.
Ingvild Hausberg Sørvoll1, Ingvild Jenssen Lægreid1, Tom Sollid1, Maria Therese Ahlen1, Silje Johansen2,3 and Håkon Reikvam2,4.
1 The
Norwegian National Unit for Platelet Immunology, Division of
Diagnostics, University Hospital of North Norway, N-9037 Tromsø, Norway.
2 K.G. Jebsen Center for Myeloid Blood Cancer, Department of Clinical Science, University of Bergen, N-5021 Bergen, Norway.
3 Section of Haematology, Department of Medicine, Haraldsplass Deaconess Hospital, N-5021 Bergen, Norway.
4 Section of Haematology, Department of Medicine, Haukeland University Hospital, N-5021 Bergen, Norway.
.
Published: July 01, 2024
Received: May 15, 2024
Accepted: June 14, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024057 DOI
10.4084/MJHID.2024.057
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Recent
years have seen an increasing interest in antibodies against platelet
factor 4 in thrombotic complications. Hence, we want to explore the
prevalence of anti-PF4/heparin antibodies in newly diagnosed acute
myelogenous leukaemia (AML) patients. In the present study, we
demonstrated that the existence of PF4/heparin antibodies is a rare
occurrence at the time of diagnosis in AML patients.
AML is an
aggressive and highly malignant haematological disease characterized by
bone marrow infiltration of immature leukemic blasts suppressing normal
haematopoiesis, leading to severe cytopenia.[1] The
disease is associated with a high degree of mortality and morbidity,
and among commonly occurring complications, we find both bleeding and
thrombosis. The pathophysiology behind the haemostatic complications
occurring in AML is complex and multifactorial and includes bone marrow
failure with severe thrombocytopenia. Also, endothelial dysfunction,
inflammation with cytokine activation and coagulopathy with
disseminated intravascular coagulopathy (DIC) contribute to these
complications.[2] The most studied example is the coagulopathy associated with acute promyelocytic leukemia (APL),[3] although also in other AML cases haemostatic complications are of concern.[2]
Heparin-induced
thrombocytopenia (HIT) is a rare condition associated with antibodies
against platelet factor 4 in a complex with heparin, which
activate platelets, leading to thrombocytopenia accompanied by a
pronounced thrombotic state.[4] Both classical HIT and
autoimmune HIT variants like delayed-onset- and refractory or
persistent HIT are observed after exposure to heparins. However, other
HIT-like conditions can, in rare cases, occur in the absence of recent
heparin exposure such as spontaneous HIT,[5] or vaccine-induced immune thrombotic thrombocytopenia (VITT) following COVID-19 vaccines, [6,7] and in rare cases after other vaccines,[8] adenovirus infection,[9,10] and monoclonal gammopathy of clinical significance.[11]
A relatively high number of anti-PF4/heparin antibody-positive patients
have been observed among patients with myeloproliferative neoplasms
(MPNs).[12] Based on these considerations, we wanted
to examine the presence of anti-PF4/heparin antibody-positive patients
in a cohort of newly diagnosed AML patients.
The collection and
use of samples for this study were approved by the regional committees
for medical and health research ethics (REK) both for biobanking and in
vitro experimental research (REK Vest 1750/2015 and REK Nord
480847/2022). Registration of collected samples was also approved by
the Norwegian Data Protection Authority (reference 02/1118-5). Serum
samples from 136 AML patients at the time of diagnosis and before
initiating treatment (Table 1)
were collected and aliquoted before being frozen and stored at -80°C.
Antibodies to PF4/heparin were tested for by enzyme-linked
immunosorbent assay (ELISA) using LIFECODES PF4 IgG (Immucor), dilution
1:50, with an optical density cutoff value ≥0.400.
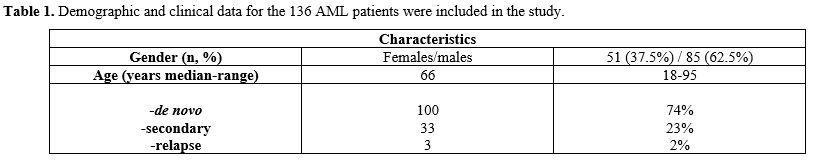 |
- Table
1. Demographic and clinical data for the 136 AML patients were included in the study.
|
Only
one of the 136 patients (0.7%) had a positive test for anti-PF4/heparin
antibodies: OD value 0.959, and in the other 135 samples, no
anti-PF4/heparin antibodies were detected (Figure 1).
The ELISA-positive patient was a male patient of 49 years with a
diagnosis of AML FAB M4, karyotype, 45; X, -Y, and with the NPM1
mutation without the FLT3 mutations. Medical records indicate no
previous exposure to heparins. Coagulation parameters at the time of
diagnosis demonstrated prothrombin time- INR 1.1 (normal range
0.9-1.2), activated partial thromboplastin time (APTT) 38 seconds
(30-44), fibrinogen 4.6 g/L (1.9-4.0), and D-dimer 2.33 mg/L
(<0.50). He received induction therapy with the 3+7 regimen, with
additional consolidation with cytarabine, and importantly, he had no
bleeding or thrombotic complications during the treatment period.
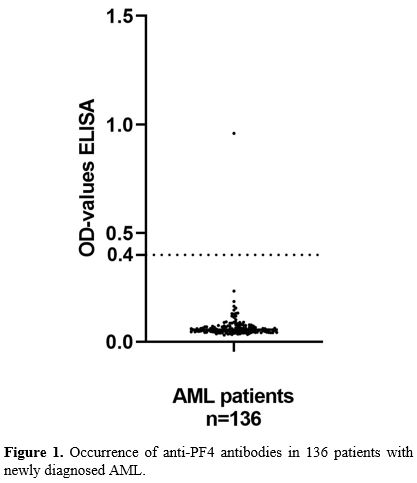 |
- Figure 1. Occurrence of anti-PF4 antibodies in 136 patients with newly diagnosed AML.
|
For
further analysis of the ELISA-positive patient, the AcuStar HIT-IgG
test (Instrumentation Laboratory) with cutoff value ≥ 1.0 U/ml and the
Heparin‐induced multiple electrode aggregometry (HIMEA) run on the
multiple analyzers (Dynabyte Medical), as described in Schultz et al,[7] and a PF4‐dependent P‐selectin expression assay, modified from Samuelson Bannow et al.,[13]
were used as functional platelet activation assays. For the
ELISA-positive patient, the AcuStar HIT-IgG test and the two functional
tests were all negative.
In the present study, we demonstrated
that the existence of PF4/heparin antibodies is a rare occurrence in
AML patients at the time of diagnosis, with an estimated occurrence
< 1%, an incidence lower than the existence of such antibodies in a
normal population.[14] Hence, the contribution of
anti-PF4/heparin antibodies in the coagulopathy seen in the acute phase
of AML patients is very low. It should be emphasized that the presence
of anti-PF4/heparin antibodies without functional platelet assay has
scarce significance. Functional assay should always be performed with
the suspicion of PF4-mediated thrombotic conditions. Furthermore, a
significant number of AML patients develop thrombosis, including
catheter-associated thrombosis (CAT),[15] during their treatment period, and hence often are exposed to heparins.[15]
If these patients are more vulnerable to developing anti-PF4/heparin
antibodies later in the treatment course and eventually HIT, it remains
unanswered. Physicians treating acute leukaemia patients should be
reassured by the low occurrence of these antibodies in this study.
However, be aware of the potential association after exposure to
heparins and other triggers, and the low antibody occurrence does not
exclude the possibility of later development of HIT or anti-PF4-driven
disease.
Acknowledgement
The technical support of Kristin Paulsen Rye is greatly appreciated.
References
- DiNardo CD, Erba HP, Freeman SD, Wei AH: Acute myeloid leukaemia. Lancet 2023, 401(10393):2073-2086. https://doi.org/10.1016/S0140-6736(23)00108-3 PMid:37068505
- Wang
TF, Makar RS, Antic D, Levy JH, Douketis JD, Connors JM, Carrier M,
Zwicker JI: Management of hemostatic complications in acute leukemia:
Guidance from the SSC of the ISTH. J Thromb Haemost 2020,
18(12):3174-3183. https://doi.org/10.1111/jth.15074 PMid:33433069 PMCid:PMC7909744
- Yilmaz M, Kantarjian H, Ravandi F: Acute promyelocytic leukemia current treatment algorithms. Blood Cancer J 2021, 11(6):123. https://doi.org/10.1038/s41408-021-00514-3 PMid:34193815 PMCid:PMC8245494
- Arepally GM: Heparin-induced thrombocytopenia. Blood 2017, 129(21):2864-2872. https://doi.org/10.1182/blood-2016-11-709873 PMid:28416511 PMCid:PMC5445568
- Schonborn
L, Esteban O, Wesche J, Dobosz P, Broto M, Puig SR, Fuhrmann J, Torres
R, Serra J, Llevadot R et al: Anti-PF4 immunothrombosis without
proximate heparin or adenovirus vector vaccine exposure. Blood 2023,
142(26):2305-2314. https://doi.org/10.1182/blood.2023022136 PMid:37883798 PMCid:PMC10862238
- Greinacher
A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S: Thrombotic
Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med 2021,
384(22):2092-2101. https://doi.org/10.1056/NEJMoa2104840 PMid:33835769 PMCid:PMC8095372
- Schultz
NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT,
Wiedmann M, Aamodt AH, Skattor TH, Tjonnfjord GE, Holme PA: Thrombosis
and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med
2021, 384(22):2124-2130. https://doi.org/10.1056/NEJMoa2104882 PMid:33835768 PMCid:PMC8112568
- Johansen
S, Laegreid IJ, Ernstsen SL, Azrakhsh NA, Kittang AO, Lindas R,
Gjertsen BT, Vetti N, Mortberg TV, Sorvoll IH et al: Thrombosis and
thrombocytopenia after HPV vaccination. J Thromb Haemost 2022,
20(3):700-704. https://doi.org/10.1111/jth.15604 PMid:34817130 PMCid:PMC9906134
- Warkentin
TE, Baskin-Miller J, Raybould AL, Sheppard JI, Daka M, Nazy I, Moll S:
Adenovirus-Associated Thrombocytopenia, Thrombosis, and VITT-like
Antibodies. N Engl J Med 2023, 389(6):574-577. https://doi.org/10.1056/NEJMc2307721 PMid:37590457
- Wang
JJ, Schonborn L, Warkentin TE, Chataway T, Grosse L, Simioni P, Moll S,
Greinacher A, Gordon TP: Antibody Fingerprints Linking Adenoviral
Anti-PF4 Disorders. N Engl J Med 2024, 390(19):1827-1829. https://doi.org/10.1056/NEJMc2402592 PMid:38749041
- Warkentin TE: Autoimmune Heparin-Induced Thrombocytopenia. J Clin Med 2023, 12(21). https://doi.org/10.3390/jcm12216921 PMid:37959386 PMCid:PMC10649402
- Meyer
SC, Steinmann E, Lehmann T, Muesser P, Passweg JR, Skoda RC, Tsakiris
DA: Anti-Platelet Factor 4/Heparin Antibody Formation Occurs
Endogenously and at Unexpected High Frequency in Polycythemia Vera.
Biomed Res Int 2017, 2017:9876819. https://doi.org/10.1155/2017/9876819 PMid:28698883 PMCid:PMC5494054
- Samuelson
Bannow B, Warad DM, Jones CG, Pechauer SM, Curtis BR, Bougie DW, Sharma
R, Grill DE, Redman MW, Khalighi PR et al: A prospective, blinded study
of a PF4-dependent assay for HIT diagnosis. Blood 2021,
137(8):1082-1089. https://doi.org/10.1182/blood.2020008195 PMid:32898858 PMCid:PMC7907721
- Arepally
GM, Hursting MJ: Platelet factor 4/heparin antibody (IgG/M/A) in
healthy subjects: a literature analysis of commercial immunoassay
results. J Thromb Thrombolysis 2008, 26(1):55-61. https://doi.org/10.1007/s11239-008-0217-y PMid:18369708 PMCid:PMC3606554
- Marin
A, Bull L, Kinzie M, Andresen M: Central catheter-associated deep vein
thrombosis in cancer: clinical course, prophylaxis, treatment. BMJ
Support Palliat Care 2021, 11(4):371-380. https://doi.org/10.1136/bmjspcare-2019-002106 PMid:34413028 PMCid:PMC8606430

