessments
under local norms and study requirements. Induction treatment consists
of one course (cycle 1) of daratumumab sc as a single agent, followed
by eight courses (cycles 2–9) of daratumumab sc in combination with
bortezomib sc and dexamethasone (DVd regimen). A maximum of 6 cycles
(cycles 10-15) of daratumumab SC as a single agent will be administered
to patients who achieve at least an SD after the induction phase. Every
21 days, induction cycles will be administered, while every 28 days,
maintenance cycles will be administered.
Polatuzumab Vedotin
Nearly
40% of all cases of plasmablastic lymphoma have CD79a expression,
ranging from 35% in HIV-negative patients to 45% in HIV-positive
patients and 68% in post-transplant patients.[5-12] Thus, CD79a has been identified as a desirable therapeutic target for PBL.
Polatuzumab
vedotin (Pola) is an innovative antibody-drug combination. It comprises
a monoclonal antibody that targets CD79b, a component of the B-cell
receptor found on normal B cells. This antibody is chemically linked to
the microtubule-disrupting anti-mitotic agent, monomethyl auristatin.[89,90]
A phase 1 trial demonstrated the safety of using polatuzumab vedotin
alone in patients with severely treated B-cell malignancies, including
non-Hodgkin lymphoma and chronic lymphocytic leukemia.[91,92]
In real-world research, two patients with PBL were recently treated
with a combination of polatuzumab, vedotin, and bendamustine. However,
there is currently no available data on the outcomes of this treatment.[93] It is plausible that Pola could be used in a clinical trial for patients with relapsed or refractory PBL.
PD-1/PD-L1 Blocking Therapies
PD-1
binds to PD-L1 or PD-L2 on the surface of tumor cells and/or
tumor-associated macrophages (TAM) in the tumor microenvironment,
transmitting inhibitory signals to the T-cell receptor (TCR) pathway.
Consequently, TCR-mediated signaling activation and cellular
proliferation are inhibited.[94-101]
The
inhibition of the PD-1/PD-L1 pathway can free T-cells from the
inhibitory effects of tumor cells and restore the T-cell-mediated
antitumor immune response. In recent years, significant progress has
been made in developing cancer immunotherapies, such as PD1/PD-L1
inhibition. James Allison (MD Anderson Cancer Center, Houston, Texas,
U.S.A.) and Tasuku Honjo (Kyoto University, Kyoto, Japan) were awarded
the Nobel Prize in Physiology or Medicine in 2018 for their discoveries
that made cancer immunotherapy possible.
PD-L1 levels expressed by
tumor cells are generally associated with a response to PD-1/PD-L1
inhibitor therapies, widely used to treat patients with non-hematologic
and hematologic malignancies, such as lung cancer, melanoma, and
lymphoma. These inhibitors can prevent the binding of PD-1 to its
ligands, thereby restoring the T-cell immune response and resulting in
substantial and durable patient responses. The highest response rates
have been observed in patients with classic Hodgkin lymphoma (CHL)
among hematolymphoid neoplasms. In contrast, patients with non-Hodgkin
lymphomas, including diffuse large B-cell lymphoma (DLBCL) and T-cell
lymphomas with heterogeneous PD-L1/PD-L2 expression, have exhibited
variable responses. Immunohistochemistry has been the primary method
for assessing PD-L1 positivity in neoplastic cells based on PD-L1
expression. The positivity threshold values for PD-L1 vary between
studies. For instance, a 5% cut-off was used in an early study, whereas
different cut-offs were used in different lymphoma studies.[102-111]
An
analysis of 82 patients with PBL revealed that almost all cases
exhibited the presence of programmed death ligand 1 (PDL1) and
programmed cell death protein 1 (PD1) in the immune infiltrate.
Furthermore, a significant proportion of these cases, specifically one
quarter, demonstrated substantial expression of PDL1 in both tumor
cells and immune cells.[112]
In Epstein-Barr virus-positive PBL (EBV+ PBL), there was a higher level of overexpression of PD1/PDL1 in the microenvironment.[113]
Immunotherapy
is thus becoming a viable treatment choice for this condition. A case
study documented the use of nivolumab as salvage therapy in a patient
with PDL1+ PBL, which successfully allowed an allogeneic stem cell
transplant.[114]
A case study involving a
patient with chemoresistant EBV + PBL showed that treatment with
tislelizumab plus lenalidomide resulted in complete remission (CR) and
an overall survival of 18 months.[115] Moreover, a
recent case study documented a successful treatment outcome using
pembrolizumab and radiotherapy in a patient with HIV-negative,
EBV-positive recurrent plasmablastic lymphoma.[116]
Chimeric Antigen Receptor T-Cell Therapy
The
field of therapeutic T cell engineering has gained significant
attention recently because of the remarkable achievements of CD19
(chimeric antigen receptor) CAR treatment. Chimeric antigen receptors
(CARs) are artificial receptors that alter the specificity and modify
the function of T cells into which they are genetically inserted.[117-119]
The
CD19 antigen is a member of the immunoglobulin superfamily and is
specifically expressed in B-lymphocytes. Its expression is limited to
the B-cell lineage, beginning in the early stages of B cell
development, which coincide with heavy chain immunoglobulin
rearrangement, and continuing until the later stages of B cell
differentiation. Notably, the expression of CD19 increases as B cells
differentiate. CD19, CD21, CD81, and Leuk-13.5 combine on the cell
membrane of B-lymphoid cells to create a transduction complex. In
addition, CD81 controls the level of CD19 expression during B cell
growth.[117-119]
Over the past ten years,
research has clearly shown and emphasized the important role of
CD19-CAR-T cells in treating people with DLBCL that has relapsed or are
not responding to treatment. The therapeutic function was designated
for patients with a resistant disease and experience an early
recurrence. CD19-CAR-T cells have demonstrated consistent therapeutic
action in DLBCL patients with only a partial response after salvage
therapy.[119-124]
CD19-CAR-T cells have
effectively treated high-risk DLBCL patients as a first-line treatment.
However, further research is necessary to evaluate their efficacy
compared to traditional treatments. There isn't enough information yet
to say which of the four commercially available CAR products for people
with B cell lymphomas—Axi-Cel, Brexa-Cel, Liso-Cel, and
Tisagenlecleucel—works best and causes the fewest side effects.[119-124]
Sadly,
the majority of plasmablastic lymphomas lack CD19 expression.
Nevertheless, only a small proportion of those patients can express
this receptor, making CAR-T therapy plausible.
Raychaudhuri et al.[125]
demonstrated that PBL can respond positively to axi-cel when CD19 is
expressed. This patient's response was transient but clinically
significant. Pain and transfusion-dependent cytopenias resolved, and
the patient's performance status returned to normal. Longer in vivo
persistence of axi-cell activity may have allowed for a more robust
response.[125]
Autologous Bone Marrow Transplantation (ABMT)
Multiple studies have shown that up to 60 percent of patients with relapsed or refractory lymphoma will progress before ABMT.[126] In addition, the prognosis for patients with recurrent or resistant PBL is dismal.[127]
Since the outcome appears significantly better in patients with
relapsed NHL who received ABMT compared to those who did not, it seems
reasonable to investigate the use of ABMT earlier in the course of
disease, at least in the high-risk subgroup of patients.
A study
using the EBMT registry found that some types of NHL, like
plasmablastic lymphoma, had a higher risk of relapse compared to DLBCL
(relative risk, 3.4%; 95% confidence interval, 1.1% to 10.4%; P.03) and
a possible trend toward worse survival. It was hypothesized that ABMT
in CR1 could be advantageous for this subgroup of patients.[128]
GICAT
(Gruppo Italiano Cooperativo AIDS e Tumori) presented an interim
analysis of a phase II multicenter trial of early consolidation with
ABMT in HIV-positive patients with NHL and subsequently published their
experience in HIV-positive patients with PBL.[129,130]
Four
patients had PBL in this study. One patient was excluded from the study
due to prolonged cytopenia during induction therapy (she is still in
CR1 at +34 months). The last three patients got transplants with the
BEAM (BCNU, etoposide, cytarabine, and melphalan) conditioning regimen
in CR1 after induction therapy. At +24, +19, and +13 months, all of
them are alive and in remission. The 2-year PFS and OS were 73% and
76%, respectively, across the entire series, with a median follow-up of
19.5 months (range: 4 to 65).[129,130]
In the
Italian GICAT center, two additional HIV-positive patients with
high-risk PBL were treated with ABMT as upfront consolidation following
CHOP induction, and the results were reported separately. One patient
is still alive in CR at +83 months, while the other died 4 months after
transplantation due to progression.[130]
Similar
to the Italian cooperative group and the Moffitt Cancer Center, the
Center for International Blood and Marrow Transplant Research (CIBMTR)
grouped patients with PBL by disease status. Seven of eleven (64%)
patients who had ABMT in CR1 were still alive at the most recent
follow-up, after an average of 25 months (range, 4 to 43 months); only
four of nine (44%) patients who had ABMT in CR2 were still alive after
an average of 62 months (range, 12 months). (CIBMTR, unpublished data).
In a recent multicenter study involving 281 patients diagnosed with
plasmablastic lymphoma, 13 participants had autologous bone marrow
transplantation (ABMT) as a therapeutic consolidation following their
initial complete remission. The current investigation lacks data
regarding the responses or survival of these patients.[21]
Ideally, randomized studies are required to establish the efficacy of
ABMT in PBL. People with PBL who have any of the following high-risk
factors should be considered for consolidation with ABMT as a first
option: an aaIPI score greater than 2, no HIV, MYC gene rearrangement,
TP53 gene deletion, or any response to induction chemotherapy other
than CR (partial response or refractory disease).
Double Autologous Stem Cell Transplantation
There
is only one report in the literature of an HIV-negative person with
extraoral PBL who went into and stayed in complete remission for a very
long time after intensive therapy with thalidomide and dexamethasone,
followed by consolidation with two autologous stem cell transplants.
The authors chose the intensive multiple myeloma-like treatment at
random because antimyeloma drugs have been used in PBL case reports.
The author stated that despite the inherent aggressiveness of the
disease, the early presentation stage may have had a positive effect on
the patient's long-term prognosis.[131]
Allotransplant for PBL
Compared
to ABMT, the literature on allogeneic hematopoietic cell transplant
(allo-HCT) PBL is considerably more limited. It's very important to
know about the risks of opportunistic infections, having multiple
infections at the same time (like viral hepatitis), the complicated
drug interactions between antiretroviral drugs and transplant-related
drugs, and how HIV affects T cell numbers and functions, the bone
marrow microenvironment, and the cytokine milieu. These factors lead to
both higher transplant-related mortality (TRM) and HIV-related
mortality.Because
they lower TRM, reduced-intensity conditioning regimens have made
allogeneic hematopoietic cell transplantation more successful overall,
but it's still not clear what role they play in HIV-positive patients.
A 51-year-old HIV-positive man with PBL and a hematopoietic cell
transplant comorbidity index of 4 (high risk) underwent Allo HSCT from
a matched unrelated donor to demonstrate the feasibility of the
procedure.[132] He was given fludarabine, busulfan,
and antithymocyte globulin to condition him. For the prevention of
GVHD, methotrexate and tacrolimus were used. At the time of the report,
he was two years post-transplantation, disease-free, and off
immunosuppression.[132]In
a recent multicenter study involving 281 patients diagnosed with
plasmablastic lymphoma, 5 participants had allo-HCT for relapsed PBL
HIV negative. Out of these patients, four died away, with three deaths
attributed to complications from the transplant and one death
attributed to a second malignancy. One patient who received allo-HCT
after one previous treatment is currently alive, as of the most recent
follow-up, which occurred 26 months after the allo-HCT procedure. The
patient is experiencing an important chronic graft-versus-host disease.[21]Another report describing allo-HSCT in an HIV-negative PBL patient was found in the literature.[133]
The patient experienced a recurrence following a consolidated ABMT.
Then, he receives a salvage allo-HSCT from his daughter and achieves a
favorable outcome. He has been in long-term, complete remission and is
still alive. It may be attributable to his younger age, low IPI score,
and prompt allo-HCT treatment following relapse. Thus, allo-HSCT has
the potential to enhance the likelihood of long-term survival for young
patients with PBL who have experienced a relapse.
Radiation Therapy (RT)
Radiotherapy
is less considered a treatment option for PBL patients, as it has only
been reported in approximately 200 published cases (Table 5).
 |
- Table 5. Literature review of Radiation Therapy reports in PBL.
|
In
2024, systemic therapy will be used to treat most PBL cases. However,
combined modality therapy (CMT), which includes systemic
chemoimmunotherapy followed by consolidation radiation therapy (RT), is
still a well-proven way to treat the disease. RT has multiple
applications, either alone or in combination with multi-agent
chemotherapy.[134-140]After
initial treatment, nearly half of PBL patients may experience either
primary refractoriness or disease progression. Relapsed or refractory
PBL remains exceedingly difficult to treat, with persistently dismal
outcomes. Notably, RT still works well even for diseases that don't
respond to chemotherapy. It has traditionally been an important part of
clinical practice for these patients, with or without systemic therapy,
as part of both curative and palliative-intent programs.[134-140]Although
radiation treatment (RT) remains a potential option for some patients
who cannot undergo systemic therapy, it is primarily employed as a
consolidation therapy following chemoimmunotherapy. Consolidation
radiation is often recommended following multiple courses of systemic
therapy in patients with advanced or bulky illness, several risk
factors, or a partial response.[140-142]Even
in patients who achieve complete remission, the most common pattern of
PBL relapse after chemotherapy involves the original sites of disease.
RT may result in a benefit for event-free survival and, eventually, an
overall survival (OS) benefit.[142-144]Short
courses of RT can alleviate a variety of symptoms, including pain,
bleeding, airway or bowel obstruction, and neurologic compromise.
Diseases that pose a threat to vital organs, such as the spinal cord or
airway, may also be treated with RT to prevent impending complications.
Finally, radiation therapy (RT) can be utilized as a potent treatment
method for localized progression, with the aim of postponing the
requirement for systemic therapy. This is particularly beneficial, as
systemic therapy is often linked to a greater degree of adverse
effects. The ideal doses for palliating R/R DLBCL are still unknown,
and the most suitable treatment regimen may eventually vary depending
on the specific clinical situation. Typically, hypofractionated doses
ranging from 20 to 30 Gy are delivered. Various strategies may be
suitable, depending on the clinical situation. Patients with a limited
life expectancy are recommended to undergo short treatment regimens,
such as receiving 4 Gy of radiation over 5 days, 8 Gy in a single day,
or even 2 Gy over 2 days or 4 Gy in one day. Extended treatment
schedules (e.g., 3 Gy for 10 days or 2.5 Gy for 15 days) may be more
suitable for patients with a more positive outlook, particularly those
with a smaller amount of disease. Generally, it is advisable to
restrict therapy volumes to the bulk of the disease with the smallest
possible margin.[142-148]
CNS Prophylaxis
CNS
relapse is a relatively uncommon but frequently devastating
complication of DLBCL. Most CNS relapses occur during or shortly after
first-line immune-chemotherapy, with a median time of 6 to 8 months, as
reported in a recent prospective clinical trial.[149]Treating
secondary CNS lymphoma (SCNSL) is sometimes challenging, and
historically, the outcomes have been unsatisfactory. Consequently,
significant attention has been given to identifying patients with the
highest propensity to this problem, as well as implementing preventive
measures aimed at minimizing risk as much as feasible. A better
understanding of DLBCL's molecular biology and the CNS-IPI238 trial
have helped find people at high risk for secondary central nervous
system lymphoma (SCNSL). However, decisions about preventing this
disease are still based on looking at past cases or extrapolating data
from other types of the disease.[150-152] There have
been no prospective randomized trials conducted to directly assess the
effectiveness of CNS prophylaxis. Physicians often encounter the
difficult task of preventing a potential consequence without exposing
the patient to further treatment that may have harmful side effects and
lack substantial evidence of its effectiveness.Numerous
studies have investigated possible CNS relapse risk factors in DLBCL.
In 2016, the German High-Grade Non-Hodgkin Lymphoma Study Group
(DSHNHL) created a prognostic model (CNS-IPI) that sorts patients into
three risk groups based on the five standard IPI factors as well as
whether the disease has spread to the kidneys or adrenal glands.[150,152]Significantly,
patients who had five or six risk variables had a respective
probability of CNS relapse of 15% and 32.5%. Specific extranodal (EN)
locations have been linked to a higher likelihood of central nervous
system (CNS) recurrence. The CNS-IPI model considers the involvement of
the kidneys and adrenal glands. However, intravascular lymphoma is
known to have a high risk of involving the CNS, either at the start or
during a relapse. The association between testicular involvement and
CNS recurrence probability, ranging from 10% to 25% over a 10-year
period, has been well-established in both limited and advanced stages.[150-154]In
a retrospective series, breast involvement was associated with a higher
risk of CNS relapse (15%), whereas other EN sites, such as the uterus,
blood, bone marrow, and epidural area, exhibited inconsistent results
and are unlikely to be independently predictive of CNS relapse.[155]
A
new systematic review looked at stand-alone IT prophylaxis in 7357
patients who were getting chemoimmunotherapy. The review included three
post-hoc trial analyses and 10 retrospective investigations.[156]
In univariable or multivariable analyses, IT prophylaxis was not
associated with a reduction in CNS relapse rate. The administration of
IT therapy can be difficult and uncomfortable for the patient, with
some evidence suggesting an association with hospitalization for
infection-related reasons in older patients.[150-157]The
utilization of brain imaging and lumbar puncture/cerebrospinal fluid
(CSF) analysis to detect individuals at high risk of central nervous
system (CNS) involvement who could potentially benefit from treatments
targeting the CNS is increasing.[158]Several
studies have demonstrated that flow cytometric analysis of CSF is more
sensitive than cytology for detecting occult CNS involvement.[159,161]
Nonetheless, a percentage of patients with negative flow cytometry
result in CNS relapse shortly after treatment, indicating the need for
more sensitive techniques. The incidence of CNS relapse is estimated to
occur in approximately 3–5% of PBL patients.[3-10]Further,
PBL in people living with HIV (PLWH) has an increased risk of
aggressive disease, with CNS involvement occurring more frequently than
other extra-nodal involvement.886 people who were newly diagnosed with AIDS-related lymphomas (DLBCL and BL) were looked at in detail.[162]
It was found that the central nervous system (CNS) was involved in
between 5% and 30% of the cases at the start of the therapy. The
recurrence of central nervous system (CNS) cancer happens promptly,
typically within a median period of 4.2 months following diagnosis, and
is associated with a very poor survival rate of only 1.6 months. Over
90% of patients underwent intrathecal (IT) and central nervous system
(CNS) prophylaxis, while 5% encountered CNS recurrence. In this case,
it has not been determined what the best treatment plan, dosage
frequency, and ways to avoid complications in the central nervous
system (CNS), such as choosing between intravenous medications that can
reach the CNS and intrathecal therapy. The guidelines from the US
National Comprehensive Cancer Network recomment the use of intrathecal
methotrexate for central nervous system prophylaxis in all people
living with HIV (PLWH) who have lymphoma.[22]In
conclusion, people with PBL are likely to get leptomeningeal disease
because of the high rate of proliferation, the strong link to HIV
infection, the high rate of extranodal involvement, and the presence of
MYC translocations. CNS prophylaxis should be considered in a
case-by-case basis.
Antiretroviral Treatment During Chemotherapy for HIV+ PBL
It
is crucial to note that more than 60% of individuals diagnosed with
plasmablastic lymphoma are also infected with HIV. Combined
antiretroviral therapy (cART) has independently contributed to
improving the response to chemotherapy and the survival of HIV-infected
patients with lymphoma.[163-166]All
HIV-positive patients with PBL should, therefore, receive cART
concurrently with chemotherapy. The antiretroviral treatment history,
HIV strain sensitivity, HLAB5701 result, and hepatitis B virus (HBV)
infection markers should be considered when choosing a cART regimen. In
certain instances, it is recommended to obtain the HIV strain's tropism
result (R5, X4, or dual tropism). In addition, it is essential to
always consider the potential pharmacological interactions and
cross-toxicity between antiretrovirals and antitumor drugs or other
commonly used drugs in this patient population, such as antifungals.
There is limited clinical evidence regarding the efficacy and safety of
chemotherapy and antiretroviral therapy.In
a series of 150 HIV-infected patients with cancer (mostly hematological
malignancies), protease inhibitor-based cART regimens were less
effective and less safe than those based on non-nucleoside reverse
transcriptase inhibitors (NNRTI) and integrase strand transfer
inhibitors (ISTI) (INSTI).[167] Between 40 and 60
percent of HIV-infected patients have been exposed to HBV, and between
3 and 10 percent have chronic hepatitis B, which is defined by the
presence of HBV surface antigen (HBsAg).[168-172] Reactivation of HBV can occur during chemotherapy, particularly when rituximab is employed as a therapeutic agent.[172-175]
Patients co-infected with HIV and HBV should receive a combination
antiretroviral therapy (cART) regimen that is also effective against
HBV. In addition to tenofovir and emtricitabine (FTC) or lamivudine
(3TC), a third HIV drug should be administered.[174,175]
Tenofovir alafenamide (TAF) should be preferred over tenofovir
disoproxilfumarate (TDF) because it has a more favorable safety profile
and is equally effective against HBV.[175]
Therefore, these antiretroviral drugs can alter the pharmacokinetics of
antitumor drugs that are substrates of this isoenzyme, particularly
taxanes and alkylating agents like cyclophosphamide and etoposide and,
to a lesser extent, vinca alkaloids, antitumor antibiotics, and
platinum. There are no significant interactions between antiretrovirals
and anthracyclines because aldose reductase metabolizes both
substances. In patients receiving concomitant CHOP, doxorubicin,
etoposide, and PI-based cART, cyclophosphamide clearance was decreased,
and the frequency of severe anemia and neutropenia was increased
compared to patients receiving CHOP alone. Severe cases of neutropenia
and mucositis were reported in patients receiving concomitant CHOP and
cART.[176]It
is unlikely that nucleoside or nucleotide reverse transcriptase
inhibitors (NRTIs) will interact pharmacokinetically with cytostatics.
The CCR5 antagonist maraviroc is a substrate for the CYP3A enzyme and
the P glycoprotein. This means that strong CYP3A inducers or inhibitors
may change the levels of maraviroc. Insti (raltegravir and
dolutegravir) metabolizes through glucuronidation in the liver and has
minimal interactions with cytostatic medications. Elvitegravir, a third
member of this class, must be co-administered with the cobicistat
enhancer so that their interactions are functionally equivalent to
those of the PI. No pharmacokinetic interactions between
antiretrovirals and rituximab, the most widely used monoclonal antibody
for NHL, have been described.[175-180]When
selecting a combination antiretroviral therapy (cART) regimen, it is
also crucial to consider the drugs' safety profile. 3TC, FTC, abacavir
(AUC), and TDF or TAF are the most commonly used NRTIs for the
treatment of HIV at present. 3TC, FTC, TDF, and TAF are also effective
anti-HBV agents. 3TC, FTC, and ABC are not cross-toxic with anticancer
drugs. TDF can cause proximal tubular nephropathy and a decreased
glomerular filtration rate; therefore, patients with renal
insufficiency, tumor lysis syndrome, or those receiving antitumor drugs
with nephrotoxic potential should avoid using TDF. TAF is equivalent to
TDF in terms of efficacy and lacks nephrotoxicity, at least in the
short term.[181,182] Other NRTIs, such as zidovudine
(AZT), didanosine (ddI), and stavudine (d4T), are rarely used today and
have significant cross-toxicity with certain anti-tumor drugs. AZT has
the potential to be myelotoxic and may increase the hematological
toxicity of various chemotherapeutic regimens. Certain antitumor
medications, such as platinum, taxanes, and vinca alkaloids, can worsen
peripheral neuropathy caused by DdI and d4T and cause mitochondrial
toxicity.[183] Some PIs, like atazanavir, lopinavir,
and saquinavir, may make the CT interval longer. This is something that
should be thought about when these drugs are combined with
anthracyclines, which are also known to make the CT interval longer.
The bilirubin levels of patients with hepatopathy can be used to adjust
the dosage of certain antitumor drugs. Although atazanavir can cause
unconjugated hyperbilirubinemia because it blocks the uridine
diphosphate glucuronosyltransferase 1A1 enzyme (UGT1A1), this can make
it harder to make the necessary changes when taking this
antiretroviral.[184]
Complementary Treatments
At
the time of diagnosis or after beginning chemotherapy, patients with a
large tumor mass may exhibit complications resulting from tumor lysis
syndrome. In cases where this complication is likely because of the
size of the tumor or very high levels of LDH and uric acid,
hyperhydration, forced diuresis, and allopurinol should be given before
chemotherapy. Additionally available is rasburicase, a recombinant
version of the urate oxidase enzyme that turns uric acid into
allantoin, which the kidneys excrete more effectively. This medication
is more effective than allopurinol at reducing plasma uric acid levels
and can prevent chemotherapy from beginning too late.[185,186]
The recommended intravenous dose of rasburicase is 0.20 mg/kg/day in 50
mL of normal saline for 30 minutes. The duration of treatment ranges
between 5 and 7 days, but shorter-duration regimens appear to be
equally effective. Giving granulocyte colony-stimulating factors
(G-CSF) is suggested to shorten the time of neutropenia after
chemotherapy, improve cytostatic tolerance, and allow full doses and
proper chemotherapy intervals.[187]
Prophylaxis of Opportunistic Infections Associated with HIV+PBL
After
the administration of chemotherapy, the total number of CD4+ T
lymphocytes decreases by 30–50% with respect to the baseline, depending
on the intensity of the treatment and the moment at which the analysis
is carried out. This is why the risk of opportunistic infections
associated with HIV is higher in these patients than in patients with
PBL at a similar stage.[188]In
principle, it should be said that primary or secondary prophylaxis
indicated according to the CD4+ T lymphocyte count and the previous
history of opportunistic infections should be performed. However, it is
recommended to consider that the degree of immunosuppression in
patients is greater than the one shown by the CD4+ T lymphocyte count
at the time of tumor diagnosis and that these lymphocyte markers should
be monitored throughout the treatment of the lymphoma and act
accordingly.[189]Pneumocystis
jirovecii prophylaxis is recommended for all patients. However, we must
consider the effects that systematic implementation of this practice
can have on antibiotic resistance and other undesirable outcomes, such
as Clostridium difficile colitis.[189-190] Anti-CMV
prophylaxis is generally not recommended, but close monitoring with
periodic blood PCR determinations for this virus is recommended in
those patients with a low CD4+ T lymphocyte count (every 7 days).
Vaccinations
The annual inactivated influenza vaccination and COVID-19 are recommended for both HIV-positive and HIV-negative PBL patients.The
COVID-19 vaccine is particularly important for those patients due to
the frequent prolonged positivity and virus shedding of the SARS-Cov-2
and the impact of this on delaying the chemotherapy.[191,192]
The vaccination of close contacts against influenza and COVID-19 is
also recommended. 278 As with all HIV-infected patients, PBL patients
should also receive vaccinations against pneumococcus, HBV, and the
hepatitis A virus. Although the optimal time to administer the vaccines
is unknown, it is recommended to do so at least two weeks prior to
beginning chemotherapy or at least one week after the last cycle.[193,194]
First Line Post-Treatment Assessment
After
the initial treatment, if patients achieve a PET-negative remission, we
schedule additional monitoring. Regrettably, even after discontinuation
of cytotoxic treatment and achieving complete remission, numerous
patients have fatigue, polyneuropathy, or anxiety. Thus, we promptly
direct patients experiencing anxiety to receive psychological treatment
and, if judged required, commence the administration of psychotropic
medications. For patients diagnosed with polyneuropathy, we advise
decreasing the dosage of vincristine and providing symptomatic relief
using gabapentin or duloxetine, even though there is limited evidence
supporting their effectiveness. Moreover, cancer rehabilitation clinics
recommend that patients reside there for a period of time to improve
their overall quality of life. For additional monitoring, we refrain
from using routine CT scans on asymptomatic individuals because the
American Society of Hematology has determined that they are not
effective. We suggest performing a blood count, renal and liver
function tests, LDH measurement, and a clinical examination every 3
months during the initial 2-year period following treatment. Patients
with prominent mediastinal or retroperitoneal disease at diagnosis
should undergo chest X-rays and ultrasonography. The NCCN guidelines
continue to recommend surveillance CT scans for follow-up in patients
with aggressive lymphoma, although we only utilize this approach for
specific patients who have an increased risk of experiencing a relapse.
Relapsed/Refractory Plasmablastic Lymphoma
In
patients with persistent PET-positive disease, new lymphadenopathy, or
organ lesions after first-line treatment, we perform a new biopsy to
confirm malignancy and the former diagnosis. Refractory PBL has a
significantly worse prognosis than other aggressive non-Hodgkin B-cell
lymphomas, irrespective of whether the patient has HIV or not. The
reported median PFS ranges from 6–7 months, while the median OS ranges
from 11–13 months.[5-12]
However,
due to the rarity of this disease, there is insufficient evidence to
support one particular salvage therapy for patients with relapsed or
refractory disease, particularly for those who do not achieve at least
a partial response. Furthermore, the same treatment methods used as the
first line of therapy can also be employed in later stages of
treatment. If accessible, we suggest enrolling these patients in
experimental clinical trials. At the moment, there aren't many
prospective randomized studies that directly compare the different
second-line treatment options for people with PBL who can't get
autologous stem cell transplantation (ASCT) or anti-CD19 CAR T-cell
therapy. Such a comparison would yield valuable data for treatment
sequencing and evaluate treatment effectiveness in various patient
subgroups, including those with high clinical and biological risk
factors. Managing these patients in this context is currently a
significant unmet medical need. However, situations where randomized
studies are scarce are increasingly seeing prevalent actual comparisons
across different groups, particularly those that are carefully matched.Ultimately,
we have created a comprehensive table that aims to condense the complex
therapy recommendations for this uncommon and aggressive lymphoma (Figure 1).
 |
- Figure 1. Complex therapy recommendations for Relapsed/Refractory Plasmablastic Lymphoma.
|
Conclusions
In
terms of both diagnosis and treatment, PBL is a challenging disease.
The majority of long-term survivors had limited disease or were
eligible for autologous stem cell transplants as consolidation
following combination chemotherapy responses. Regimens for myeloma that
include proteasome inhibitors, immunomodulators, and targeted therapy
pave the way for improved outcomes. Now that we have gained more
knowledge about the mutational landscape of PBL, researchers have
suggested numerous potential new targets. These include pan-TRK
inhibitors like larotrectinib or entrectinib for NTRK3 mutations. It's
also important to look at other effective treatments that have been
used for MM, like CAR-T and bispecific antibodies against CD38, CD138,
or B-cell maturation antigen (BCMA), especially for patients with
relapsed and resistant PBL.
References
- Stein H. & Dallenbach F. in Neoplastic
Hematopathology (ed.Knowles, D.M.) 675-714 (Williams & Wilkins,
Baltimore, MA, 1992).
- Delecluse
HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of
the oral cavity: a new entity associated with the human
immunodeficiency virus infection. Blood. 1997;89:1413-1420. https://doi.org/10.1182/blood.V89.4.1413 PMid:9028965
- Bibas
M. Plasmablastic Lymphoma. A State-of-the-Art Review: Part
1-Epidemiology, Pathogenesis, Clinicopathologic Characteristics,
Differential Diagnosis, Prognostic Factors, and Special Populations.
Mediterr J Hematol Infect Dis. 2024 Jan 1;16(1):e2024007. https://doi.org/10.4084/MJHID.2024.007 PMid:38223486 PMCid:PMC10786126
- Alaggio
R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E,
Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk
W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong
D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart
J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM,
Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S,
Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu
WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN,
Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam
Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV,
Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C,
Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A,
Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th
edition of the World Health Organization Classification of
Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022
Jul;36(7):1720-1748. https://doi.org/10.1038/s41375-022-01620-2 PMid:35732829 PMCid:PMC9214472
- Campo
E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC,
Brousset P, Cerroni L, de Leval L, Dirnhofer S, Dogan A, Feldman AL,
Fend F, Friedberg JW, Gaulard P, Ghia P, Horwitz SM, King RL, Salles G,
San-Miguel J, Seymour JF, Treon SP, Vose JM, Zucca E, Advani R, Ansell
S, Au WY, Barrionuevo C, Bergsagel L, Chan WC, Cohen JI, d'Amore F,
Davies A, Falini B, Ghobrial IM, Goodlad JR, Gribben JG, Hsi ED, Kahl
BS, Kim WS, Kumar S, LaCasce AS, Laurent C, Lenz G, Leonard JP, Link
MP, Lopez-Guillermo A, Mateos MV, Macintyre E, Melnick AM, Morschhauser
F, Nakamura S, Narbaitz M, Pavlovsky A, Pileri SA, Piris M, Pro B,
Rajkumar V, Rosen ST, Sander B, Sehn L, Shipp MA, Smith SM, Staudt LM,
Thieblemont C, Tousseyn T, Wilson WH, Yoshino T, Zinzani PL, Dreyling
M, Scott DW, Winter JN, Zelenetz AD. The International Consensus
Classification of Mature Lymphoid Neoplasms: a report from the Clinical
Advisory Committee. Blood. 2022 Sep 15;140(11):1229-1253. https://doi.org/10.1182/blood.2022015851 PMid:35653592 PMCid:PMC9479027
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015 Apr 9;125(15):2323-30. https://doi.org/10.1182/blood-2014-10-567479 PMid:25636338
- Bibas
M, Castillo JJ. Current knowledge on HIVassociated Plasmablastic
Lymphoma. Mediterr J Hematol Infect Dis. 2014;6(1):e2014064. https://doi.org/10.4084/mjhid.2014.064 PMid:25408850 PMCid:PMC4235470
- Castillo
JJ, Furman M, Beltrán BE,et al.: Human immunodeficiency
virus-associated plasmablastic lymphoma: poor prognosis in the era of
highly active antiretroviral therapy. Cancer. 2012 Nov
1;118(21):5270-7. https://doi.org/10.1002/cncr.27551 PMid:22510767
- Florindez
JA, Alderuccio JP, Reis IM, Lossos IS. Survival analysis in treated
plasmablastic lymphoma patients: a population-based study. Am J
Hematol. Nov 2020;95(11):1344-1351. https://doi.org/10.1002/ajh.25955 PMid:32777103
- Alec
R. Hansen , Victoria A. Vardell , Lindsey A. Fitzgerald , Epidemiologic
characteristics, treatment patterns, and survival analysis of
Plasmablastic Lymphoma in the US: A SEER and NCDB analysis., Clinical
Lymphoma, Myeloma and Leukemia (2023), https://doi.org/10.1016/j.clml.2023.12.014 PMid:38262787
- Castillo
J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma:
lessons learned from 112 published cases. Am J Hematol. 2008
Oct;83(10):804-9. https://doi.org/10.1002/ajh.21250 PMid:18756521
- Castillo
JJ, Winer ES, Stachurski D, Perez K, Jabbour M, Milani C, Colvin G,
Butera JN. Prognostic factors in chemotherapy-treated patients with
HIV-associated Plasmablastic lymphoma. Oncologist. 2010;15(3):293-9. https://doi.org/10.1634/theoncologist.2009-0304 PMid:20167839 PMCid:PMC3227958
- Ibrahim
IF, Shapiro GA, Naina HVK. Treatment of HIV-associated plasmablastic
lymphoma: a single-center experience with 25 patients. J Clin Oncol
2014; 32(15Suppl):8583. https://doi.org/10.1200/jco.2014.32.15_suppl.8583
- Loghavi
S, Alayed K, Aladily TN, Zuo Z, Ng SB, Tang G, Hu S, Yin CC, Miranda
RN, Medeiros LJ, Khoury JD. Stage, age, and EBV status impact outcomes
of plasmablastic lymphoma patients: a clinicopathologic analysis of 61
patients. J Hematol Oncol. 2015 Jun 10;8:65. https://doi.org/10.1186/s13045-015-0163-z PMid:26055271 PMCid:PMC4472407
- Pinnix
CC, Shah JJ, Chuang H, et al. Doxorubicin-based chemotherapy and
radiation therapy produces favorable outcomes in limited-stage
plasmablastic lymphoma: a single-institution review. Clin Lymphoma
Myeloma Leuk 2016; 16:122-8. https://doi.org/10.1016/j.clml.2015.12.008 PMid:26795083 PMCid:PMC9754636
- Rudresha
AH, Lakshmaiah KC, Agarwal A, et al. Plasmablastic lymphoma in
immunocompetent and in immunocompromised patients: experience at a
regional cancer centre in India. South Asian J Cancer 2017; 6:69-71. https://doi.org/10.4103/sajc.sajc_186_16 PMid:28702410 PMCid:PMC5506813
- Focà
E, Cavaglià G, Rusconi S, et al. Survival in HIV-infected patients with
lymphoma according to the choice of antiretroviral treatment: an
observational multicentre study. HIV Med 2018, Online ahead of print https://doi.org/10.1111/hiv.12624 PMid:29862615
- Al
Tabaa Y, Tchernonog E, Faurie P, et al. Post-treatment positron
emission tomography-computed tomography is highly predictive of outcome
in plasmablastic lymphoma. Eur J Nucl Med Mol Imaging 2018; 45:1705-9. https://doi.org/10.1007/s00259-018-4020-5 PMid:29679112
- Zuze T, Painschab MS, Seguin R, et al. Plasmablastic lymphoma in Malawi. Infect Agent Cancer 2018; 13:22. https://doi.org/10.1186/s13027-018-0195-4 PMid:29988350 PMCid:PMC6022505
- Rodrigues-Fernandes
CI, de Souza LL, Dos Santos-Costa SF, et al. Clinicopathological
analysis of oral plasmablastic lymphoma: a systematic review. J Oral
Pathol Med 2018; 47:915-22. https://doi.org/10.1111/jop.12753 PMid:29917262
- Di
Ciaccio PR, Polizzotto MN, Cwynarski K, Gerrie AS, Burton C, Bower M,
Kuruvilla J, Montoto S, McKay P, Fox CP, Milliken S, Jiamsakul A,
Osborne W, Collins GP, Manos K, Linton KM, Iyengar S, Kassam S, Limei
MP, Kliman D, Wong Doo N, Watson AM, Fedele P, Yannakou CK, Hunt S, Ku
M, Sehn LH, Smith A, Renshaw H, Maxwell A, Liu Q, Dhairyawan R,
Ferguson G, Pickard K, Painter D, Thakrar N, Song KW, Hamad N. The
influence of immunodeficiency, disease features, and patient
characteristics on survival in plasmablastic lymphoma. Blood. 2024 Jan
11;143(2):152-165. https://doi.org/10.1182/blood.2023021348 PMid:37832030
- NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas. NCCN 2024;Version 1..2024- 18 January 2024
- Robak P, Robak T. Bortezomib for the Treatment of Hematologic Malignancies: 15 Years Later. Drugs R D. 2019 Jun;19(2):73-92. https://doi.org/10.1007/s40268-019-0269-9 PMid:30993606 PMCid:PMC6544598
- Leonard
JP, Kolibaba K, Reeves JA, et al. Randomized phase 2 openlabel study of
R-CHOP ± bortezomib in patients (Pts) with untreated non-germinal
center B-cell-like (Non-GCB) subtype diffuse large cell lymphoma
(DLBCL): results from the Pyramid trial (NCT00931918). Blood.
2015;126(23):811. 44. https://doi.org/10.1182/blood.V126.23.811.811
- Robak
T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed
mantle-cell lymphoma. N Engl J Med. 2015;372(10):944-953. https://doi.org/10.1056/NEJMoa1412096 PMid:25738670
- Dunleavy
K, Pittaluga S, Czuczman MS, Dave SS, Wright G, Grant N, Shovlin M,
Jaffe ES, Janik JE, Staudt LM, Wilson WH. Differential efficacy of
bortezomib plus chemotherapy within molecular subtypes of diffuse large
B-cell lymphoma. Blood. 2009 Jun 11;113(24):6069-76. https://doi.org/10.1182/blood-2009-01-199679 PMid:19380866 PMCid:PMC2699229
- Jambusaria
A, Shafer D, Wu H, Al-Saleem T, Perlis C. Cutaneous plasmablastic
lymphoma. J Am Acad Dermatol. 2008 Apr;58(4):676-8. https://doi.org/10.1016/j.jaad.2007.08.009 PMid:18342714
- Bose
P, Thompson C, Gandhi D, Ghabach B, Ozer H. AIDS-related plasmablastic
lymphoma with dramatic, early response to bortezomib. Eur J Haematol.
2009 Jun;82(6):490-2. https://doi.org/10.1111/j.1600-0609.2009.01235.x PMid:19220417
- Bibas
M, Grisetti S, Alba L, Picchi G, Del Nonno F, Antinori A. Patient with
HIV-associated plasmablastic lymphoma responding to bortezomib alone
and in combination with dexamethasone, gemcitabine, oxaliplatin,
cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone. J
Clin Oncol. 2010 Dec 1;28(34):e704-8. https://doi.org/10.1200/JCO.2010.30.0038 PMid:20823416
- Lipstein
M, O'Connor O, Montanari F, Paoluzzi L, Bongero D, Bhagat G.
Bortezomib-induced tumor lysis syndrome in a patient with HIV-negative
plasmablastic lymphoma. Clin Lymphoma Myeloma Leuk. 2010
Oct;10(5):E43-6. https://doi.org/10.3816/CLML.2010.n.074 PMid:21856550
- Dasanu
CA, Bauer F, Codreanu I, Padmanabhan P, Rampurwala M. Plasmablastic
haemato-lymphoid neoplasm with a complex genetic signature of Burkitt
lymphoma responding to bortezomib. Hematol Oncol. 2013 Sep;31(3):164-6.
https://doi.org/10.1002/hon.2024 PMid:22899491
- Saba
NS, Dang D, Saba J, Cao C, Janbain M, Maalouf B, Safah H. Bortezomib in
plasmablastic lymphoma: a case report and review of the literature.
Onkologie. 2013;36(5):287-91. https://doi.org/10.1159/000350325 PMid:23689224
- Cao
C, Liu T, Zhu H, Wang L, Kai S, Xiang B. Bortezomib-contained
chemotherapy and thalidomide combined with CHOP (Cyclophosphamide,
Doxorubicin, Vincristine, and Prednisone) play promising roles in
plasmablastic lymphoma: a case report and literature review. Clin
Lymphoma Myeloma Leuk. 2014 Oct;14(5):e145-50. https://doi.org/10.1016/j.clml.2014.03.002 PMid:25225082
- Cencini
E, Fabbri A, Guerrini S, Mazzei MA, Rossi V, Bocchia M. Long-term
remission in a case of plasmablastic lymphoma treated with COMP
(cyclophosphamide, liposomal doxorubicin, vincristine, prednisone) and
bortezomib. Eur J Haematol. 2016 Jun;96(6):650-654. https://doi.org/10.1111/ejh.12732 PMid:26715026
- Hirosawa
M, Morimoto H, Shibuya R, Shimajiri S, Tsukada J. A striking response
of plasmablastic lymphoma of the oral cavity to bortezomib: a case
report. Biomark Res. 2015 Nov 4;3:28. https://doi.org/10.1186/s40364-015-0053-0 PMid:26543559 PMCid:PMC4634743
- Castillo
JJ, Reagan JL, Sikov WM, Winer ES. Bortezomib in combination with
infusional dose-adjusted EPOCH for the treatment of plasmablastic
lymphoma. Br J Haematol. 2015;169(3):352-355 https://doi.org/10.1111/bjh.13300 PMid:25612847
- Fernandez-Alvarez
R, Gonzalez-Rodriguez AP, Rubio-Castro A, et al. Bortezomib plus CHOP
for the treatment of HIV-associated plasmablastic lymphoma: clinical
experience in three patients. Leuk Lymphoma. 2016;57(2):463-466. https://doi.org/10.3109/10428194.2015.1050666 PMid:25976108
- Fedele
PL, Gregory GP, Gilbertson M, Shortt J, Kumar B, Opat S, Grigoriadis G.
Infusional dose-adjusted epoch plus bortezomib for the treatment of
plasmablastic lymphoma. Ann Hematol. 2016 Mar;95(4):667-8. https://doi.org/10.1007/s00277-016-2601-6 PMid:26801792
- Arora
N, Gupta A, Sadeghi N. Durable complete remission with combination
chemotherapy and bortezomib in HIV-associated plasmablastic lymphoma.
BMJ Case Rep. 2017 Oct 9;2017:bcr2017222063. https://doi.org/10.1136/bcr-2017-222063 PMid:28993364 PMCid:PMC5652596
- Guerrero-Garcia
TA, Mogollon RJ, Castillo JJ. Bortezomib in plasmablastic lymphoma: a
glimpse of hope for a hard-to-treat disease. Leuk Res.
2017;62(September):12-16 https://doi.org/10.1016/j.leukres.2017.09.020 PMid:28963907
- Dittus
C, Grover N, Ellsworth S, Tan X, Park SI. Bortezomib in combination
with dose-adjusted EPOCH (etoposide, prednisone, vincristine,
cyclophosphamide, and doxorubicin) induces long-term survival in
patients with plasmablastic lymphoma: a retrospective analysis. Leuk
Lymphoma. 2018 Sep;59(9):2121-2127. https://doi.org/10.1080/10428194.2017.1416365 PMid:29303024
- Castillo
JJ, Guerrero-Garcia T, Baldini F, Tchernonog E, Cartron G, Ninkovic S,
Cwynarski K, Dierickx D, Tousseyn T, Lansigan F, Linnik Y, Mogollon R,
Navarro JT, Olszewski AJ, Reagan JL, Fedele P, Gilbertson M,
Grigoriadis G, Bibas M. Bortezomib plus EPOCH is effective as frontline
treatment in patients with plasmablastic lymphoma. Br J Haematol. 2019
Feb;184(4):679-682. https://doi.org/10.1111/bjh.15156 PMid:29527667
- Ando
K, Imaizumi Y, Kobayashi Y, Niino D, Hourai M, Sato S, Sawayama Y, Hata
T, Ohshima K, Miyazaki Y. Bortezomib- and Lenalidomide-Based Treatment
of Refractory Plasmablastic Lymphoma. Oncol Res Treat.
2020;43(3):112-116. https://doi.org/10.1159/000504608 PMid:31842017
- Umeanaeto
O, Gamboa J, Diaz J, Hakim MN, Corral J, Philipovskiy A, Gaur S.
Incorporating Bortezomib in the Management of Plasmablastic Lymphoma.
Anticancer Res. 2019 Sep;39(9):5003-5007. https://doi.org/10.21873/anticanres.13690 PMid:31519607
- Cai
J, Qiu L, Ma L, Zhang N, Fan FY. Case Report: Bortezomib Plus CDOP
Followed by Sequential Autologous Hematopoietic Stem Cell
Transplantation and Lenalidomide-Based Maintenance Therapy in
Plasmablastic Lymphoma. Front Med (Lausanne). 2021 Dec 3;8:749863. https://doi.org/10.3389/fmed.2021.749863 PMid:34926499 PMCid:PMC8677941
- Sabry
W, Wu Y, Kodad SG. Bortezomib, Lenalidomide and Dexamethasone
Combination Induced Complete Remission in Relapsed/Refractory
Plasmablastic Lymphoma: Case Report of a Potential Novel Treatment
Approach. Curr Oncol. 2022 Jul 18;29(7):5042-5053. https://doi.org/10.3390/curroncol29070399 PMid:35877259 PMCid:PMC9323819
- Gribben
JG, Fowler N, Morschhauser F. Mechanisms of Action of Lenalidomide in
B-Cell Non-Hodgkin Lymphoma. J Clin Oncol. 2015 Sep 1;33(25):2803-11. https://doi.org/10.1200/JCO.2014.59.5363 PMid:26195701 PMCid:PMC5320950
- Carras,
S.; Regny, C.; Peoc'h, M.; Gervasoni, J.; Gressin, R.; Cahn, J.Y.;
Molina, L. Dramatic efficacy of low dose lenalidomide as single agent
in a patient with refractory gastric nonhuman immunodeficiency
virus-associated plasmablastic lymphoma. Leuk. Lymphoma 2015, 56,
2986-2988. [CrossRef] [PubMed] https://doi.org/10.3109/10428194.2015.1016931 PMid:25676034
- Yanamandra,
U.; Sahu, K.K.; Jain, N.; Prakash, G.; Saikia, U.; Malhotra, P.
Plasmablastic lymphoma: Successful management with CHOP and
lenalidomide in resource constraint settings. Ann. Hematol. 2016, 95,
1715-1717. https://doi.org/10.1007/s00277-016-2732-9 PMid:27324386
- Schmit
J.M.; Delaune, J.; Norkin, M.; Grosbach, A. A case of plasmablastic
lymphoma achieving complete response and durable remission after
lenalidomide-based therapy. Oncol. Res. Treat. 2017, 40, 46-48. https://doi.org/10.1159/000455146 PMid:28095384
- Marrero,
W.D.; Cruz-Chacón, A.; Castillo, C.; Cabanillas, F. Successful Use of
Bortezomib-Lenalidomide Combination as Treatment for a Patient with
Plasmablastic Lymphoma. Clin. Lymphoma Myeloma Leuk. 2018, 18,
e275-e277. https://doi.org/10.1016/j.clml.2018.04.011 PMid:29753690
- Cheng,
L.; Song, Q.; Liu, M.; Wang, Y.; Yi, H.; Qian, Y.; Xu, P.; Cheng, S.;
Wang, C.; Wang, L.; et al. Case Report: Successful Management of a
Refractory Plasmablastic Lymphoma Patient with Tislelizumab and
Lenalidomide. Front. Immunol. 2021, 12, 702593. https://doi.org/10.3389/fimmu.2021.702593 PMid:34322131 PMCid:PMC8312258
- Lee
M, Martin BA, Abdulhaq H. Daratumumab, Lenalidomide, and Dexamethasone
(DRD), an Active Regimen in the Treatment of
Immunosuppression-Associated Plasmablastic Lymphoma (PBL) in the
Setting of Gorham's Lymphangiomatosis: Review of the Literature. Case
Rep Hematol. 2022 Jun 27;2022:8331766. https://doi.org/10.1155/2022/8331766 PMid:35795542 PMCid:PMC9252825
- Yi JH, Kim SJ, Kim WS. Brentuximab vedotin: clinical updates and practical guidance. Blood Res. 2017 Dec;52(4):243-253. https://doi.org/10.5045/br.2017.52.4.243 PMid:29333400 PMCid:PMC5762734
- Donato
EM, Fernández-Zarzoso M, Hueso JA, de la Rubia J. Brentuximab vedotin
in Hodgkin lymphoma and anaplastic large-cell lymphoma: an
evidence-based review. Onco Targets Ther. 2018 Aug 6;11:4583-4590. https://doi.org/10.2147/OTT.S141053 PMid:30122950 PMCid:PMC6084082
- Holderness
BM, Malhotra S, Levy NB, Danilov AV. Brentuximab vedotin demonstrates
activity in a patient with plasmablastic lymphoma arising from a
background of chronic lymphocytic leukemia. J Clin Oncol. 2013 Apr
20;31(12):e197-9. https://doi.org/10.1200/JCO.2012.46.9593 PMid:23509308
- Luo
B, Huang L, Gu Y, et al. Expression of exportin-1 in diffuse large
B-cell lymphoma: immunohistochemistry and TCGA analyses. Int J Clin Exp
Pathol 2018; 11: 5547-60.
- Culjkovic-Kraljacic
B, Fernando TM, Marullo R, et al. Combinatorial targeting of nuclear
export and translation of RNA inhibits aggressive B-cell lymphomas.
Blood 2016; 127: 858-68. https://doi.org/10.1182/blood-2015-05-645069 PMid:26603836 PMCid:PMC4760090
- Kuruvilla
J, Savona M, Baz R, et al. Selective inhibition of nuclear export with
selinexor in patients with non-Hodgkin lymphoma. Blood 2017; 129:
3175-83. https://doi.org/10.1182/blood-2016-11-750174 PMid:28468797
- Laín
S, Xirodimas D, Lane DP. Accumulating active p53 in the nucleus by
inhibition of nuclear export: a novel strategy to promote the p53 tumor
suppressor function. Exp Cell Res 1999; 253: 315-24 https://doi.org/10.1006/excr.1999.4672 PMid:10585254
- Chari
A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor dexamethasone for
triple-class refractory multiple myeloma. N Engl J Med 2019; 381:
727-38 https://doi.org/10.1056/NEJMoa1903455 PMid:31433920
- Ben-Barouch
S, Kuruvilla J. Selinexor (KTP-330) - a selective inhibitor of nuclear
export (SINE): anti-tumor activity in diffuse large B-cell lymphoma
(DLBCL). Expert Opin Investig Drugs 2019; 29: 15-21. https://doi.org/10.1080/13543784.2020.1706087 PMid:31847605
- Kasamon
YL, Price LSL, Okusanya OO, Richardson NC, Li RJ, Ma L, et al. FDA
Approval Summary: Selinexor for Relapsed or Refractory Diffuse Large
B-Cell Lymphoma. Oncologist. 2021;26(10):879-86. https://doi.org/10.1002/onco.13859 PMid:34132444 PMCid:PMC8488790
- Podar
K, Shah J, Chari A, Richardson PG, Jagannath S. Selinexor for the
treatment of multiple myeloma. Expert Opin Pharmacother.
2020;21(4):399-408 PMid:31957504
- Yuhua
F, Renjun B, Yonghua Y, Zhang T, Chen L. Selinexor in the Treatment of
a Patient with Refractory Plasmablastic Lymphoma: A Case Reports. Ann
Short Rep Clin Image. 2022; 3(3): 1028.
- Lin
P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic
analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004
Apr;121(4):482-8. https://doi.org/10.1309/74R4TB90BUWH27JX PMid:15080299
- de
Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen
LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson
KC, Lokhorst HM, van de Winkel JG, Parren PW. Daratumumab, a novel
therapeutic human CD38 monoclonal antibody, induces killing of multiple
myeloma and other hematological tumors. J Immunol. 2011 Feb
1;186(3):1840-8. https://doi.org/10.4049/jimmunol.1003032 PMid:21187443
- Krejcik
J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, Liu K,
van de Donk NW, Weiss BM, Ahmadi T, Lokhorst HM, Mutis T, Sasser AK.
Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell
expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016
Jul 21;128(3):384-94. https://doi.org/10.1182/blood-2015-12-687749 PMid:27222480 PMCid:PMC4957162
- Morabito
F, Damle RN, Deaglio S et al. The CD38 ectoenzyme family: advances in
basic science and clinical practice. Mol. Med. 2006; 12(11-12):342-4. https://doi.org/10.2119/2006-00110.Morabito PMid:17380202 PMCid:PMC1829202
- Patten
PEM, Buggins AGS, Richards J et al. CD38 expression in chronic
lymphocytic leukemia is regulated by the tumor microenvironment. Blood
2008; 111(10):5173-81. https://doi.org/10.1182/blood-2007-08-108605 PMid:18326821
- Konoplev
S, Medeiros LJ, Bueso-Ramos CE et al. Immunophenotypic profile of
lymphoplasmacytic lymphoma/Waldenström macroglobulinemia. Am. J. Clin.
Pathol. 2005;124(3):414-20. https://doi.org/10.1309/3G1XDX0DVHBNVKB4 PMid:16191510
- Parry-Jones
N, Matutes E, Morilla R et al. Cytogenetic abnormalities additional to
t(11;14) correlate with clinical features in leukaemic presentation of
mantle cell lymphoma, and may influence prognosis: A study of 60 cases
by FISH. Br. J. Haematol. 2007; 137(2):117-124. https://doi.org/10.1111/j.1365-2141.2007.06526.x PMid:17391491
- Keyhani
A, Huh YO, Jendiroba D et al. Increased CD38 expression is associated
with favorable prognosis in adult acute leukemia. Leuk. Res. 2000;
24(2):153-9. https://doi.org/10.1016/S0145-2126(99)00147-2 PMid:10654451
- Marinov
J, Koubek K, Starý J. Immunophenotypic significance of the "lymphoid"
CD38 antigenin myeloid blood malignancies. Neoplasma 1993; 40(6):355-8.
- Suzuki
R, Suzumiya J, Nakamura S et al. Aggressive natural killer-cell
leukemia revisited:large granular lymphocyte leukemia of cytotoxic NK
cells. Leukemia 2004; 18(4):763-70. https://doi.org/10.1038/sj.leu.2403262 PMid:14961041
- Wang
L, Wang H, Li P et al. CD38 expression predicts poor prognosis and
might be a potentialtherapy target in extranodal NK/T cell lymphoma,
nasal type. Ann. Hematol. 2015;94(8):1381-8. https://doi.org/10.1007/s00277-015-2359-2 PMid:25865943
- Hari
P, Raj R V, Olteanu H. Targeting CD38 in Refractory Extranodal Natural
Killer Cell-T-CellLymphoma. N. Engl. J. Med. 2016; 375(15):1501-1502. https://doi.org/10.1056/NEJMc1605684 PMid:27732828
- Bride
KL, Vincent TL, Im S-Y et al. Preclinical efficacy of daratumumab in
T-cell acute lymphoblastic leukemia. Blood 2018; 131(9):995-999. https://doi.org/10.1182/blood-2017-07-794214 PMid:29305553 PMCid:PMC5833263
- Overdijk
MB, Verploegen S, Bögels M et al. Antibody-mediated phagocytosis
contributes to the anti-tumor activity of the therapeutic antibody
daratumumab in lymphoma and multiple myeloma. MAbs 2015; 7(2):311-21. https://doi.org/10.1080/19420862.2015.1007813 PMid:25760767 PMCid:PMC4622648
- Chikeka
I, Grossman M, Deng C, Jacob AT, Husain S. Plasmablastic lymphoma in an
HIV patient with cutaneous presentation: A case of remarkable remission
in a typically refractory disease. JAAD Case Rep. 2020 Feb
12;6(3):161-165. https://doi.org/10.1016/j.jdcr.2019.11.007 PMid:32083160 PMCid:PMC7019043
- Marvyin
K, Tjønnfjord EB, Breland UM, Tjønnfjord GE. Transformation to
plasmablastic lymphoma in CLL upon ibrutinib treatment. BMJ Case Rep.
2020 Sep 29;13(9):e235816. https://doi.org/10.1136/bcr-2020-235816 PMid:32994268 PMCid:PMC7526319
- Roché
P, Venton G, Berda-Haddad Y, Fritz S, Ivanov V, Mercier C, Colle J,
Tichadou A, Fanciullino R, Lepidi H, Costello R, Farnault L. Could
daratumumab induce the maturation of plasmablasts in Plasmablastic
lymphoma?-Potential therapeutic applications. Eur J Haematol. 2021
Apr;106(4):589-592. https://doi.org/10.1111/ejh.13584 PMid:33469987
- Ricker
E, Yun Kyoung Ryu, Jennifer E. Amengual, Daratumumab Plus Chemotherapy
Induces Complete Responses in a Consecutive Series of Four Patients
with Plasmablastic Lymphoma, Blood,Volume 138, Supplement 1,2021,Page
4573 https://doi.org/10.1182/blood-2021-150800
- Ramadas
P, Williams M, Duggan DB. Plasmablastic Lymphoma or Plasmablastic
Myeloma: A Case of Post-Transplant Lymphoproliferative Disorder. Case
Rep Hematol. 2021 Sep 27;2021:4354941. https://doi.org/10.1155/2021/4354941 PMid:34616575 PMCid:PMC8490046
- Kathrotiya
M, Radhakrishnan VS, Bhave SJ, Kumar J, Roychowdhury M, Arun I, Das J,
Chandy M, Nair R. Relapsed plasmablastic lymphoma in a HIV-negative
patient: Pushing the envelope. Clin Case Rep. 2020 Dec 20;9(2):873-877.
https://doi.org/10.1002/ccr3.3673 PMid:33598263 PMCid:PMC7869339
- Ryu
YK, Ricker EC, Soderquist CR, Francescone MA, Lipsky AH, Amengual JE.
Targeting CD38 with Daratumumab Plus Chemotherapy for Patients with
Advanced-Stage Plasmablastoid Large B-Cell Lymphoma. J Clin Med. 2022
Aug 22;11(16):4928. https://doi.org/10.3390/jcm11164928 PMid:36013165 PMCid:PMC9409851
- Bhatt
P, Kloock C, Comenzo R. Relapsed/Refractory Multiple Myeloma: A Review
of Available Therapies and Clinical Scenarios Encountered in Myeloma
Relapse. Curr Oncol. 2023 Feb 15;30(2):2322-2347. https://doi.org/10.3390/curroncol30020179 PMid:36826140 PMCid:PMC9954856
- Pinto
MP, Thorneloe NS, Brown MR, Stalons ML, Stoll KE, Holmes AR, Pathan M,
Gonzales PA. The devolution of a mature plasma cell dyscrasia into a
fatal plasmablastic lymphoma. J Case Rep Images Oncology
2023;9(2):7-14. https://doi.org/10.5348/100124Z10MP2023CR
- Dornan
D, Bennett F, Chen Y et al (2009) Therapeutic potential of an
anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the
treatment of non-Hodgkinlymphoma. Blood 114:2721-2729 https://doi.org/10.1182/blood-2009-02-205500 PMid:19633198
- Deeks ED (2019) Polatuzumab vedotin: first global approval. Drugs 79:1467-1475. https://doi.org/10.1007/s40265-019-01175-0 PMid:31352604 PMCid:PMC6794237
- Palanca-Wessels
MCA, Czuczman M, Salles G et al (2015) Safety and activity of the
anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or
refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic
leukaemia: a phase 1 study. https://doi.org/10.1016/S1470-2045(15)70128-2 PMid:25925619
- Sehn
L, Herrera A, Flowers C et al (2020) Polatuzumab vedotin in relapsed or
refractory diffuse large B-cell lymphoma. J Clin Oncol 38(2):155-165 https://doi.org/10.1200/JCO.19.00172 PMid:31693429 PMCid:PMC7032881
- Northend
M, Wilson W, Osborne W. et al.: Results of a United Kingdom real-world
study of polatuzumab vedotin, bendamustine, and rituximab for
relapsed/refractory DLBCL. Blood Adv. 2022 May 10;6(9):2920-2926. https://doi.org/10.1182/bloodadvances.2021005953 PMid:35020818 PMCid:PMC9092410
- Alsaab,
H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.;
Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer
Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front.
Pharmacol. 2017, 8, 561. https://doi.org/10.3389/fphar.2017.00561 PMid:28878676 PMCid:PMC5572324
- Ishida,
Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a
novel member of the immunoglobulin gene superfamily, upon programmed
cell death. EMBO J. 1992, 11, 3887-3895. https://doi.org/10.1002/j.1460-2075.1992.tb05481.x PMid:1396582 PMCid:PMC556898
- Ishida, Y. PD-1: Its Discovery, Involvement in Cancer Immunotherapy, and Beyond. Cells 2020, 9, 1376. https://doi.org/10.3390/cells9061376 PMid:32492969 PMCid:PMC7349669
- Jalali,
S.; Price-Troska, T.; Bothun, C.; Villasboas, J.; Kim, H.J.; Yang,
Z.Z.; Novak, A.J.; Dong, H.; Ansell, S.M. Reverse signaling via PD-L1
supports malignant cell growth and survival in classical Hodgkin
lymphoma. Blood Cancer J. 2019, 9, 22. https://doi.org/10.1038/s41408-019-0185-9 PMid:30783096 PMCid:PMC6381098
- Xu-Monette, Z.Y.; Zhou, J.; Young, K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood 2018, 131, 68-83. https://doi.org/10.1182/blood-2017-07-740993 PMid:29118007 PMCid:PMC5755041
- Garcia-Lacarte,
M.; Grijalba, S.C.; Melchor, J.; Arnaiz-Leche, A.; Roa, S. The
PD-1/PD-L1 Checkpoint in Normal Germinal Centers and Diffuse Large
B-Cell Lymphomas. Cancers 2021, 13, 4683. https://doi.org/10.3390/cancers13184683 PMid:34572910 PMCid:PMC8471895
- Latchman,
Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.;
Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second
ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2,
261-268. https://doi.org/10.1038/85330 PMid:11224527
- Xia,
Y.; Medeiros, L.J.; Young, K.H. Immune checkpoint blockade: Releasing
the brake towards hematological malignancies. Blood Rev. 2016, 30,
189-200. https://doi.org/10.1016/j.blre.2015.11.003 PMid:26699946
- Bardhan,
K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from
Discovery to Clinical Implementation. Front.Immunol. 2016, 7, 550. https://doi.org/10.3389/fimmu.2016.00550
- Azuma,
T.; Yao, S.; Zhu, G.; Flies, A.S.; Flies, S.J.; Chen, L. B7-H1 is a
ubiquitous antiapoptotic receptor on cancer cells. Blood 2008,111,
3635-3643. https://doi.org/10.1182/blood-2007-11-123141 PMid:18223165 PMCid:PMC2275025
- Zhang,
J.P.; Song, Z.; Wang, H.B.; Lang, L.; Yang, Y.Z.; Xiao, W.; Webster,
D.E.; Wei, W.; Barta, S.K.; Kadin, M.E.; et al. A novel model of
controlling PD-L1 expression in ALK(+) anaplastic large cell lymphoma
revealed by CRISPR screening. Blood 2019, 134,171-185. https://doi.org/10.1182/blood.2019001043 PMid:31151983 PMCid:PMC6624970
- Herbst,
R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.;
Sosman, J.A.; McDermott, D.F.; Powderly, J.D.;Gettinger, S.N.; et al.
Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A
in cancer patients. Nature 2014,515, 563-567. https://doi.org/10.1038/nature14011 PMid:25428504 PMCid:PMC4836193
- Xie,
W.; Medeiros, L.J.; Li, S.; Yin, C.C.; Khoury, J.D.; Xu, J. PD-1/PD-L1
Pathway and Its Blockade in Patients with ClassicHodgkin Lymphoma and
Non-Hodgkin Large-Cell Lymphomas. Curr. Hematol. Malig. Rep. 2020, 15,
372-381. https://doi.org/10.1007/s11899-020-00589-y PMid:32394185
- Ansell, S.M. PD-1 Blockade in Classic Hodgkin Lymphoma. JCO Oncol. Pract. 2021, 17, 72-73. [CrossRef] https://doi.org/10.1200/OP.20.01020 PMid:33439697
- Chen,
B.J.; Chapuy, B.; Ouyang, J.; Sun, H.H.; Roemer, M.G.; Xu, M.L.; Yu,
H.; Fletcher, C.D.; Freeman, G.J.; Shipp, M.A.; et al.PD-L1 expression
is characteristic of a subset of aggressive B-cell lymphomas and
virus-associated malignancies. Clin. Cancer Res.2013, 19, 3462-3473. https://doi.org/10.1158/1078-0432.CCR-13-0855 PMid:23674495 PMCid:PMC4102335
- Kiyasu,
J.; Miyoshi, H.; Hirata, A.; Arakawa, F.; Ichikawa, A.; Niino, D.;
Sugita, Y.; Yufu, Y.; Choi, I.; Abe, Y.; et al. Expression of
programmed cell death ligand 1 is associated with poor overall survival
in patients with diffuse large B-cell lymphoma. Blood2015, 126,
2193-2201 https://doi.org/10.1182/blood-2015-02-629600 PMid:26239088 PMCid:PMC4635115
- Granai
M, Mundo L, Akarca AU, Siciliano MC, Rizvi H, Mancini V, Onyango N,
Nyagol J, Abinya NO, Maha I, Margielewska S, Wi W, Bibas M, Piccaluga
PP, Quintanilla-Martinez L, Fend F, Lazzi S, Leoncini L, Marafioti T.
Immune landscape in Burkitt lymphoma reveals M2-macrophage polarization
and correlation between PD-L1 expression and non-canonical EBV latency
program. Infect Agent Cancer. 2020 May 6;15:28. https://doi.org/10.1186/s13027-020-00292-w PMid:32391073 PMCid:PMC7201729
- Xing,
W.; Dresser, K.; Zhang, R.; Evens, A.M.; Yu, H.; Woda, B.A.; Chen, B.J.
PD-L1 expression in EBV-negative diffuse large B-cell lymphoma:
Clinicopathologic features and prognostic implications. Oncotarget
2016, 7, 59976-59986. https://doi.org/10.18632/oncotarget.11045 PMid:27527850 PMCid:PMC5312363
- Laurent
C, Fabiani B, Do C, Tchernonog E, Cartron G, Gravelle P, Amara N, Malot
S, Palisoc MM, Copie-Bergman C, Glehen AT, Copin MC, Brousset P,
Pittaluga S, Jaffe ES, Coppo P. Immune-checkpoint expression in
Epstein-Barr virus positive and negative plasmablastic lymphoma: a
clinical and pathological study in 82 patients. Haematologica. 2016
Aug;101(8):976-84. https://doi.org/10.3324/haematol.2016.141978 PMid:27175027 PMCid:PMC4967577
- Gravelle
P, Péricart S, Tosolini M, Fabiani B, Coppo P, Amara N, Traverse-Gléhen
A, Van Acker N, Brousset P, Fournie JJ, Laurent C. EBV infection
determines the immune hallmarks of plasmablastic lymphoma.
Oncoimmunology. 2018 Jul 30;7(10):e1486950. https://doi.org/10.1080/2162402X.2018.1486950 PMid:30288350 PMCid:PMC6169584
- Damlaj
M., Alzayed M., Alahmari B., Alhejazi A., Alaskar A., Alzahrani M.
Therapeutic potential of checkpoint inhibitors in refractory
plasmablastic lymphoma. Clinical Lymphoma Myeloma and Leukemia .
2019;19(10):e559-e563. https://doi.org/10.1016/j.clml.2019.06.008 PMid:31377210
- Cheng
L., Song Q., Liu M., et al. Case report: successful management of a
refractory plasmablastic lymphoma patient with tislelizumab and
lenalidomide. Frontiers in Immunology . 2021;12 https://doi.org/10.3389/fimmu.2021.702593 PMid:34322131 PMCid:PMC8312258
- Castillo
JJ, Lamacchia J, Silver J, Flynn CA, Sarosiek S. Complete response to
pembrolizumab and radiation in a patient with HIV-negative,
EBV-positive plasmablastic lymphoma. Am J Hematol. 2021 Oct
1;96(10):E390-E392. https://doi.org/10.1002/ajh.26291 PMCid:PMC8252623
- Schuster,
S.J. , Svoboda, J. , Chong, E.A. , Nasta, S.D. , Mato, A.R. , Anak, O.,
Brogdon, J.L., Pruteanu-Malinici, I., Bhoj, V., Landsburg, D. , Wasik,
M., Levine, B.L., Lacey, S.F., Melenhorst, J.J., Porter, D.L. &
June, C.H. (2017) Chimeric antigen receptor T cells in refractory
B-cell lymphomas. The New England Journal of Medicine, 377, 2545-2554. https://doi.org/10.1056/NEJMoa1708566 PMid:29226764 PMCid:PMC5788566
- Abramson,
J.S. & Chen, Y.B. (2017) More on anti-CD19 CAR T cells in CNS
diffuse large-B-cell lymphoma. The New England Journal of Medicine,
377, 2102. https://doi.org/10.1056/NEJMc1704610
- Testa
U, Leone G, Pelosi E, Castelli G, Hohaus S. CAR-T Cell Therapy in Large
B Cell Lymphoma. Mediterr J Hematol Infect Dis. 2023 Nov
1;15(1):e2023066. https://doi.org/10.4084/MJHID.2023.066 PMid:38028399 PMCid:PMC10631715
- Parker
C, Liu FF, Deger KA, Franco-Villalobos C, Proskorovsky I, Keating SJ,
Sorensen S. Cost-Effectiveness of Lisocabtagene Maraleucel Versus
Axicabtagene Ciloleucel and Tisagenlecleucel in the Third-Line or Later
Treatment Setting for Relapsed or Refractory Large B-cell Lymphoma in
the United States. Adv Ther. 2023 May;40(5):2355-2374. https://doi.org/10.1007/s12325-023-02444-x PMid:36947328 PMCid:PMC10129927
- Neelapu
SS, Locke FL, Bartlett NL, Lekakis LJ, Reagan PM, Miklos DB, Jacobson
CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Crump M, Kuruvilla J,
Van Den Neste E, Farooq U, Navale L, DePuy V, Kim JJ, Gisselbrecht C.
Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage
chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021 Oct
26;5(20):4149-4155. https://doi.org/10.1182/bloodadvances.2020003848 PMid:34478487 PMCid:PMC8945634
- Maziarz
RT, Zhang J, Yang H, Chai X, Yuan C, Schwartz E, Jakovach M,
Martinez-Prieto M, Agarwal A, Degtyarev E, et al. Indirect comparison
of tisagenlecleucel and historical treatments for relapsed/refractory
diffuse large B-cell lymphoma. Blood Adv 2022; 6: 2536-2542. https://doi.org/10.1182/bloodadvances.2021006280 PMid:35030634 PMCid:PMC9043930
- Bishop
MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, Kato K, Sureda
A, Greil R, Thieblemont C. Second-line tisagenlecleucel or standard
care in aggressive B-cell lymphoma. N Engl J Med 2022, 386(7): 629-639.
https://doi.org/10.1056/NEJMoa2116596 PMid:34904798
- Manson
G, Cartron G, Beauvais D, Roulin L, Gros FX, et al. A real-world
comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells
in relapsed or refractory diffuse large B cell lymphoma. Nature Med
2022; 28: 2145- https://doi.org/10.1038/s41591-022-01969-y PMid:36138152 PMCid:PMC9556323
- Raychaudhuri
R, Qualtieri J, Garfall AL. Axicabtagene ciloleucel for CD19+
plasmablastic lymphoma. Am J Hematol. 2020 Jan;95(1):E28-E30. https://doi.org/10.1002/ajh.25682 PMid:31725923
- Al-Malki
MM, Castillo JJ, Sloan JM, Re A. Hematopoietic cell transplantation for
plasmablastic lymphoma: a review. Biol Blood Marrow Transplant. 2014
Dec;20(12):1877-84. https://doi.org/10.1016/j.bbmt.2014.06.009 PMid:24946718
- Cattaneo
C, Finel H, McQuaker G, Vandenberghe E, Rossi G, Dreger P. Autologous
hematopoietic stem cell transplantation for plasmablastic lymphoma: the
European Society for Blood and Marrow Transplantation experience. Biol
Blood Marrow Transplant. 2015 Jun;21(6):1146-7. https://doi.org/10.1016/j.bbmt.2015.03.008 PMid:25783635
- Balsalobre
P, Diez-Martin J, Re A, et al. Autologous stem cell transplantation in
patients with HIV related lymphoma. J Clin Oncol. 2009; 27:2192-2198 https://doi.org/10.1200/JCO.2008.18.2683 PMid:19332732
- Re
A, Michieli M, Allione B, et al. Early consolidation with high dose
therapy and autologous stem cell transplantation in HIV-associated Non
Hodgkin Lymphoma at high risk (aa-IPI 2-3), an interim report of a
multicenter phase II trial. Blood. 2012;120. Abstract 731. https://doi.org/10.1182/blood.V120.21.3124.3124
- Cattaneo
C, Re A, Ungari M, et al. Plasmablastic lymphoma among HIVpositive
patients: results of a single centre's experience. Leuk Lymphoma. 2014
Apr 9 [Epub ahead of print]. https://doi.org/10.3109/10428194.2014.911867 PMid:24712980
- Broccoli
A, Nanni L, Stefoni V, Agostinelli C, Argnani L, Cavo M, Zinzani PL. A
patient with plasmablastic lymphoma achieving long-term complete
remission after thalidomide-dexamethasone induction and double
autologous stem cell transplantation: a case report. BMC Cancer. 2018
Jun 8;18(1):645. https://doi.org/10.1186/s12885-018-4561-9 PMid:29879938 PMCid:PMC5992724
- M.
Hamadani, S.M. Devine.Reduced-intensity conditioning allogeneic stem
cell transplantation in HIV patients with hematologic malignancies:
yes, we can. Blood,114 (2009), pp. 2564-2566 https://doi.org/10.1182/blood-2009-06-229666 PMid:19762505
- Rong
C, Sheng L, Wu A, Sun Y, Ouyang G. Allogeneic hematopoietic stem cell
transplantation in a patient with HIV-negative recurrent plasmablastic
lymphoma: A case report. Medicine (Baltimore). 2021 Feb
19;100(7):e24498. https://doi.org/10.1097/MD.0000000000024498 PMid:33607779 PMCid:PMC7899902
- Illidge
T, Specht L, Yahalom J, et al. Modern Radiation Therapy for Nodal
Non-Hodgkin Lymphoma-Target Definition and Dose Guidelines From the
International Lymphoma Radiation Oncology Group. International Journal
of Radiation Oncology*Biology*Physics 2014;89(1):49-58 https://doi.org/10.1016/j.ijrobp.2014.01.006 PMid:24725689
- Ng
AK, Yahalom J, Goda JS, et al. Role of Radiation Therapy in Patients
With Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Guidelines from
the International Lymphoma Radiation Oncology Group. International
Journal of Radiation Oncology*Biology*Physics 2018;100(3):652-69. https://doi.org/10.1016/j.ijrobp.2017.12.005 PMid:29413279
- Shi
Z, Das S, Okwan-Duodu D, et al. Patterns of failure in advanced stage
diffuse large B-cell lymphoma patients after complete response to
R-CHOP immunochemotherapy and the emerging role of consolidative
radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):569-577. https://doi.org/10.1016/j.ijrobp.2013.02.007 PMid:23540349
- Phan
J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation
therapy in patients with diffuse large B-cell lymphoma treated with
R-CHOP chemotherapy. J Clin Oncol. 2010;28(27):4170-4176. https://doi.org/10.1200/JCO.2009.27.3441 PMid:20713859
- Horning
SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy
in limited-stage diffuse aggressive non-Hodgkin's lymphoma: Eastern
Cooperative Oncology Group study 1484. J Clin Oncol.
2004;22(15):3032-3038. https://doi.org/10.1200/JCO.2004.06.088 PMid:15210738
- Miller
TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with
chemotherapy plus radiotherapy for localized intermediate- and
high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998;339(1):21-26. https://doi.org/10.1056/NEJM199807023390104 PMid:9647875
- Held
G, Murawski N, Ziepert M, et al. Role of radiotherapy to bulky disease
in elderly patients with aggressive B-cell lymphoma. J Clin Oncol.
2014;32(11):1112-1118. https://doi.org/10.1200/JCO.2013.51.4505 PMid:24493716
- Pfreundschuh
M, Murawski N, Ziepert M, et al. Radiotherapy (RT) to bulky (B) and
extralymphatic (E) disease in combination with 6xR-CHOP-14 or R-CHOP-21
in young good-prognosis DLBCL patients: results of the 2x2 randomized
UNFOLDER trial of the DSHNHL/GLA. J Clin Oncol.
2018;36(15_suppl):7574-7574. https://doi.org/10.1200/JCO.2018.36.15_suppl.7574
- Held
G, Zeynalova S, Murawski N, et al. Impact of rituximab and radiotherapy
on outcome of patients with aggressive B-cell lymphoma and skeletal
involvement. J Clin Oncol. 2013;31(32):4115-4122. https://doi.org/10.1200/JCO.2012.48.0467 PMid:24062391
- Ng
AK, Yahalom J, Goda JS, et al. Role of radiation therapy in patients
with relapsed/refractory diffuse large B-cell lymphoma: guidelines from
the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol
Biol Phys. 2018;100(3):652-669. https://doi.org/10.1016/j.ijrobp.2017.12.005 PMid:29413279
- Wright
CM, Koroulakis AI, Baron JA, et al. Palliative radiotherapy for diffuse
large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21(10):650-658.
https://doi.org/10.1016/j.clml.2021.05.007 PMid:34127417
- Liu
M, Liu B, Liu B, Wang Q, Ding L, Xia C, Dong L. Human immunodeficiency
virus-negative plasmablastic lymphoma: a comprehensive analysis of 114
cases. Oncol Rep. 2015 Apr;33(4):1615-20. https://doi.org/10.3892/or.2015.3808 PMid:25695332 PMCid:PMC4358079
- Tchernonog
E, Faurie P, Coppo P, Monjanel H, Bonnet A, Algarte Génin M, Mercier M,
Dupuis J, Bijou F, Herbaux C, Delmer A, Fabiani B, Besson C, Le Gouill
S, Gyan E, Laurent C, Ghesquieres H, Cartron G. Clinical
characteristics and prognostic factors of plasmablastic lymphoma
patients: analysis of 135 patients from the LYSA group. Ann Oncol. 2017
Apr 1;28(4):843-848. https://doi.org/10.1093/annonc/mdw684 PMid:28031174
- Hess
BT, Giri A, Park Y, Patel KK, Link BK, Nowakowski GS, Maliske SM,
Fortin S, Chavez JC, Saeed H, Hill BT, Mejia Garcia AV, Maddocks KJ,
Hanel W, Wagner-Johnston ND, Messmer MR, Kahl BS, Watkins M, Alderuccio
JP, Lossos IS, Nathan S, Orellana-Noia VM, Portell CA, Landsburg DJ,
Ayers EC, Castillo JJ. Outcomes of patients with limited-stage
plasmablastic lymphoma: A multi-institutional retrospective study. Am J
Hematol. 2023 Feb;98(2):300-308. https://doi.org/10.1002/ajh.26784 PMid:36588409 PMCid:PMC10107934
- Gleeson
M, Counsell N, Cunningham D, et al. Central nervous system relapse of
diffuse large B-cell lymphoma in the rituximab era: results of the UK
NCRI R-CHOP-14 versus 21 trial. Ann Oncol. 2017;28(10):2511-2516. https://doi.org/10.1093/annonc/mdx353 PMid:28961838 PMCid:PMC5834096
- Ghose
A, Elias HK, Guha G, Yellu M, Kundu R, Latif T. Influence of rituximab
on central nervous system relapse in diffuse large B-cell lymphoma and
role of prophylaxis-a systematic review of prospective studies. Clin
Lymphoma Myeloma Leuk. 2015;15(8):451-457. https://doi.org/10.1016/j.clml.2015.02.026 PMid:25816933
- Klanova
M, Sehn LH, Bence-Bruckler I, et al. Integration of cell of origin into
the clinical CNS International Prognostic Index improves CNS relapse
prediction in DLBCL. Blood. 2019;133(9):919-926. https://doi.org/10.1182/blood-2018-07-862862 PMid:30617197 PMCid:PMC6396175
- Schmitz
N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index:
a risk model for CNS relapse in patients with diffuse large B-cell
lymphoma treated with R-CHOP. J Clin Oncol. 2016;34(26):3150-3156. https://doi.org/10.1200/JCO.2015.65.6520 PMid:27382100
- Savage
KJ. Secondary CNS relapse in diffuse large B-cell lymphoma: defining
high-risk patients and optimization of prophylaxis strategies.
Hematology Am Soc Hematol Educ Program. 2017;2017(1):578-586. https://doi.org/10.1182/asheducation-2017.1.578 PMid:29222307 PMCid:PMC6142549
- Kridel
R, Telio D, Villa D, et al. Diffuse large B-cell lymphoma with
testicular involvement: outcome and risk of CNS relapse in the
rituximab era. Br JHaematol. 2017;176(2):210-221. https://doi.org/10.1111/bjh.14392 PMid:27739058
- Hu
S, Song Y, Li Y, et al. Primary breast diffuse large B cell lymphoma in
the rituximab era: outcomes of a multicenter retrospective study by the
Lymphoma and Leukemia Committee of Chinese Geriatric Oncology Society
(LLC-CGOS). Paper presented at: 58th Annual Meeting of the American
Society of Hematology; 3-6 December 2016; San Diego, CA.
- McKay
P, Wilson MR, Chaganti S, et al; British Society of Haematology. The
prevention of central nervous system relapse in diffuse large B-cell
lymphoma: a British Society for Haematology Good Practice Paper. Br J
Haematol. 2020;190(5):708-714. https://doi.org/10.1111/bjh.16866 PMid:32433789
- El-Galaly
TC, Villa D, Michaelsen TY, et al. The number of extranodal sites
assessed by PET/CT scan is a powerful predictor of CNS relapse for
patients with diffuse large B-cell lymphoma: an international
multicenter study of 1532 patients treated with chemoimmunotherapy. Eur
J Cancer.2017;75(April):195-203. https://doi.org/10.1016/j.ejca.2016.12.029 PMid:28237865
- Savage
KJ, Slack GW, Mottok A, et al. Impact of dual expression of MYC and
BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL.Blood.
2016;127(18):2182-2188 https://doi.org/10.1182/blood-2015-10-676700 PMid:26834242
- Wilson
WH, Bromberg JE, Stetler-Stevenson M, et al. Detection and outcome of
occult leptomeningeal disease in diffuse large B-cell lymphoma and
Burkitt lymphoma. Haematologica. 2014;99(7):1228-1235. https://doi.org/10.3324/haematol.2013.101741 PMid:24727817 PMCid:PMC4077085
- Eyre
TA, Djebbari F, Kirkwood AA, Collins GP. Efficacy of central nervous
system prophylaxis with stand-alone intrathecal chemotherapy in diffuse
large B-cell lymphoma patients treated with anthracycline-based
chemotherapy in the rituximab era: a systematic review.
Haematologica.2020;105(7):1914-1924. https://doi.org/10.3324/haematol.2019.229948 PMid:31488560 PMCid:PMC7327624
- Eyre
TA, Kirkwood AA, Wolf J, et al. Stand-alone intrathecal central nervous
system (CNS) prophylaxis provide unclear benefit in reducing CNS
relapse risk in elderly DLBCL patients treated with R-CHOP and is
associated increased infection-related toxicity. Br J Haematol.
2019;187(2):185-194 https://doi.org/10.1111/bjh.16070 PMid:31222719
- Barta
SK, Joshi J, Mounier N, et al. Central nervous system involvement in
AIDS-related lymphomas. Br J Haematol 2016;173:857-66. https://doi.org/10.1111/bjh.13998 PMid:27062389 PMCid:PMC4900917
- Hoffmann
C, Wolf E, Fatkenheuer G, Buhk T, Stoehr A, Plettenberg A, et
al.Response to highly active antiretroviral therapy strongly predicts
outcome in patients with AIDS-related lymphoma. AIDS. 2003;17:1521-9. https://doi.org/10.1097/00002030-200307040-00013 PMid:12824790
- Lim
ST, Karim R, Nathwani BN, Tulpule A, Espina B, Levine AM. AIDS-related
Burkitt's lymphoma versus diffuse large-cell lymphoma in the pre-highly
active antiretroviral therapy (HAART) and HAART eras: significant
differences in survival with standard chemotherapy. J Clin Oncol.
2005;23:4430-8. https://doi.org/10.1200/JCO.2005.11.973 PMid:15883411
- Navarro
JT, Ribera JM, Oriol A, Vaquero M, Romeu J, Batlle M, et al. Influence
of highly active anti-retroviral therapy on response to treatment and
survival in patients with acquired immunodeficiency syndrome-related
non-Hodgkin's lymphoma treated with cyclophosphamide,
hydroxydoxorubicin, vincristine and prednisone. Br J Haematol.
2001;112:909-15. https://doi.org/10.1046/j.1365-2141.2001.02656.x PMid:11298585
- Vaccher
E, Spina M, di Gennaro G, Talamini R, Nasti G, Schioppa O, et al
Concomitant cyclophosphamide, doxorubicin, vincristine, and prednisone
chemotherapy plus highly active antiretroviral therapy in patients with
human immunodeficiency virus-related, non-Hodgkin lymphoma. Cancer.
2001;91:155-63. https://doi.org/10.1002/1097-0142(20010101)91:1<155::AID-CNCR20>3.0.CO;2-B PMid:11148572
- Torres
HA, Rallapalli V, Saxena A, Granwehr BP, Viola GM, Ariza-Heredia E, et
al Efficacy and safety of antiretrovirals in HIV-infected patients with
cancer. Clin Microbiol Infect. 2014;20:O672-9. https://doi.org/10.1111/1469-0691.12589 PMid:24529214
- Gonzalez-Garcia
JJ, Mahillo B, Hernandez S, Pacheco R, Diz S, Garcia P, et al.
[Prevalences of hepatitis virus coinfection and indications for chronic
hepatitis C virus treatment and liver transplantation in Spanish
HIV-infected patients.The GESIDA 29/02 and FIPSE 12185/01 Multicenter
Study]. Enferm Infecc Microbiol Clin. 2005;23:340-8. https://doi.org/10.1157/13076173 PMid:15970166
- Konopnicki
D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, et al.
Hepatitis B and HIV: prevalence, AIDS progression, response to highly
active antiretroviral therapy and increased mortality in the EuroSIDA
cohort. AIDS.2005;19:593-601. https://doi.org/10.1097/01.aids.0000163936.99401.fe PMid:15802978
- Berenguer
J, Rivero A, Jarrin I, Nunez MJ, Vivancos MJ, Crespo M, et al. Human
immunodeficiency virus/hepatitis C virus coinfection in Spain:
prevalence and patient characteristics. Open Forum Infect Dis.
2016;3:ofw059.
- Stebbing
J, Atkins M, Nelson M, Rajpopat S, Newsom-Davis T, Gazzard B,et al.
Hepatitis B reactivation during combination chemotherapy for
AIDSrelated lymphoma is uncommon and does not adversely affect outcome.
Blood.2004;103:2431-2. https://doi.org/10.1182/blood-2003-12-4222
PMid:14998920
- Stroffolini
T, Andriani A, Bibas M, Barlatani A. Successful treatment with
lamivudine for reactivated hepatitis B infection following chemotherapy
for non-Hodgkin's lymphoma. Ann Hematol. 2002 Jan;81(1):48-9. https://doi.org/10.1007/s00277-001-0393-8 PMid:11807636
- Lalazar
G, Rund D, Shouval D. Screening, prevention and treatment of viral
hepatitis B reactivation in patients with haematological malignancies.
Br JHaematol. 2007;136:699-712. https://doi.org/10.1111/j.1365-2141.2006.06465.x PMid:17338776
- Evens
AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, et
al.Rituximab-associated hepatitis B virus (HBV) reactivation in
lymphoproliferative diseases: meta-analysis and examination of FDA
safety reports. Ann Oncol.2011;22:1170-80. https://doi.org/10.1093/annonc/mdq583 PMid:21115603 PMCid:PMC3082161
- Price
H, Dunn D, Pillay D, Bani-Sadr F, de Vries-Sluijs T, Jain MK, et al.
Suppression of HBV by tenofovir in HBV/HIV coinfected patients: a
systematic review and meta-analysis. PLOS ONE. 2013;8:e68152. https://doi.org/10.1371/journal.pone.0068152 PMid:23874527 PMCid:PMC3707972
- Panel
de expertos de GeSIDA y Plan Nacional sobre el Sida. Documento de
consenso de GeSIDA/Plan Nacional sobre el Sida respect al tratamiento
antirretroviral en adultos infecta- dos por el virus dela
inmunodeficiencia humana. Available from: http://gesida-seimc.org/wp-content/uploads/2017/02/gesida-guiasclinicas-2017-TAR.pdf
- Buti
M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir
alafenamide versus tenofovir disoproxil fumarate for the treatment of
patients with HBeAg-negative chronic hepatitis B virus infection: a
randomised, double-blind, phase 3, non-inferiority trial. Lancet
Gastroenterol Hepatol. 2016;1:196-206. https://doi.org/10.1016/S2468-1253(16)30107-8 PMid:28404092
- Chan
HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, et al. Tenofovir
alafenamide versus tenofovir disoproxil fumarate for the treatment of
HBeAgpositive chronic hepatitis B virus infection: a randomised,
double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol
Hepatol. 2016;1:185-95. https://doi.org/10.1016/S2468-1253(16)30024-3 PMid:28404091
- Alwan
F, He A, Montoto S, Kassam S, Mee M, Burns F, et al. Adding rituximab
to CODOX-M/IVAC chemotherapy in the treatment of HIV-associated
Burkittlymphoma is safe when used with concurrent combination
antiretroviral therapy.AIDS. 2015;29:903-10. https://doi.org/10.1097/QAD.0000000000000623 PMid:25730506
- Bower
M, McCall-Peat N, Ryan N, Davies L, Young AM, Gupta S, et al. Protease
inhibitors potentiate chemotherapy-induced neutropenia.
Blood.2004;104:2943-6. https://doi.org/10.1182/blood-2004-05-1747 PMid:15238428
- Barta
SK, Xue X, Wang D, Tamari R, Lee JY, Mounier N, et al. Treatment
factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a
pooledanalysis of 1546 patients. Blood. 2013;122:3251-62. https://doi.org/10.1182/blood-2013-04-498964 PMid:24014242 PMCid:PMC3821722
- Rudek
MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients
with cancer who have HIV/AIDS. Lancet Oncol. 2011;12:905-12. https://doi.org/10.1016/S1470-2045(11)70056-0 PMid:21570912
- Tourret
J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of
HIVinfected patients: a double-edged sword? J Am Soc Nephrol.
2013;24:1519-27. https://doi.org/10.1681/ASN.2012080857 PMid:24052632 PMCid:PMC3785270
- Antela
A, Aguiar C, Compston J, Hendry BM, Boffito M, Mallon P, et al. The
role of tenofovir alafenamide in future HIV management. HIV Med.
2016;17 Suppl. 2:4-16. https://doi.org/10.1111/hiv.12401 PMid:26952360
- Goldman
SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson
FL, et al. A randomized comparison between rasburicase and allopurinol
in children with lymphoma or leukemia at high risk for tumor lysis.
Blood.2001;97:2998-3003. https://doi.org/10.1182/blood.V97.10.2998 PMid:11342423
- Pui
CH, Mahmoud HH, Wiley JM, Woods GM, Leverger G, Camitta B, et al.
Recombinant urate oxidase for the prophylaxis or treatment of
hyperuricemia in patients With leukemia or lymphoma. J Clin Oncol.
2001;19:697-704. https://doi.org/10.1200/JCO.2001.19.3.697 PMid:11157020
- Smith
TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al.
2006 update of recommendations for the use of white blood cell
growthfactors: an evidence-based clinical practice guideline. J Clin
Oncol. 2006;24:3187-205. https://doi.org/10.1200/JCO.2006.06.4451 PMid:16682719
- Tomblyn
M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al.
Guidelines for preventing infectious complications among hematopoietic
cell transplantation recipients: a global perspective. Biol Blood
Marrow Transplant.2009;15:1143-238. https://doi.org/10.1016/j.bbmt.2009.06.019 PMid:19747629 PMCid:PMC3103296
- Freifeld
AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical
practice guideline for the use of antimicrobial agents in neutropenic
patientswith cancer: 2010 update by the Infectious Diseases Society of
America. ClinInfect Dis. 2011;52:e56-93. https://doi.org/10.1093/cid/cir073 PMid:21258094
- Flowers
CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK, et al.
Antimicrobial prophylaxis and outpatient management of fever and
neutropenia in adults treated for malignancy: American Society of
Clinical Oncology clinical practice guideline. J Clin Oncol.
2013;31:794-810. https://doi.org/10.1200/JCO.2012.45.8661 PMid:23319691
- Beck
CR, McKenzie BC, Hashim AB, Harris RC, University of Nottingham
Influenza and the ImmunoCompromised Study Group, Nguyen-Van-Tam JS.
Influenza vaccination for immunocompromised patients: systematic review
and meta-analysis by etiology. J Infect Dis. 2012;206:1250-9. https://doi.org/10.1093/infdis/jis487 PMid:22904335
- Rubin
LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013
IDSA clinical practice guideline for vaccination of the
immunocompromised host. Clin Infect Dis. 2014;58:309-18 https://doi.org/10.1093/cid/cit816 PMid:24421306
- Mondi
A, Lorenzini P, Castilletti C, .:INMI Recovery study group. Risk and
predictive factors of prolonged viral RNA shedding in upper respiratory
specimens in a large cohort of COVID-19 patients admitted to an Italian
reference hospital. Int J Infect Dis. 2021 Apr;105:532-539.
- Buske
C, Dreyling M, Alvarez-Larrán A, et al.: Managing hematological cancer
patients during the COVID-19 pandemic: an ESMO-EHA Interdisciplinary
Expert Consensus. ESMO Open. 2022 Apr;7(2):100403. https://doi.org/10.1016/j.esmoop.2022.100403 PMid:35272130 PMCid:PMC8795783





