A
prerequisite for a successful allogeneic hematopoietic stem cell
transplantation (HSCT) is the availability of a human leukocyte antigen
(HLA) identical stem cell donor. Due to the fact that the HLA system is
inherited independently of the blood group system, approximately 40–50%
of all HSCTs are performed across the ABO blood group barrier.[1,2]
The expected immune-hematological consequences after transplantation of
an ABO-mismatched stem cell graft are immediate and delayed hemolytic
complications due to presence of isohemagglutinins or passenger
lymphocyte syndrome (PLS).[3] The risks of these
complications can partially be prevented by graft manipulation and
appropriate transfusion support. Here, we report a rare case of
haploidentical HSCT in which a donor’s residual high-titer anti-A
antibody induced intravascular hemolysis and hyperbilirubinemia during
group O RBC transfusion during the blood group transition phase.
An
11-year-old boy was undergoing treatment with dasatinib for chronic
myeloid leukemia, onset May 2022. He subsequently underwent acute
transformation, and allogeneic peripheral stem cell transplantation
from a HLA 5/10-matched donor with ABO incompatibility was performed at
our medical center in September 2023. The patient and donor blood
groups were A positive and O positive, respectively. The boy was
treated with a myeloablative conditioning regimen consisting of
fludarabine, busulfan, and melphalan, and a graft-versus-host disease
(GVHD) prevention and treatment scheme including post-transplant
cyclophosphamide, methotrexate, cyclosporine, and anti-lymphocyte
immunoglobulin. The nucleated cell dose infused was 11.8 × 108/kg and the CD34+ cell dose was 8.5 × 106/kg.
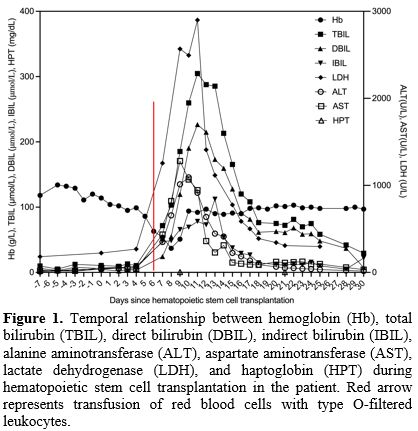
On
day 6 post-transplantation, the patient’s hemoglobin was 63 g/L (normal
range, 120–150 g/dL) and he therefore received a transfusion of 2 units
of red blood cells (RBCs) with type O-filtered leukocytes, after which
he developed fever and hemolysis. Routine blood tests and serum
biochemical tests revealed hemoglobin, 54 g/L; total bilirubin (TBIL),
71.8 μmol/L (normal range, 0–23 μmol/L); direct bilirubin (DBIL), 24.4
μmol/L (normal range, 5–18 μmol/L); indirect bilirubin (IBIL), 47.4
μmol/L (normal range, 5–18 μmol/L); alanine aminotransferase (ALT), 656
U/L (normal range, 7–40 U/L); aspartate aminotransferase (AST), 802 U/L
(normal range, 13–35 U/L); and lactate dehydrogenase (LDH), 1255 U/L
(normal range, 114–240 U/L). Blood analysis at day 8
post-transplantation showed a further drop in hemoglobin to 37 g/L.
Coombs test was positive. The anti-A isohemoagglutitin titer of the
blood donor was >1:1080. Intravascular hemolytic anemia was
considered with elevated bilirubin and aminotransferases caused by
hemolysis, and hydration, alkalinization, diuretic therapy, and type O
washed RBC transfusion were administered, after which the patient’s
hemoglobin gradually increased to 94 g/L. Serum biochemical tests at
day 10 post-transplantation showed TBIL, 259.7 μmol/L; DBIL, 190.4
μmol/L; IBIL, 69.3 μmol/L; ALT, 1092 U/L; AST, 1068 U/L; and LDH, 2494
U/L (Figure 1).
Hepatic
veno-occlusive disease, sinusoidal obstruction syndrome, thrombotic
microangiopathy, and viral infections were excluded. His bilirubin and
transaminase levels gradually decreased to normal after treatment with
anti-thymocyte immunoglobulin and basiliximab. The boy had full donor
chimerism of 99.8% at day 28 post-transplantation.
Decreased
hemoglobin levels are a common complication after transplantation with
secondary blood group antigen incompatibility, and may be associated
with PLS. PLS is caused by the transfer of B-lymphocytes present in the
donor graft into the recipient circulation following HSCT.[4]
These cells may produce antibodies against the recipient’s RBCs,
thereby triggering antibody-dependent cytotoxicity and erythroid
clearance, with potential resulting hemolysis and jaundice.[4]
ABO and Rh antibodies are the most common antibodies identified in PLS,
with a type O donor and type A recipient posing the greatest risk of
hemolysis, as in the current patient.[5] Ambulatory monitoring of blood group antibody titers can aid patient monitoring and diagnosis.[6]
The disease is self-limiting and does not require special treatment,
but RBC transfusions may be given in cases of severe anemia. During the
transition phase, transfusion of type O RBCs or recipient RBCs is
recommended for ABO-incompatible transplants.[7] In
the present case, the patient developed intravascular hemolytic anemia
after transfusion of type O RBCs, and it was considered that a small
amount of residual plasma during RBC preparation contained high-titer
anti-A antibodies, which combined with the patient’s own group A RBCs
by an acute hemolytic reaction. The patient’s hemolysis did not worsen
after the transfusion of type O washed RBCs and his hemoglobin level
increased.
Although the patient’s hemolysis resolved and
hemoglobin increased, his bilirubin tended to remain stable; however,
there was a rapid rise in bilirubin, predominantly direct bilirubin, at
day 10 post-transplantation. There was no significant change in the
patient’s weight at this time and platelet transfusions were effective,
which were not consistent with veno-occlusive disease/sinusoidal
obstruction syndrome.[8] Although the patient had
intravascular hemolysis, he had no symptoms such as hypertension,
fragmented RBC, proteinuria, or worsening renal failure, and therefore
did not meet the diagnostic criteria for thrombotic microangiopathy.[9]
Viral tests, including hepatitis B virus, hepatitis C virus,
cytomegalovirus, and Epstein-Barr virus were all negative, and viral
infection was therefore not considered. However, his bilirubin and
aminotransferase levels decreased rapidly after treatment with
anti-thymocyte immunoglobulin and basiliximab, indicating the
possibility of hepatic aGVHD. The blood group-associated antigens are
expressed, not only in RBC membranes, but also in other tissues. Blood
group-associated antigens are widely distributed in intrahepatic and
extrahepatic epithelial cells and hepatocytes, and may be adequate to
induce rejection.[10] It follows the development of severe hyperbilirubinemia during HSCT may be caused by a combination of the factors.