Ugo
Testa1, Elvira Pelosi1,
Germana Castelli1, Alberto Fresa2,3,
and Luca Laurenti2,3.
1 Istituto
Superiore di Sanità, Roma, Italy.
2
Dipartimento di Diagnostica per Immagini,
Radioterapia Oncologica ed
Ematologia,
Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma,
Italy. Sezione Di Ematologia. Roma, Italy.
3 Dipartimento di Scienze Radiologiche Ed
Ematologiche, Università Cattolica Del Sacro Cuore, Roma, Italy.
Published: May 01, 2024
Received: April 02, 2024
Accepted: April 18, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024045 DOI
10.4084/MJHID.2024.045
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
treatment outcomes of patients with chronic lymphocytic leukemia (CLL)
have considerably improved with the introduction of targeted therapies
based on Bruton kinase inhibitors (BTKIs), venetoclax, and anti-CD20
monoclonal antibodies. However, despite these consistent improvements,
patients who become resistant to these agents have poor outcomes and
need new and more efficacious therapeutic strategies.
Among these
new treatments, a potentially curative approach consists of the use of
chimeric antigen receptor T (CAR-T) cell therapy, which achieved
remarkable success in various B-cell malignancies, including B-cell
Non-Hodgkin Lymphomas (NHLs) and B-acute lymphoblastic Leukemia (ALL).
However, although CAR-T cells were initially used for the treatment of
CLL, their efficacy in CLL patients was lower than in other B-cell
malignancies. This review analyses possible mechanisms of these
failures, highlighting some recent developments that could offer the
perspective of the incorporation of CAR-T cells in treatment protocols
for relapsed/refractory CLL patients.
|
Introduction
Chronic
lymphocytic leukemia (CLL) is the most frequent leukemia in adult
subjects in Western countries, with a mean age at diagnosis around 69
years.[1] At the cellular level, this leukemia is
characterized by the progressive accumulation of mature CD5-positive
B-lymphocytes in the peripheral blood, bone marrow, and secondary
lymphoid organs.[2] The clinical stratification of CLL
patients currently relies on the Rai and Binet classification: low-risk
(Rai 0/Binet A), intermediate-risk (Rai I and II or Binet B), and
high-risk (Rai III and IV or Binet C). The molecular stratification
relies on molecular prognostic markers that include cytogenetic
abnormalities and the assessment of the mutational status of
immunoglobulin heavy-chain variable (IGVH) and TP53 genes. High-risk
markers are represented by the presence of TP53 mutations, del(17p), a
complex karyotype, or unmutated IGVH.[2]
The
treatment algorithm of CLL radically changed over the past few years,
becoming almost completely chemo-free. Therefore, according to the
current treatment recommendations, patients with the symptomatic
disease receive a covalent Bruton tyrosine kinase inhibitor (i.e.,
ibrutinib, acalabrutinib, zanubrutinib), the BCL-2 inhibitor venetoclax
+/- anti-CD20 monoclonal antibody, or a combination of the classes.[2,3]
In relapsed/refractory (R/R) patients, exposure to one class of drug
does not prevent the patient from being treated with the other one,
with effective results.[1] However, the outcomes of double-resistant patients are poor, with an overall survival of only a few months.[4-7]
Therefore,
there is an absolute need to develop novel therapies for these
double-resistant patients who have a dismal prognosis. Concerning
cellular therapy, slightly better results were obtained from allogeneic
stem cell transplantation.[7,8] Studies carried out
in the last years have suggested that chimeric antigen receptors (CAR)
designed to target T cells to antigens expressed on CLL cells may offer
a therapeutic opportunity for these patients. In this review, we
explored and analyzed the current available evidence on the use of
CAR-T cell therapy in CLL patients. The most significant results of
trials with CAR-T in CLL and Richter's transformation are summarized in
Table 1.
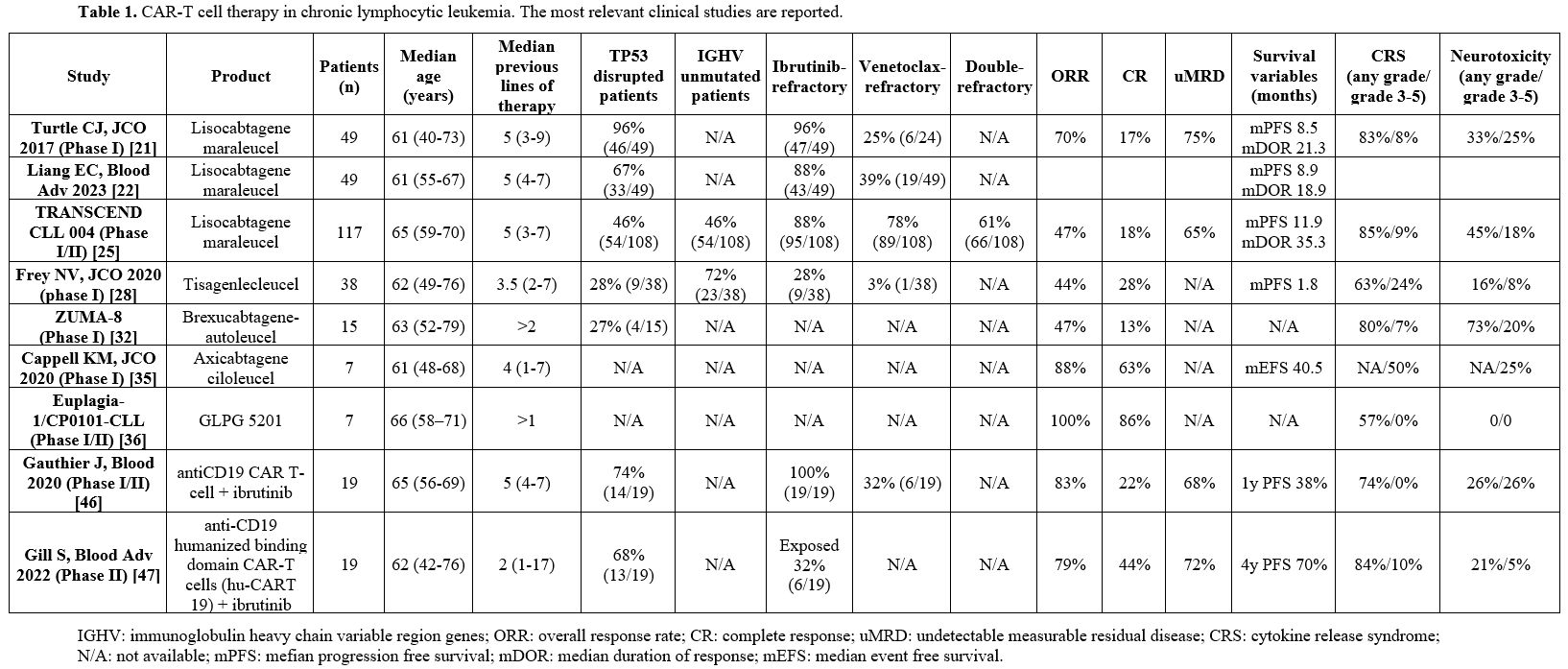 |
- Table 1. CAR-T cell therapy in chronic lymphocytic leukemia. The most relevant clinical studies are reported.
|
CD19-targeted CAR-T Cells in CLL
CD19-targeted
CAR-T cell therapy was successfully experimented with for the treatment
of several B-cell malignancies, including B-cell lymphomas and B-acute
lymphoblastic leukemia.[9-19] Four CAR-T cell products
are commercially available and approved by the FDA for clinical use:
Axicabtagene ciloleucel (Axi-Cel), Brexucabtagene-autoleucel
(Brexu-Cel), Tisagenlecleucel (Tisa-Cel) and Lisocabtagene maraleucel
(Liso-Cel). These CAR-T products were also evaluated in CLL patients.
Liso-Cel.
Liso-Cel is a second-generation anti-CD19 CAR-T cell that utilizes the
4-1BB costimulatory domain and is produced from separated subsets of
CD4- and CD8-positive cells to make a 1:1 CD4/CD8 ratio in CAR-T.[20]
The
first study involving the investigation of Liso-Cel in CLL patients was
performed on 24 R/R CLL patients, in large part with high-risk features
(such as complex karyotype and/or 17p deletion).[21] The overall response rate (ORR) was 74%, with 21% complete response (CR) and 53% partial response (PR).[21]
Recently, long-term follow-up of this study was reported, including the
analysis of phase I and II of this study; the study included a total of
49 patients with R/R CLL and 10 patients with Richter’s transformation.[22]
Patients received CAR-T cell therapy without (30 patients) or with
concurrent ibrutinib treatment (19 patients); patients were evaluated
on day +28 for response by standard criteria, and their measurable
residual disease (MRD) status was assessed by multiparameter flow
cytometry (MFC) and IGH next-generation sequencing.[22]
Of the enrolled patients, 96% had complex karyotype and/or 17p
deletion, and 94% were ibrutinib-refractory. ORR and CRR were 70% and
17%, respectively, and the median duration of response (mDoR) was 21.3
months; 5-year overall survival (OS) and progression-free survival
(PFS) were 35% and 21%, respectively. MRD by MFC was observed in 75% of
patients, and 72% of these patients were negative for NGS; in uMRD
patients by NGS, the mDoR was 55.9 months.[22]
In
2018, Siddiqi et al. reported the first results of the monotherapy
section of the TRASCEND CLL 004 phase I/II clinical trial, which
initially involved 10 R/R CLL patients.[23] The
patients with standard risk received 3 and those with high risk 2 prior
lines of therapy, including a Bruton’s tyrosine kinase inhibitor
(BTKi); after lymphodepletion, the patients received a single-infusion
of Liso-Cel at either dosage 1 (DL1, 5x107CAR-T cells) or dosage 2 (DL2, 1x108CAR-T cells).[23]
At 30 days post-CAR-T cell infusion, 75% of patients displayed an OR,
with 50% of CR; all patients with CR remained with a negative MRD
status at 3 months post-dose.[4] In 2022, the same authors reported the
results of phase I of the TRASCEND CLL 004 study, involving a total of
23 R/R CLL patients, including 100% of patients who had prior ibrutinib
and 65% venetoclax treatment; 83% of these patients had high-risk
features, including TP53 mutations and del (17p).[24]
In this study, 74% of patients had cytokine release syndrome (CRS, 9%
of grade 3) and 39% of neurological events (22% of grade 3). ORR was
42.9%, and CR was 11%; the patients who achieved MRD negativity were
75% and 60% in peripheral blood and bone marrow, respectively.[24]
The results of this study have represented the basis for a phase II
study at 1x108CAR-T cell dosage. A more recent report of this phase
I/II study reported the results of 117 CLL patients.[25] In the patients treated with dosage level 2 (DL2, 1x108CAR-T
cells), 88% (95/108) were BTKi refractory, 78% (89/108) were venetoclax
refractory, 61% (66/108) were double-refractory. The efficacy
evaluation at DL2 showed an ORR of 42.9% with 18.4% CR; the median DoR
was 35.3 months, with a median follow-up of 19.7 months.[25]
The same efficacy was reported in the evaluable double-refractory
patients [ORR was 42% (21/49), with 18% (9/49) of CR, median DoR 35.25
months]. Undetectable MRD was obtained in 64% of double refractory
patients in peripheral blood and 59% in bone marrow. The median
duration of response among patients with CR was not reached. In
exploratory analyses of undetectable MRD and progression-free survival,
PFS was 26.2 months in patients with uMRD and 2.8 months in those with
detectable MRD in blood. Grade 3 CRS occurred in 8.5% of cases, and
grade 3 neurological events in 17.9% of cases.[25]
With a longer follow-up (median follow-up of 23.5 months), Lico-Cel
continues to demonstrate durable CR, high MRD rates, and a manageable
safety profile in this population of heavily pretreated CLL patients.[26]
Tisa-Cel.
Tisa-Cel is a second-generation anti-CD19 CAR-T cell
with 4-1BB costimulatory domain and CD8a hinge region.[27]
The
initial study carried out using autologous T cell transduced with a
CD19-directed CAR (CTL-109, Tisa-Cel) reported the results observed in
14 R/R CLL patients: the ORR was 57%, with 4 CR and 4PR.[28]
A final evaluation of this study involved the treatment of 38 R/R CLL
patients with CTL109, showing an ORR of 44%, with 28% of CR.[29] Patients achieving a CR showed an OS longer than those who did not, with a mOS not reached vs 64 months.[8] The median PFS was 40.2 months in patients with CR, compared to 1 month in those without CR.[29]
Interestingly,
Melenhorst et al. reported the cellular and molecular analysis of two
CLL patients exhibiting long-term remission after a CTL 109 infusion
performed in 2010.[9] The anti-leukemia activity of
anti-CD19 CTL 109 cells displayed two distinct phases: an initial phase
characterized by CD8+or CD4-CD8-Helioshi
γδ CAR-T cells, followed by the predominance of proliferative CD4+CAR-T
cells in the ensuing years.[30] Surprisingly, CD4+T cells seem
responsible for mediating anti-CD19 cytotoxic activity; functional
characterization of these cells, as well as upregulation of
antigen-mediated signaling pathways and upregulation of G 2MK, G2MA,
and PRF1, support this view.[30]
Brexu-Cel.
Brexu-Cel is a second-generation anti-CD19 CAR-T cell that utilizes the
CD28 costimulatory domain and CD28 hinge region; an additional step is
introduced in the manufacturing of these CAR-T cells to remove
malignant cells from the leukapheresis product.[31]
The
ZUMA-8 phase I/II clinical trial explored the safety and efficacy of
Brexu-Cel (KTE-19), anti-CD19 CAR-T cells. In phase I, the enrolment of
12-18 patients was planned to assess dose-limiting toxicities, while in
phase II, 60 patients would be enrolled to assess safety and efficacy.[32]
Phase I results of the ZUMA-8 trial involving 15 R/R CLL patients have
involved four cohorts of patients: cohorts 1 and 2 were treated with
1x106 and 2x106 KTE-19 cells/Kg, cohort 3 involved the enrolment of low-tumor burden patients and cohort 4 any patients.[33]
The enrolled patients were heavily pretreated. Seven out of fifteen
patients exhibited an objective response, with 3 CR; two of these
patients with a low tumor burden had a CR.[33] Significant CAR-T cell expansion occurred in 4 patients, mostly in those with low tumor burden.[33]
Axi-Cel. Axi-Cel is a second-generation anti-CD19 CAR-T cell utilizing CD28 costimulatory domain and CD28 hinge region.[34]
Axi-Cel, autologous anti-CD19 CAR-T cells have been explored in a few
R/R CLL patients. An initial study by Kochenderfer et al. also included
4 R/R CLL patients.[35] A later study included 8 R/R
CLL patients and explored the long-term effects of Axi-Cel CAR-T cell
therapy. The ORR was 88%, with 63% of CR and 25% of PR; 3/5 of the
patients who achieved a CR maintained a response ≥3 years.[36] The median duration of ongoing responses was 82 months; median EFS was 40.5 months.[36]
CAR-T GLPG 5201.
A recent study reported the first results obtained using GLPC 5201, a
second-generation anti-CD19/4-1BB CAR-T product, administered as a
fresh product in a single dose, without the need for cryopreservation
within 7 days of apheresis.[36] Seven patients were
enrolled in the ongoing phase I study; all these 7 patients were
diagnosed as R/R CLL, and 4/7 had Richter's transformation (RT); no
grade 3-4 CRS or neurologic events were observed; 7/7 patients
responded to the treatment, with 6/7 CRs; responding patients remained
in CR at a 7-month follow-up.[37] These results, although still preliminary, are considered very promising.
A
more extensive report of this study was presented at the ASH Meeting
2023 and involved a total of 12 patients: 5 with R/R CLL and 7 with
RT.[38] ORR and CRR were 86% and 7!% for RT patients.[38]
Robust expansion of CAR-T cells was observed, with detectable CAR-T
cells up to 9 months after CAR-T cell infusion.[38] Infused CAR-T cells
exhibited a preserved early memory phenotype for CD4+ and CD8+ CAR-T
cells.[38]
Varnicambtagene autoleucel.
Varnicambtagene autoleucel (Var-Cel), an academic anti-CD19 CAR product
manufactured by the Hospital Clinic of Barcelona, Spain, was used
primarily for the treatment of non-Hodgkin lymphoma in the CART19-BE-01
trial. Recently, the safety profile and the efficacy of Var-Cel were
explored in 20 R/R CLL patients, including 63% of patients with RT.[39]
Var-Cel manufacturing was performed in a median of 8 days; with a
median follow-up of 8.4 months, the ORR was 83%, with 83% of patients
achieving an MRD negativity; CR in BM and PB was 67%, achieving CR at
the level of extramedullary sites.[39] PFS was shorter in patients with RT and with higher BM infiltration.[39] These preliminary observations support further development of Var-Cel in these high-risk populations of CLL patients.
CD19-targeted CAR-T Cell Therapy in Richter’s Transformation
Richter’s
transformation is a relatively rare event involving the development of
aggressive B-cell lymphoma in patients with CLL or SLL; the prognosis
is dismal, with a short OS.[40] Table 2 reports the results of the trials involving CAR-T use in Richter's transformation.
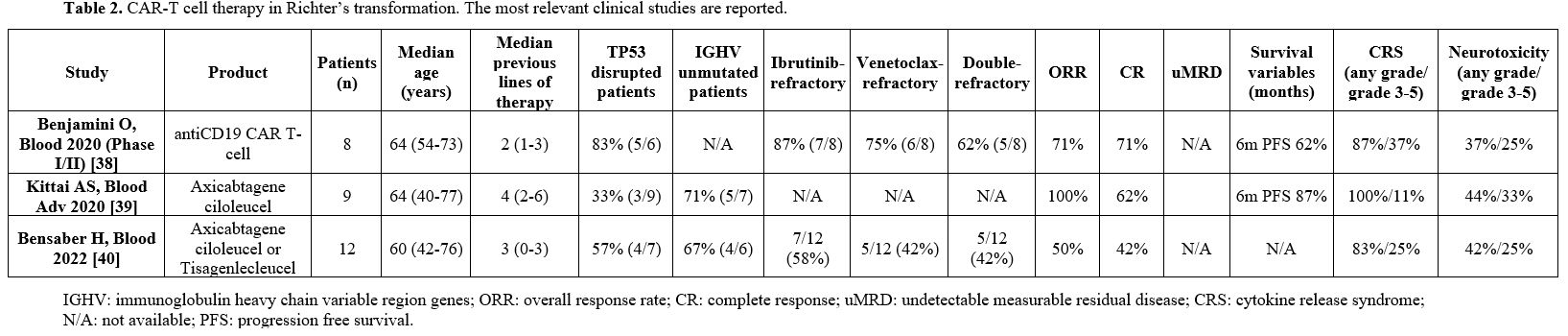 |
- Table 2. CAR-T cell therapy in Richter’s transformation. The most relevant clinical studies are reported.
|
Benjamini
and coworkers reported the analysis of the safety and efficacy of CD19
CAR-T cells in 8 patients with Richter’s transformation generated using
a retroviral vector encoding a CAR comprising FMC63 anti-CD19 ScFv,
linked to a CD28 costimulatory domain and CD3-zeta intracellular
signaling domain.[38] All these patients had a prior history of CLL, and 83% of them had del 17p/TP53 mutation.[41] Patients had at least 3 previous lines of therapy, including ibrutinib and venetoclax treatment.[41] Of these patients, 71% had a CR following treatment with CAR-t cells, and 2 patients proceeded to allo-HSCT.[38] Three patients had grade 3-4 CRS.[41]
Kittai
et al. reported a retrospective analysis of 9 patients with Richter's
transformation treated with Axi-Cel in the context of an institutional
experience of off-label use of these CAR-T cells.[39] Eight of these 9 patients were evaluated for response, and most of them continued treatment with BTK inhibitor.[42] All of these patients displayed a response, with 5 CR and 3 PR.[42]
The median follow-up was 6 months: one patient relapsed during these 6
months, while the remaining 7 patients remained in remission.[42]
Bensader
et al. have retrospectively analyzed 12 patients with Richter’s
transformation treated with either Axi-Cel (5 patients) or Tisa-Cel (7
patients), with 50% ORR and 42 CRs.[43] Of the treated patients, 25% had grade 3 CRS. All the patients included in this analysis were heavily pretreated.
Blackmon
reported 7 patients who have been treated with Liso-Cel and relapsed
after CAR-T cell therapy with Richter’s syndrome.[41] All these patients
had CLL with high-risk factors prior to CAR-T cell therapy (including TP53 mutations, del17p, NOTCH1 mutations, and SF3B1 mutations.[44]
CD19-targeted CAR-T Cell Therapy in Combination with Ibrutinib
Several
clinical observations have strongly supported the rationale of
associating CAR-T cells with ibrutinib administration: (i) ibrutinib
treatment of CLL patients increases in vivo persistence of activated T
cells, decreases Treg/CD4+T cell ratio, and decreases the
immunosuppressive properties of CLL cells;[45] (ii)
previous cycles of ibrutinib therapy improve the in vivo expansion of
CD19-directed CAR-T cells, in association with decreased expression of
the immunosuppressive molecules PD-1 and CD200 on the membrane of
T-lymphocytes;[46] (iii) Ibrutinib administration after CAR-T cell infusion decreases cytokine release syndrome;[47]
assays demonstrated enhanced anti-CLL T-cell killing function during
ibrutinib-rituximab treatment, including a switch from predominantly
CD4+ T-cell: CLL immune synapses at baseline to increased CD8+ lytic
synapses on-therapy.[48]
Gauthier et al. have
conducted a pilot study to evaluate the safety and feasibility of
administering ibrutinib concurrently with CD19 CAR-T cell therapy.[49]
Thus, 19 R/R CLL patients, with a median number of 5 prior therapies,
have been treated with Liso-Cel and ibrutinib; 13 of these patients
received the scheduled ibrutinib treatment and were evaluated for
safety and response to the treatment. The 4-week ORR was 83%, with 61%
of patients achieving an MRD-negativity in bone marrow, as assessed by
NGS.[46] In these patients, the 1-yr OS and PFS were
86% and 59% respectively. Patients treated with ibrutinib and CAR-T
cells displayed lower CRS severity and lower serum concentrations of
CRS-associated cytokines.[49]
Gill et al.
reported the results of a phase II clinical study in which anti-CD19
humanized binding domain CAR-T cells (hu-CART 19) were used in
combination with ibrutinib in 19R/R CLL patients not in CR despite ≥6
months of ibrutinib.[50] CRR evaluated at 3 months was 44%; at 12 months, 72% of patients had no measurable MRD.[47] Of 15 patients with undetectable MRD at 6 months, 13 remained in CR at the last follow-up.[50]
Mechanisms of Resistance to CD19 CAR-T Cell Therapy in CLL
As
mentioned above, clinical trials of CD19-targeted CAR-T cell therapy
have shown a durable antitumor response in a limited proportion of R/R
CLL patients, around 20-25%, while most patients are resistant to this
treatment.
Several studies have explored the characteristics of
CAR-T cells, as well as the genomic abnormalities and other molecular
features of CLL that could correlate with response or resistance to
CAR-T cells.[51]
Frazetta and coworkers have
investigated the transcriptomic profile of CAR-T cells of R/R CLL
patients undergoing CAR-T cell therapy: responding patients were
enriched in memory-related genes, whereas T cells from non-responders
upregulated the expression of genes involved in effector
differentiation, glycolysis, exhaustion, and apoptosis.[52]
Furthermore, patients with sustained mission showed an elevated
frequency of CD27+CD45RO-CD8+T cells with memory-like characteristics
before CAR-T cell generation.[52] An additional
feature of functionally active CAR-T cells consisted of the production
of STAT3-related cytokines and serum IL6 levels correlated with CAR-T
cell expansion.[52]
Resting CD8+lymphocytes in
CLL have an altered mitochondrial profile (increased mitochondrial
respiration, membrane potential, and level of reactive oxygen species),
which impairs the development of efficacious CAR-T cells in these
patients.[53] In line with these observations,
CD8+CAR-T cells of CLL patients responding to CAR-T cell therapy have
increased mitochondrial mass with respect to non-responders, and this
property is correlated with CAR-T cell expansion in vivo.[53]
CAR-T
cells harbor an engineered receptor that is delivered through
lentiviral vector integration and may modify the cellular genome by
insertional mutagenesis. In some patients, retroviral integration
within the host genes, such as the TET2 gene[50] or genes involved in cell signaling and chromatin modification pathways,[54] may promote therapeutic T cell proliferation.
As
discussed above, non-responding or partially responding CLL patients
exhibited marginal or no expansion of their transformed CAR-T cells; in
contrast, patients with full response possessed CAR-T cells with
pronounced proliferative capacity and sustained persistence.[55]
In addition to these observations, the level and the duration of CAR
expression on the surface of T cells are a key determinant of clinical
efficacy since patients exhibiting complete remission have a permanent
CAR expression. In contrast, non-responding patients lost their
cell-surface CAR detection at the time of relapse, thus suggesting that
CAR extinction at the cell surface is an important mechanism of
resistance to therapy.[56] Epigenetic mechanisms seem
to be responsible for the extinction of CAR expression; particularly,
the bromodomain and extra-terminal (BET) family of chromatin adaptors
seem to be involved in this epigenetic silencing of CAR expression.[56]
BET protein inhibition decreased TET2 levels and improved the
proliferative capacity of exhausted CAR-T cells and their antitumor
activity.[56]
The reduced CAR-T cell expansion
and persistence may also be attributed to the activation of naturally
occurring negative immune checkpoint molecules (such as PD-1, TM-3,
LAG-3, and CTLA-4). Agarwal et al. have explored whether the disruption
of the co-inhibitory receptors CTLA-4 or PD-1 could restore CAR-T
function.[57] CRISPR-Cas9-mediated deletion of CTLA-4
in preclinical models of leukemia and in T-cells from patients with CLL
who previously failed CAR-T cell treatment reinvigorates dysfunctional
T cells, thus suggesting a strategy for increasing patient responses to
CAR-T cell therapy.[57]
CD20-targeted CAR-T Cells in CLL
Only
an ongoing phase I clinical trial is evaluating the safety and efficacy
of CD20-targeted CAR-T cells in CLL patients.[54] MB-106 is a fully human
third-generation CD20-targeted CAR-T product with both CD28 and 4-1BB
costimulatory domains; an ongoing phase I/II clinical trial is
evaluating the safety and the efficacy of MB-106 in R/R CD20+ B-cell
malignancies.[58] The results on the first 16
patients included 1 patient with R/R CLL achieving CR, with
MRD-negative status following treatment with MB-106.[58]
Other
clinical trials have started the evaluation of bispecific CAR-T cells
targeting both CD19 and CD20 antigens. In phase I clinical trial
enrolling patients with B-cell lymphomas and CC, it was evaluated the
safety and the efficacy of LV20.19, a CAR construct targeting both CD20
and CD19: 3 patients with R/R CLL were treated, with two patients
achieving CR and one patient achieving PR.[59]
Another study of tandem CAR-T targeting CD19 and CD20 also included one R/R CLL patient, who achieved a CR to this therapy.[60]
Park
et al. reported the initial evaluation of a third-generation CAR-T
targeting CD19 and containing two costimulatory domains, CD28 and
4-1BB.[61] In the context of a phase I evaluation of
CAR-T cells developed using this CAR construct, 9 patients with R/R
CLLL and 2 with Richter’s transformation were evaluated: 2/9 R/R CLL
and 1 / 2 patients with Richter’s transformation achieved a CR.[61] In
the same phase I study, 7/8 and 2/2 patients with large B cell lymphoma
and follicular lymphoma, respectively, achieved a CR.[61]
Allogeneic CAR-T Cells
Most
of the studies performed with CAR-T cells in CLL, as well as in other
tumors, involve the use of autologous, patient-derived CAR-T cells.
However, allogeneic CAR-T (alloCAR-T) cells may represent a treatment
alternative to autoCAR-T cells. Two types of allogeneic CAR-T cells can
be utilized: donor-compatible donors and off-the-shelf CAR-T cells.
Donor-derived CAR-T cells are CAR-T cells obtained from HLA-compatible
donors or donors in patients with a history of allo-HSCT. HLA
compatibility is required for donor-relative CAR-T, while
"off-the-shelf" CAR-T cells require techniques to reduce the rejection
risk of non-HLA-matched products. An example of the technology used for
off-the-shelf CAR-T cells is given by UCART 19 cells, recently used for
the treatment of B-ALL patients.[62] UCART 19 is a
first-in-class “off-the-shelf” alloCAR-T cell based on genetic
engineering of T cells from a normal donor to express an anti-CD19
(murine 4G7 scFv)/4-1BB/CD3ζ CAR together with RQR8 safety switch (a
suicide gene); T cells were further genome-edited through disruption of
T cell receptor alpha chain (to prevent GVHD) and CD52 gene knockout
(to protect donor cells from early rejection).[63]
Studies
with "off-the-shelf" CAR-T cells have not yet been reported in CLL.
Only one study with donor-derived alloCAR-T cells reported the
treatment of a few R/R CLL patients.[64] In fact,
Brudno et al. reported the results of a phase I study involving the
treatment of 20 patients with B-cell malignancies, including 5 R/R CLL
patients.[60] In this study, CAR-T cells were
generated from the patient's prior allo-HSCT donor. They were based on
a CAR composed of a murine scFv anti-CD19, a CD28 costimulatory domain,
and a CD3ζ T-cell activation domain.[64] None of the
treated patients developed new onset GVHD. Among the 5 CLL patients
included in the study, 1 achieved a CR, 1 a PR, and 3 did not respond
to the treatment.[64] Interestingly, the CLL patient
achieving a CR showed rapid elimination of leukemic cells after
alloCAR-T at the level of bone marrow, peripheral blood, and lymph
nodes.[64]
Conclusions
CLL
was the first hematopoietic malignancy for which CAR-T cells were
administered for therapeutic purposes. However, subsequent development
was challenged by the finding of inferior responses observed in CLL
patients compared to those observed in other B-cell malignancies. These
more limited responses are related to the presence of patient
comorbidities, immunodeficiency, and immunosubversion of the CAR-T cell
product.
Thus, none of the four approved CAR-T cell products,
Axi-Cel, Brexu-Cel, Lisi-Cell, and Tisa-Cel for NHL and B-ALL, are
currently approved for CLL. The recent data reported for Liso-Cel could
provide sufficient support for its approval for the treatment of R/R
CLL patients.
Although preliminary and based on only 12 R/R CLL
patients, the results observed using fresh, unfrozen CAR-T cells GLPG
5201 were particularly promising, manufactured, and reinfused to the
patients within 7 days of blood draw. Frozen peripheral blood
mononucleated cells as starting material and frozen CAR-T infusion
products maintain high antitumor activity, but fresh CAR-T infusion
product exhibits higher antitumor reactivity.[65]
Future studies will assess whether freshly manufactured CAR-T cells
have a more potent antitumor activity compared to CAR-T cells
manufactured using frozen cell preparations for the treatment of R/R
CLL patients.
The development of allogeneic CAR-T cells and
natural killer cells from healthy donors may represent a promising
solution to address the reduced fitness of T cells observed in CLL
patients.
Combined treatment with ibrutinib and CAR-T cells
appears to be a therapeutic strategy associated with increased efficacy
due to synergistic effects between BTKi and cell therapy and provides
some safety benefits. Future studies will clarify whether
next-generation BTK inhibitors in association with CAR-T cells could
further improve the therapeutic benefit observed with ibrutinib.
At
the moment, the role of CAR-T cell therapy is limited to CLL patients
with relapsed/refractory disease. Additional improvements in the safety
and efficacy of CAR-T cells are required to integrate CAR-T cell
therapy in earlier lines of treatment and patients with Richter's
transformation. It is of interest to note that some RT patients treated
with CAR-T cell therapy achieved long-term remission with prolonged
survival.[66]
Interestingly, a very recent study
supports the targeting of CLL cells expressing a tumor-specific
antigen, a B-cell receptor light chain neoepitope defined by a
characteristic point mutation (IGLV3-21R110), for selective targeting of a poor-risk subset of CLL with CAR-T cells.[67] CAR-T cells targeting this tumor neoantigen exert a significant anti-leukemia effect, sparing normal B cells.
References
- SEER Cancer Stat Facts:
Chronic Lymphocytic Leukemia/Small
Lymphocytic Lymphoma. National Cancer Institute. Bethesda, MD,
https://seer.cancer.gov/statfacts/html/cllsll.html
- Eichhorst
B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO
Clinical Practice Guidelines for diagnosis, treatment and follow-up.
Ann Oncol. 2021;32(1):23-33. doi:10.1016/j.annonc.2020.09.019
https://doi.org/10.1016/j.annonc.2020.09.019
PMid:33091559
- NCCN
Guidelines Update: Chronic Lymphocytic Leukemia/Small Lymphocytic
Lymphoma. J Natl Compr Canc Netw. 2023;21(5.5):563-566.
doi:10.6004/jnccn.2023.5007 https://doi.org/10.6004/jnccn.2023.5007
- Lew
TE, Lin VS, Cliff ER, Blombery P, Thompson ER, Handunnetti SM,
Westerman DA, Kuss BJ, Tam CS, Huang D, et al. Outcomes of patients
with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood
Adv 2021; 5: 4054-4063.
https://doi.org/10.1182/bloodadvances.2021005083
PMid:34478505
PMCid:PMC8945613
- Mato,
A.R.; Hess, L.M.; Chen, Y.; Abada,
P.B.; Konig, H.; Pagel, J.M.; Walgren, R. Outcomes for Patients with
Chronic Lymphocytic Leukemia (CLL) Previously Treated With Both a
Covalent BTK and BCL2 Inhibitor in the United States: A Real-World
Database Study. Clin. Lymphoma Myeloma Leuk. 2023, 23, 57-67.
https://doi.org/10.1016/j.clml.2022.09.007
PMid:36335022
- Eyre,
T.A.; Hess, L.M.; Sugihara, T.; He, D.; Khanal, M.; Pagel, J.M.;
Walgren, R.; Abada, P.B.; Konig, H.; Roeker, L.; Mato, A. Clinical
outcomes among patients with chronic lymphocytic leukemia (CLL)/small
lymphocytic lymphoma (SLL) who received treatment with a covalent BTK
and BCL2 inhibitor in the United States: A real-world database study.
Leuk. Lymphoma 2023, 64, 1005-1016.
https://doi.org/10.1080/10428194.2023.2190436
PMid:36987650
- Innocenti
I, Fresa A, Tomasso A, Tarnani M, De Padua L, Benintende G, Pasquale R,
Galli E, Morelli F, Giannarelli D, Autore F, Laurenti L. Treatment
Sequencing and Outcome of Chronic Lymphocytic Leukemia Patients Treated
at Fondazione Policlinico Universitario Agostino Gemelli IRCCS: A
Thirty-Year Single-Center Experience. Cancers (Basel). 2023 Nov
26;15(23):5592. doi: 10.3390/cancers15235592. https://doi.org/10.3390/cancers15235592
PMid:38067296
PMCid:PMC10705134
- Roeker
LE, Dreger P, Brown JR, Lahoud
OB, Eyre TA, Brander DM, et al. Allogeneic stem cell transplantation
for chronic lymphocytic leukemia in the era of novel agents. Blood Adv
[Internet]. 2020 Aug 25;4(16):3977-89. https://doi.org/10.1182/bloodadvances.2020001956
PMid:32841336
PMCid:PMC7448605
- Neelapu
SS, Locke FL, Bartlett NL,
Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR
T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017
Dec 28;377(26):2531-2544. doi: 10.1056/NEJMoa1707447. Epub 2017 Dec 10.
https://doi.org/10.1056/NEJMoa1707447
PMid:29226797 PMCid:PMC5882485
- Jacobson
CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al.
Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin
lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet
Oncol. 2022 Jan;23(1):91-103. doi: 10.1016/S1470-2045(21)00591-X. Epub
2021 Dec 8. https://doi.org/10.1016/S1470-2045(21)00591-X
PMid:34895487
- Locke
FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al;
All ZUMA-7 Investigators and Contributing Kite Members. Axicabtagene
Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J
Med. 2022 Feb 17;386(7):640-654. doi: 10.1056/NEJMoa2116133. Epub 2021
Dec 11. https://doi.org/10.1056/NEJMoa2116133
PMid:34891224
- Wang
M, Munoz J, Goy A, Locke FL, Jacobson
CA, Hill BT, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or
Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020 Apr
2;382(14):1331-1342. doi: 10.1056/NEJMoa1914347. https://doi.org/10.1056/NEJMoa1914347
PMid:32242358
PMCid:PMC7731441
- Shah
BD, Ghobadi A, Oluwole OO, Logan
AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory
adult B-cell acute lymphoblastic leukaemia: phase 2 results of the
single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021 Aug
7;398(10299):491-502. doi: 10.1016/S0140-6736(21)01222-8. Epub 2021 Jun
4. https://doi.org/10.1016/S0140-6736(21)01222-8
PMid:34097852
- Abramson
JS, Solomon SR, Arnason J,
Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel as
second-line therapy for large B-cell lymphoma: primary analysis of the
phase 3 TRANSFORM study. Blood. 2023 Apr 6;141(14):1675-1684. doi:
10.1182/blood.2022018730. https://doi.org/10.1182/blood.2022018730
PMid:36542826
PMCid:PMC10646768
- Abramson
JS, Palomba ML, Gordon
LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for
patients with relapsed or refractory large B-cell lymphomas (TRANSCEND
NHL 001): a multicentre seamless design study. Lancet. 2020 Sep
19;396(10254):839-852. doi: 10.1016/S0140-6736(20)31366-0. Epub 2020
Sep 1. https://doi.org/10.1016/S0140-6736(20)31366-0
PMid:32888407
- Maude
SL, Laetsch TW, Buechner J,
Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children
and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018
Feb 1;378(5):439-448. doi: 10.1056/NEJMoa1709866. https://doi.org/10.1056/NEJMoa1709866
PMid:29385370
PMCid:PMC5996391
- Mueller
KT, Waldron E, Grupp SA,
Levine JE, Laetsch TW, Pulsipher MA, et al. Clinical Pharmacology of
Tisagenlecleucel in B-cell Acute Lymphoblastic Leukemia. Clin Cancer
Res. 2018 Dec 15;24(24):6175-6184. doi: 10.1158/1078-0432.CCR-18-0758.
Epub 2018 Sep 6..
https://doi.org/10.1158/1078-0432.CCR-18-0758
PMid:30190371
PMCid:PMC7433345
- Schuster
SJ, Bishop MR, Tam CS,
Waller EK, Borchmann P, McGuirk JP, et al; JULIET Investigators.
Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell
Lymphoma. N Engl J Med. 2019 Jan 3;380(1):45-56. doi:
10.1056/NEJMoa1804980. Epub 2018 Dec 1. https://doi.org/10.1056/NEJMoa1804980
PMid:30501490
- Fowler
NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et
al. Tisagenlecleucel in adult relapsed or refractory follicular
lymphoma: the phase 2 ELARA trial. Nat Med. 2022 Feb;28(2):325-332.
doi: 10.1038/s41591-021-01622-0. Epub 2021 Dec 17.
https://doi.org/10.1038/s41591-021-01622-0
PMid:34921238
- Sommermeyer
D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, Riddell
SR. Chimeric antigen receptor-modified T cells derived from defined
CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo.
Leukemia. 2016 Feb;30(2):492-500. doi: 10.1038/leu.2015.247. Epub 2015
Sep 15. https://doi.org/10.1038/leu.2015.247
PMid:26369987 PMCid:PMC4746098
- Turtle
CJ, Hay KA, Hanafi LA, Li D, Cherian S,
Chen X, Wood B, Lozanski A, Byrd JC, Heimfeld S, et al. Durable
molecular remissions in chronic lymphocytic leukemia treated with
CD19-specific chimeric antigen receptor-modified T cells after failure
of ibrutinib. J Clin Oncol 2017; 35: 3010-3020.
https://doi.org/10.1200/JCO.2017.72.8519
PMid:28715249 PMCid:PMC5590803
- Liang
E, Hirayama A, Kimble E, Portuguese,
Albittar A, Chapouis A, Shadman M, Till B, Cassaday R, Milano F, et al.
Long-term follow-up update and multivariable analyses of factors
associated with duration of response after CD19 CAR T-cell therapy for
relapsed/refractory CLL. Hemasphere 2023; 7 (suppl.1): e472395b.
https://doi.org/10.1097/01.HS9.0000969368.47239.5b
PMCid:PMC10429589
- Siddiqi
T, Soumarai JD, Wierda WG, D ubovsky JA, Gillenwater HH, Pharm LG,
Mitchell A, Thorpe J, Yang LDorritie KA, et al. Rapid MRD-negative
responses in patients with relapsed/refractory CLL treated with
Liso-Cel, a CD19-directed CAR T-cell product: preliminary results from
transcend CLL 004, a phase 1 / 2 study including patients with
high-risk disease previously treated with ibrutinib. Blood 2018; 132
(suppl. 1):300. https://doi.org/10.1182/blood-2018-99-110462
- Siddiqi
T, Soumerai JD, Dorritie KA, Stephens DM, Riedell PA, Arnason JA, Kipps
TJ, Gillenwater HH, Gong L, Yang L, et al. Phase 1 TRASCEND CELL 004
study of lisocabtagene maraleucel in patients with relapsed/refractory
CLL or SLL. Blood 2022; 139: 1794-1806.
https://doi.org/10.1182/blood.2021011895
PMid:34699592
PMCid:PMC10652916
- Siddiqi
T, Maloney D, Kenderian SS,
Brander DM, Dorritie K, Soumerai J. Lisocabtagene maraleucel in chronic
lymphocytic leukemia and small lymphocytic lymphoma (TRASCEND CLL 004):
a multicentre, open-label, single-arm, phase 1-2 study. Lancet 2023;
402: 641-654. https://doi.org/10.1016/S0140-6736(23)01052-8
PMid:37295445
- Siddiqi
T, Maloney DG, Kenderian SS,
Brander DM, Dorritie K, Soumnerai J, Riedell PA, Shah NV, Nath R,
Fakhri B, et al. Lisocabtagene Maraleucel (liso-cel) in R/R CLL/SLL:
24-median follow-up of TRASCEND CLL-004. Blood 2023; 142 (suppl.1):
330. https://doi.org/10.1182/blood-2023-179529
- Milone
MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M,
Lakhal M, Gloss B, Danet-Desnoyers G, Campana D, Riley JL, Grupp SA,
June CH. Chimeric receptors containing CD137 signal transduction
domains mediate enhanced survival of T cells and increased antileukemic
efficacy in vivo. Mol Ther. 2009 Aug;17(8):1453-64. doi:
10.1038/mt.2009.83. Epub 2009 Apr 21. Erratum in: Mol Ther. 2015
Jul;23(7):1278. PMID: 19384291; PMCID: PMC2805264.
https://doi.org/10.1038/mt.2009.83
PMid:19384291 PMCid:PMC2805264
- Porter
DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci
KT, Shen A, Gonzalez V, et al. Chimeric antigen receptor T cells
persist and induce sustained remissions in relapsed refractory chronic
lymphocytic leukemia. Sci Transl 2015; 7: 303ra139.
https://doi.org/10.1126/scitranslmed.aac5415
- Frey
NV, Gill S, Hexner EO, Schuster S, Nasta S, Loren A, Svoboda J,
Stadtmauer E, Landsburg DJ, Mato A, et al. Long-term outcomes from a
randomized dose optimization study of chimeric antigen receptor
modified T cells in relapsed chronic lymphocytic leukemia. J Clin Oncol
2020; 38: 2862-2871. https://doi.org/10.1200/JCO.19.03237
PMid:32298202
PMCid:PMC8265376
- Melenhorst
JJ, Chen GM, Wang M,
Porter DL, Chen C, Collins MA, Gao P, Brabyopadhyay S, n H, Zhao Z, et
al. Decade-long leukemia remissions with persistence of CD4+ CAR T
cells. Nature 2022; 602: 503-509.
https://doi.org/10.1038/s41586-021-04390-6
PMid:35110735
PMCid:PMC9166916
- Wang
M, Munoz J, Goy A, Locke FL,
Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW,
McSweeney PA, Miklos DB, Pagel JM, Kersten MJ, Milpied N, Fung H, Topp
MS, Houot R, Beitinjaneh A, Peng W, Zheng L, Rossi JM, Jain RK, Rao AV,
Reagan PM. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory
Mantle-Cell Lymphoma. N Engl J Med. 2020 Apr 2;382(14):1331-1342. doi:
10.1056/NEJMoa1914347.
https://doi.org/10.1056/NEJMoa1914347
PMid:32242358 PMCid:PMC7731441
- Flinn
I, Marris M, Wierda WG, Coutre S, Pagel JM, Byrd JC, Goyal L, Goodman
K, Zheng Y, Milletti F, et al. ZUMA-8: a phase 1-2 multicenter study
evaluating KTE-X19 in patients (pts) with relapsed/refractory (R/R)
chronic lymphocytic leukemia (CLL). J Clin Oncol 2019; 37(suppl. 16):
TPS7566. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS7566
- Davids
MS, Kenderian SS, Flinn IW, Hill BT, Maris M, Ghia P, Byrne M, Barlett
NL, Pagel JM, Zheng Y, et al. ZUMA-8: a phase 1 study of KTE-X19, an
anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in patients
with relapsed/refractory chronic lymphocytic leukemia. Blood 2022; 140
(suppl. 1): 7454-7456. https://doi.org/10.1182/blood-2022-167868
- Kochenderfer
JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, Wilson WH, Rosenberg
SA. Construction and preclinical evaluation of an anti-CD19 chimeric
antigen receptor. J Immunother. 2009 Sep;32(7):689-702. doi:
10.1097/CJI.0b013e3181ac6138. PMID: 19561539; PMCID: PMC2747302.
https://doi.org/10.1097/CJI.0b013e3181ac6138
PMid:19561539
PMCid:PMC2747302
- Kochenderfer
JN, Dudley ME, Kassim SH,
Somerville R, Carpenter RO, Setler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al. Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively
treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol 2015; 33: 540-549.
https://doi.org/10.1200/JCO.2014.56.2025
PMid:25154820 PMCid:PMC4322257
- Cappell
KM, Sherry RM, Yang JC, Goff SL, Vanasse
DA, McInntyre L, Rosenberg SA, Kochenderfer JN. Long-term follow-up of
anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol 2020;
38: 3805-3815. https://doi.org/10.1200/JCO.20.01467
PMid:33021872
PMCid:PMC7655016
- Martinez-Cibrian
N, Betriu S,
Ortiz-Maldonado V, Esteban D, Tovar N, Moreno AT, Alserawan L; Montoro
M, Van Muyden A, Pont M, et al. Initial clinical results of EUPLAGIA-1,
a phase 1-2 trial of point-of-care manufactured GLPG5201 in R/R CLL/SLL
with or without Richter's transformation. Hemasphere 2023; 7(S3):
2714-2715. https://doi.org/10.1097/01.HS9.0000972484.02679.4e
PMCid:PMC10430544
- Tovar
N, Ortiz-Maldonado V,
Martinez-Cibrian N, Betriu S, Esteban D, Triguero A, Verbruggen N,
Spoon M, Liefaard MC, Pont M, van Muyden A. Seven-day vein-tovein
point-of-care manufactured CD19 CAR T cells (GLPG5201) in
relapsed/refractory CLL/SLL including Richter's transformation: results
from the phase 1 Euplagia-1 trial. Blood 2023; 142 (suppl.1): 2112.
https://doi.org/10.1182/blood-2023-189321
- Ortiz-Maldonado
V, Martinez-Cibrian N, De Campo Balguerias G, Espanol-Rego M, Navarro
S, Lopez-Oreja I, Nadeu F, Cobo A, Brillembourg H, Alserawan L, et al.
Varnicambtagene autoleucel (ARI-0001) for relapsed or refractory
chronic lymphocytic leukemia (CLL) and Richter transformation (RT).
Blood 2023 142 (suppl.1): 3483.
https://doi.org/10.1182/blood-2023-182896
- Douglas
M.
Richter transformation: clinical manifestations, evaluation, and
management. J Adv Pract Oncol 2022; 13: 525-534.
https://doi.org/10.6004/jadpro.2022.13.5.6
PMid:35910504
PMCid:PMC9328451
- Benjamini
O, Shimoni A, Besser M,
Shem-Tov N, Danylesko I, Yerushalmi R, Merkel DG, Tadmor T, Lavie D, et
al. Safety and efficacy of CD19-CAR T cells in Richter's transformation
after targeted therapy for chronic lymphocytic leukemia. Blood 2020;
136(suppl.1): 40. https://doi.org/10.1182/blood-2020-138904
- Kittai
AS, Bond DA, William B, Saad A, Penza S, Efebera Y, Larkin K, Wall SA,
Choe HK, Bhatnagar B, et al. Clinical activity of axicabtagene
ciloleucel in adult patients with Richter syndrome. Blood Adv 2020; 4:
4648-4652. https://doi.org/10.1182/bloodadvances.2020002783
PMid:33002129 PMCid:PMC7556158
- Bensaber
H, Bachy E,
Beauvais D, Dulery R, Gastinne T, Villemagne B, Roulin L, Paubelle E,
Castilla-Llorente C, Longval T, et al. Anti-CD19 CAR T-cell therapy for
patients with Richter syndrome: a Lysa study from the Descar-T
registry. Blood 2022; 140 (suppl.1): 3803-3804.
https://doi.org/10.1182/blood-2022-158807
- Blackmon
A,
Danilov AV, Wang L, Pillai R, rshkarlo HB, Rosen ST, Siddiqi T.
Richter's transformation after CD-19 directed CAR-T cells for
relapsed/refractory chronic lymphocytic leukemia (CLL). Blood 2021; 138
(suppl. 1): 1430. https://doi.org/10.1182/blood-2021-149815
- Long
M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, Maddocks KJ, Cheney
C, Jones JA, et al. Ibrutinib treatment improves T cell number and
function in CLL patients. J Clin Invest 2017; 127: 3052-3064.
https://doi.org/10.1172/JCI89756
PMid:28714866 PMCid:PMC5531425
- Fraietta
JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, Lacey SF,
Melenhorst J, McGettigan SE, et al. Ibrutinib enhances chimeric antigen
receptor T-cell engraftment and efficacy in leukemia. Blood 2016; 127:
1117-1127. https://doi.org/10.1182/blood-2015-11-679134
PMid:26813675
PMCid:PMC4778162
- Ruella
M, Kenderian SS, Shestova
O, ichinsky M, Melenhorst JJ, Wasik MA, Lacey SF, June CH, Gill S, et
al. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome
after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms.
Leukemia 2017; 31: 246-248. https://doi.org/10.1038/leu.2016.262
PMid:27677739
- Papazoglou
D, Wang XV, Shanafelt TD,
Lesnick CE, Ioannou N, De Rossi G, Herter S, Bacac M, Klein C, Tallman
MS, Kay NE, Ramsay AG. Ibrutinib-based therapy reinvigorates CD8+ T
cells compared to chemoimmunotherapy: immune monitoring from the E1912
trial. Blood. 2024 Jan 4;143(1):57-63. doi: 10.1182/blood.2023020554..
https://doi.org/10.1182/blood.2023020554
PMid:37824808
PMCid:PMC10797553
- Gauthier
J, Hirayama AV, Purushe J,
Hay KA, Lymp J, Li DH, Yeung C, Sheih A, Pender BS, Hawkins RM, et al.
Feasibility and efficacy of CD19-targeted CAR T cells with concurrent
ibrutinib for CLL after ibrutinib failure. Blood 2020; 135: 1650-1660.
https://doi.org/10.1182/blood.2019002936
PMid:32076701 PMCid:PMC7205814
- Gill
S, Vides V, Frey NV, Hexner EO, Metzeger S, O'Brien
M, Hwang WT, Brogdon JL, Davis MM, Fraietta JA, et al. Anti-CD10 CAR T
cells in combination with ibrutinib for the treatment of chronic
lymphocytic leukemia. Blood Adv 2022; 6: 5774-5781.
https://doi.org/10.1182/bloodadvances.2022007317
PMid:35349631
PMCid:PMC9647791
- Vitale
C, Griggio V, Perutelli F,
Coscia M. CAR-modified cellular therapies in chronic lymphocytic
leukemia: is the uphili road getting less steep? HemaSphere 2023; 7:
12(e988). https://doi.org/10.1097/HS9.0000000000000988
PMid:38044959
PMCid:PMC10691795
- Fraietta
JA, Lacey SF, Orlando EJ,
Pruteanu-Malinci I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O'Connor
RS, Hwang WT, et al. Determinants of response and resistance to CD19
chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic
leukemia. Nat Med 2018; 24: 563-571.
https://doi.org/10.1038/s41591-018-0010-1
PMid:29713085
PMCid:PMC6117613
- van
Bruggen JAC, Martens AWJ, Fraietta JA, Hofland T, Tonino SH, Eldering
E, Levin MD, Siska PJ, Endstra S, Rathmell JC, June CH, Porter DL,
Melenhorst JJ, Kater AP, van der Windt GJW. Chronic lymphocytic
leukemia cells impair mitochondrial fitness in CD8+ T cells and impede
CAR T-cell efficacy. Blood. 2019 Jul 4;134(1):44-58. https://doi.org/10.1182/blood.2018885863
PMID: 31076448; PMCID: PMC7022375.
- Fraietta
JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ, Cogdill AP,
Morrissette JJD, DeNizio JE, Reddy S, Hwang Y, Gohil M, Kulikovskaya I,
Nazimuddin F, Gupta M, Chen F, Everett JK, Alexander KA, Lin-Shiao E,
Gee MH, Liu X, Young RM, Ambrose D, Wang Y, Xu J, Jordan MS, Marcucci
KT, Levine BL, Garcia KC, Zhao Y, Kalos M, Porter DL, Kohli RM, Lacey
SF, Berger SL, Bushman FD, June CH, Melenhorst JJ. Disruption of TET2
promotes the therapeutic efficacy of CD19-targeted T cells. Nature.
2018 Jun;558(7709):307-312. https://doi.org/10.1038/s41586-018-0178-z
PMID: 29849141; PMCID: PMC6320248.
- Nobles
CL, Sherill-Mix S, Everett JK, Reddy S, Fraietta JA, Porter DL, Frey N,
Gill S, Grupp SA, Maude SL, et al. CD19-targeting CAR T cell
immunotherapy outcomes correlate with genomic modification by vector
integration. J Clin Invest 2020; 130: 673-685.
https://doi.org/10.1172/JCI130144
PMid:31845905 PMCid:PMC6994131
- Kong
W, Dimitri A, Wang W, Jung IY, Ott CJ, Fasolino M, Wang Y, Kulikovskaya
I, Gupta M, Yoder T, et al. BET bromodomain protein inhibition reverses
chimeric antigen receptor chimeric antigen receptor extinction and
reinvigorates exhausted T cells in chronic lymphocytic leukemia. J Clin
Invest 2021; 13: e145459. https://doi.org/10.1172/JCI145459
PMid:34396987 PMCid:PMC8363276
- Agarwal
S, Aznar
MA, Rech AJ, Good CR, Kuramitsu S, Da T, Gahil M, Chen L, Hong SJ,
Ravikumur S, et al. Deletion of the co-inhibitory co-receptor CTLA4
enhances and invigorates chimer antigen receptor T cells. Immunity
2023; 56: 1-20. https://doi.org/10.1016/j.immuni.2023.09.001
PMid:37776850
- Shadman
M,Yeung CC, Redman M, Lee
SY, Lee DH, Ra S, Ujjani CS, Dezube BJ, Poh C, Warren EH, et al. High
efficacy and low toxicity of MB-106, a third generation CD20 targeted
CAR-T for treatment of relapsed/refractory b-NHL and CLL. Transplant
Cell Ther 2022; 28: S182-S183.
https://doi.org/10.1016/S2666-6367(22)00386-4
- Shah
NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, Krueger
W, Worden AA, Kadan MJ, Yim S.; et al. Bispecific anti-CD20, anti-CD19
CAR T cells for relapsed B cell malignancies: a phase 1 dose-escalation
and expansion trial. Nat Med 2020; 26: 1569-1575.
https://doi.org/10.1038/s41591-020-1081-3
PMid:33020647
- Tong
C, Zhang Y, Liu Y, Ji X, Zhang WY, Guo Y, Han X, Ti D, Dai H, Wang C.,
et al. Optimized tandem CD19/CD20 CAR-engineered T cells in
refractory/relapsed B cell lymphoma. Blood 2020; 136: 1632-1644.
https://doi.org/10.1182/blood.2020005278
PMid:32556247 PMCid:PMC7596761
- Park
JH, Palomba ML, Bellevi CL, Riviere I, Wang X,
Senechal B, Furman RR, Bernal Y, Hall M, Pineda J, et al. A phase I
first-in-human clinical trial of CD19-targeted 19-28z/4-1BBL "Armored"
CAR T cells in aptients with relapsed or refractory NHL and CLL
including Richter's transformation. Blood 2018; 132(suppl.1): 224.
https://doi.org/10.1182/blood-2018-99-117737
- Benjamin
R, Jain N, Maus MV, Boissel N, Graham C, Jorwik A, Yallop D, Konopleva
M, Frigault MJ, Teshima T, et al. UCART19, a first-in-class allogeneic
anti-CD19 chimeric antigen receptor T-cell therapy for adults with
relapsed or refractory B-cell acute lymphoblastic leukemia (CALM): a
phase 1, dose-escalation trial. Lancet Haematol 2022; 9: e833-e843.
https://doi.org/10.1016/S2352-3026(22)00245-9
PMid:36228643
- Dupouy
S, Marciq I, Derippe T, Almena-Carrasco M, Jozwik A, Fouliard S, Adimy
Y, Geronimi J, Graham C, Jain N, et al. Clinical pharmacology and
determinants of response to UCART19, an allogeneic anti-CD19 CAR-T cell
product, in adult B-cell acute lymphoblastic leukemia. Cancer Res
commun 2022; 2: 1520-1530.
https://doi.org/10.1158/2767-9764.CRC-22-0175
PMid:36970059
PMCid:PMC10035397
- Brudno
JN, Sommerville R, Shi V,
Rose J, Halverson DC, Fowler DH, Gea-Banacloche JC, Pavletic SZ,
Hickstein DD, Lu TL, et al. Allogeneic T cells that express an
anti-CD19 chimeric antigen receptor induce remissions of B-cell
malignancies that progress after allogeneic hematopoietic stem cell
transplantation without causing graft-versus-host disease. J Clin Oncol
2016; 344: 1112-1121. https://doi.org/10.1200/JCO.2015.64.5929
PMid:26811520 PMCid:PMC4872017
- Brezinger-Dayan
K,
Itzhaki O, Melnichenko J, Kubi A, Zelter L, Jacoby E, Avigdot A,
Shapira Frommer R, Besser MJ. Impact of cryopreservation on CAR T
production and clinical response. Front Oncol 2022; 12: 1024362.
https://doi.org/10.3389/fonc.2022.1024362
PMid:36276077
PMCid:PMC9582437
- Kutsch
N, Godel P, Voltin CA, Hallek
M, Scheid C, Borchmann P, Holtick U. Long-term remission in a patient
with relapsed Richter's transformation treated with CD19-directed
chimeric antigen-receptor T-cells after allogeneic stem cell
transplantation. Eur J Haematol 2024; in press.
https://doi.org/10.1111/ejh.14182
PMid:38316549
- Markl
F, Schultheib C, Ali M, Chen SS, Zintchenko M, Egli L, Mietz J,
Chijioke D, Paschold L, Spajic S, et al. Mutation-specific CAR T cells
as precision therapy for IGLV3-21R110 expressing high-risk chronic
lymphocytic leukemia. Nat Commun 2024; 15: 993.
https://doi.org/10.1038/s41467-024-45378-w
PMid:38307904
PMCid:PMC10837166

