Recently, De Winter et al. developed a model for predicting recurrent VTE, major bleeding (MB), and clinically relevant non-major bleeding (CRNMB) within 5 years in patients with VTE without active cancer who completed at least a 3-month primary anticoagulant treatment course.[1] The VTE-PREDICT risk score may be a useful tool in clinical practice but needs to be further validated with real-life data.
We recently published our single-center experience regarding secondary prophylaxis of VTE with low-dose apixaban or rivaroxaban in high-risk patients for VTE recurrence.[2] Our study population was represented by 323 non-oncologic patients receiving apixaban 2.5 mg BID or rivaroxaban 10 mg daily as VTE secondary prophylaxis with a follow-up longer than 12 months. The median low-dose DOAC administration time was 25.40 months (IQR 13.93-45.90). Twelve (3.7%) VTE recurrence events were observed after a median low-dose treatment of 27.68 months (IQR 17.0-47.3). VTE recurrence rate of 0.6% at 1 year and 1.9% at 2 years. In total, 21 patients (6,5%) had a bleeding event after a median treatment of 13.6 months (IQR 10,6-17,9): one major bleeding (0,3%), 8 CRNMB (2,5%) and 12 minor bleeding (3,7%). The only major bleeding event was registered after 11.7 months. Three CRNMB (0,9%) were registered within the first year; the other 5 CRNMB (1,5%) after more than one year.
We decided to calculate the VTE-PREDICT risk score1 with patients' data at the start date of low-dose DOAC administration, to estimate the effect of extended anticoagulant treatment on VTE recurrence and bleeding events at one year and at 5 years after low-dose DOAC initiation. We used the calculator that is available online (https://vtepredict.com/calculators/vtePredict). Every patient had completed at least a 3-month primary anticoagulant treatment before starting low-intensity DOAC.
A receiver operating characteristic (ROC) curve was performed on continuous variables to determine a cut-off predictive of increased adverse event incidence for the VTE-PREDICT score. By ROC analysis, no statistically significant cut-off was found. The cut-offs that presented the relative best area under the curve (AUC), grating the best sensitivity and specificity, were 1.5% for current 5-year recurrent VTE and 2,0% for current 5-year clinically relevant (MB + CRNMB) bleeding. No statistically significant difference was found in the thrombotic-event-free-survival (t-EFS) between patients having more or less than a calculated 1.5% VTE-PREDICT risk for current 5-year recurrent VTE risk (Figure 1 – p=0.4). No statistically significant difference was found in the bleeding-event-free-survival (b-EFS) between patients having more or less than a calculated 2.0% current 5-year clinically relevant bleeding risk (Figure 2 – p=0.11).
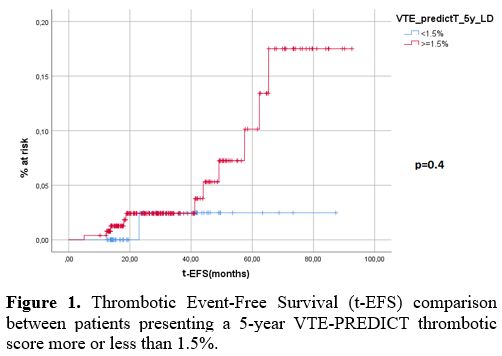 |
Figure 1. Thrombotic Event-Free Survival (t-EFS) comparison between patients presenting a 5-year VTE-PREDICT thrombotic score more or less than 1.5%. |
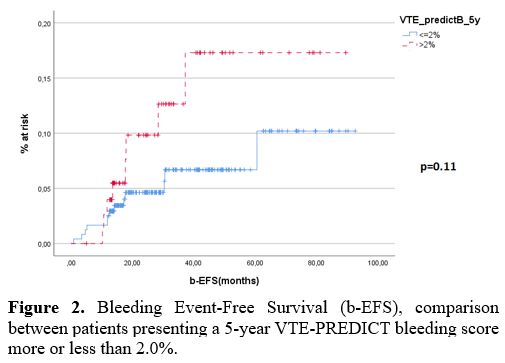 |
Figure 2. Bleeding Event-Free Survival (b-EFS), comparison between patients presenting a 5-year VTE-PREDICT bleeding score more or less than 2.0%. |
Moreover, we confronted the median odds obtained with this calculator with our real-life data. The median VTE-PREDICT thrombotic recurrence risk with low dose was 0.6% (IQR 0.5%-0,7%) at 1 year and 1.8% (IQR 1.5%-2.2%) at 5 years. The median VTE-PREDICT hemorrhagic risk for CRNMB and major bleedings with low-dose was 1.0% (IQR 0.7%-2.2%) at 1 year and 3.1% (IQR 2.4%-7.3%) at 5 years.
Comparing these VTE-PREDICT results with our real life; we can highlight that:
- Our rate of VTE recurrences (0,6%) and clinically relevant (MB + CRNMB) bleeding (1,2%) within the first 12 months were comparable with the calculator’s odds (0.6% VTE-PREDICT thrombotic risk – 1,0% VTE-PREDICT hemorrhagic risk)
- The 5-year VTE-PREDICT hemorrhagic risk might slightly underestimate the bleeding risk, considering that after a median low-dose DOAC administration of 25.40 months, we have already experienced a clinically relevant bleeding rate of 2.8% that is almost equal to the 3.1% predicted in a five-year follow-up.
- The 5-year VTE-PREDICT thrombotic risk seems to importantly underestimate the risk of VTE recurrence during a long follow-up in patients receiving low-dose DOAC, considering that after a median low-dose DOAC administration of 25.40 months, we have already experienced a VTE recurrence rate higher than the one predicted (3.7% vs 1.8%).
The VTE-PREDICT risk score can be applied to estimate the absolute benefits and harms of extended anticoagulation for individual patients in order to help clinicians in their decision on anticoagulant therapy. In the short-term follow-up, for patients receiving low-dose DOAC as secondary VTE prophylaxis, such a score is reliable in predicting the VTE recurrence and bleeding rate. In the low-dose DOAC long-term follow-up scenario, this calculator underestimates the bleeding rate and miscalculates the rate of VTE recurrence. Hence, the VTE-PREDICT risk score needs further validation in such patient cohorts and in different cohorts, especially focusing on long follow-up data.
Finally, as data derived from nonvalvular atrial fibrillation elderly patients[3-4] are progressively showing, low-dose DOACs may represent a valid and safer alternative anticoagulant treatment in the elderly. In particular, to date, in the context of a VTE, the decision on continuing or stopping anticoagulation (after 3-6 months of acute VTE treatment) in the elderly is based on clinician choice, balancing the risks of bleeding and recurrent VTE for each case. In such a population, low-dose DOACs may become the cornerstone VTE secondary prophylaxis drugs that reduce the risk of bleeding and guarantee a reduction in the risk of VTE recurrence. Of our cohort, 41 patients (12,7%) were 75 years old or older. The median low-dose DOAC administration time for such patients was 22.10 months (IQR 14.00-33.30). In this subgroup, one (2,4%) non-fatal VTE recurrence event was observed after a low-dose treatment time of 18,3 months, and 4 patients (9,75%) had a bleeding event after a median treatment of 11,7 months: 2 CRNMB (4,9%) and 2 minor bleeding (4,9%). Therefore, no statistically significant difference in VTE recurrence rate (p=0.64) or bleeding rate (p=0.37) was evidenced between patients <75 years and patients ≥ 75 years of our cohort. Moreover, we calculated the median odds by exploiting the VTE-PREDICT risk score calculator from our elderly patient's data. The median VTE-PREDICT thrombotic recurrence risk with low dose was 0.6% (IQR 0.5%-0,8%) at 1 year and 2.7% (IQR 2.4%-3.0%) at 5 years. The median VTE-PREDICT hemorrhagic risk for CRNMB and major bleedings with low-dose was 1.2% (IQR 1.0%-3.1%) at 1 year and 10.9% (IQR 10.0%-11.4%) at 5 years. Due to the paucity of our subgroup sample, no clear conclusion can be issued. Nevertheless, it may be evidenced that the predictive ability of the VTE-PREDICT risk score seems to be comparable between our whole cohort and the elderly subgroup; perhaps in the low dose DOAC long-term follow-up scenario, this calculator might better estimate the bleeding rate in the elderly. Nevertheless, as mentioned above, the VTE-PREDICT risk score needs further validation in various patient cohorts, including elderly patients.