A total of 55 patients with transfusion-dependent thalassemia (TDT) were enrolled in this study. The entry criteria of the thalassemia group were as follows: (1) thalassemia was diagnosed by hemoglobin electrophoresis and DNA analysis; (2) patient was diagnosed as TDT; (3) age ≥ 6 years old and could cooperate with blowing; (4) blowing time from the last blood transfusion time ≥7 days. The exclusion criteria were: (1) thalassemia patients with organ failure, such as heart, liver, lung, and kidney; (2) patients with fever, infection, and other conditions; (3) smokers; (4) female patients during pregnancy and lactation. All patients or their parents (if minors) provided written informed consent. CO concentration was assessed using an automatic device (Model WY-2102, WellYearn Technology Co., Ltd., Shenzhen, China), and the final result was taken as an average value after three times of measurements.
The baseline characteristics of patients are summarized in Table 1, consisting of 55 patients with TDT (29 β0/β0, 17 β+/β0, 3 β+/β+, 6 β0/HbE). The median age was 13 years (range 6–30), and 36(66.4%) patients were male, and 6 (10.9%) patients had co-inherited α-thalassemia. Nineteen (34.5%) patients underwent splenectomy. Fourty-five (81.8%) patients had transfusions more than twenty times in the past two years, that is, an average of one transfusion per month. Regarding the complications of thalassemia, a total of 49 (89.1%) patients were diagnosed with iron overload. Two (3.6%) patients were diagnosed with diabetes and 9 (16.4%) patients suffered cholecystolithiasis. Other complications, such as hypothyroidism, pulmonary hypertension, and leg ulcers, were not observed in our study.
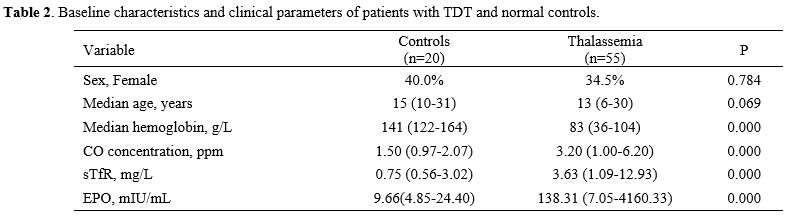
Our findings indicated that CO concentration was significantly higher in patients with TDT than in normal controls, being nearly twice as long in the latter group (median 3.2 vs. 1.5 ppm, Table 2). Patients with TDT showed higher erythropoietic activity compared with controls based on the levels of erythropoietin (EPO) and sTfR. The levels of EPO (P=0.000) and sTfR (P=0.000) were significantly higher in patients with TDT than in controls.
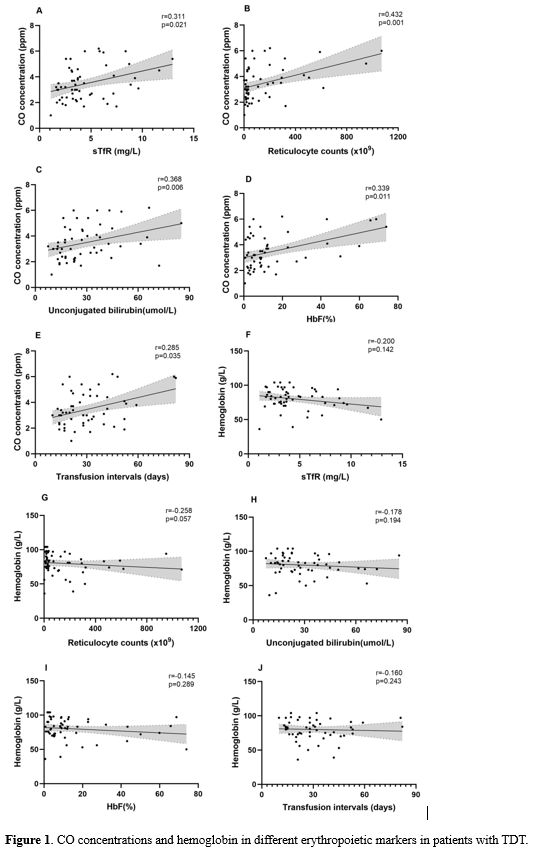
We employed Spearman’s rank correlation analysis to explore the correlations between CO concentration and various markers of ineffective erythropoiesis in patients with TDT (Figure 1). In terms of the identified correlations, CO concentration showed positive correlations with sTfR (r=0.311, p=0.021), reticulocyte counts (r=0.432, p=0.001), and unconjugated bilirubin levels (r=0.368, p=0.006). Similarly, CO concentrations were positively correlated with fetal hemoglobin (HbF) levels (r=0.339, p=0.011) and the transfusion intervals (r=0.285, p=0.035). However, we unexpectedly found that hemoglobin levels in these patients were not correlated with the above markers.
 |
|
Endogenous CO originates mainly from the heme oxidation that genesis during the hemoglobin degradation that follows natural red blood cell rupture. Sannolo et al.[5] indicated that the production of endogenous CO increased in patients with ineffective erythropoiesis. Similarly, our data also illustrated that patients with thalassemia have boosted CO concentration, which showed red blood cell destruction increasing and ineffective erythropoiesis. Blood transfusion could effectively inhibit ineffective erythropoiesis in patients with thalassemia. Our study found that CO concentration positively correlated with transfusion intervals. With the length of time between blowing and the last transfusion being longer, bone marrow suppression enhanced, CO concentration increased, and ineffective erythropoiesis gradually worsened in patients with thalassemia.
sTfR is a biomarker of erythropoiesis, originates mostly from erythroblasts and, to a lesser extent, from reticulocytes.[6] Circulating sTfR is proportional to erythroid precursor mass.[7] In the previous studies, it was demonstrated that sTfR level was a good marker for evaluating erythropoietic activity in the bone marrow of thalassemia.[8,9] Therefore, our study used sTfR as an observation marker to evaluate erythroid expansion. Interestingly, we found that the sTfR levels were correlated positively with CO concentration in patients with thalassemia, which indicated that CO concentration of patients with thalassemia was closely associated with bone marrow erythroid expansion, reflecting ineffective erythropoiesis in patients with thalassemia. In a longitudinal study conducted by James et al.,[2] no correlation between CO concentration and sTfR level was found, but the small size of the study group and only six patients may explain the result. However, it was observed that the level of CO concentration decreased on the fourth day after blood transfusion, and the level of sTfR decreased accordingly, reflecting an inhibition of erythropoiesis.
In β-thalassemia, ineffective erythropoiesis leads to an unconjugated bilirubin level increase, followed by aggravated red blood cell destruction and an increased number of reticulocytes in the peripheral circulation caused by bone marrow erythroid expansion. Except for sTfR, reticulocyte counts and unbound bilirubin are also believed to be potentially useful laboratory measurements in the study of ineffective erythropoiesis.[1] We observed that the CO concentrations were positively correlated with the reticulocyte counts and unbound bilirubin levels, which would help reflect ineffective erythropoiesis. Similarly, James et al.[2] also reported that the CO concentration correlated with reticulocyte counts, which supported our findings. The level of HbF in patients with β-thalassemia is higher than that of normal people.[10] Blood transfusion therapy effectively inhibited ineffective erythropoiesis, and patients with β-thalassemia showed decreased HbF levels.[11] Our study showed that CO concentration is positively correlated with HbF levels, reflecting ineffective erythropoiesis and blood transfusion therapy could inhibit ineffective erythropoiesis and reduce CO production. These data suggested that CO concentration could effectively evaluate ineffective erythropoiesis.
Conventionally, studies on the efficacy of thalassemia treatments have focused on changes in hemoglobin levels. However, the current results suggest that compared with hemoglobin, CO concentration is closely related to the ineffective erythropoiesis-related indicators of patients with TDT, indicating its potential value for evaluating the severity and therapeutic effect of β-thalassemia. Therefore, CO concentration determined in this way could thus be a useful indicator for evaluating patient condition and treatment efficacy in patients with β-thalassemia.
In summary, as a noninvasive and rapid examination method, the CO breath test could better reflect the ineffective erythropoiesis in patients with thalassemia. This would benefit clinicians by helping them better manage patients with TDT and provide better individualized clinical treatment decisions for them.
Ethics approval and consent to participate
All procedures were carried out according to the relevant guidelines. This study was approved by the ethics committee of the 923rd Hospital of the Joint Logistics Support Force of the People's Liberation Army. The patients or their parents involved in this study provided informed consent.Acknowledgments
We want to thank all participants involved in this study. This study was supported by the Scientific Research Project of Guangxi Zhuang Autonomous Region Health Committee (Z-A20231086) and the Scientific Research Fund Project of Guangzhou City Life Oasis Public Welfare Service Center (GZLZ-HEMA-008).References
- Cazzola M. Ineffective erythropoiesis and its treatment[J]. Blood, 2022, 139(16):2460-2470. https://doi.org/10.1182/blood.2021011045 PMid:34932791
- Ellen
Butensky J, Hendrik J V, Ronald J W, David K S, Elliott V, Laurie S,
Judith Y H, Julie S, Daniel W G Paul H. Elevated exhaled carbon
monoxide concentration in hemoglobinopathies and its relation to red
blood cell transfusion therapy[J]. Pediatr Hematol Oncol, 2010,
27(2):112-121. https://doi.org/10.3109/08880010903536227 PMid:20201692
- Zhang
H, Ma Y, Liu Q, Ye T, Meng F, Zhou Y, Yu G, Yang J, Jiang H, Wang Q, Li
G, Ji Y, Zhu G, Du L Ji K. Human erythrocyte lifespan measured by
Levitt's CO breath test with newly developed automatic instrument[J]. J
Breath Res, 2018, 12(3):036003 https://doi.org/10.1088/1752-7163/aaacf1 PMid:29400658
- Zhou
Y., Li J., Wei M., Lu L., Luo J., Jin X., Yang S., Yang L., Liao G.,
Zhou T., Huang J., Chen Y., Yin X. Measurement of erythrocyte lifespan
using a CO breath test in patients with thalassemia and the impact of
treatment. Mediterr J Hematol Infect Dis 2023, 15(1): e2023050 https://doi.org/10.4084/MJHID.2023.050 PMid:37705519 PMCid:PMC10497307
- Sannolo
N, Farina V Fiorillo A. Abnormal endogenous carbon monoxide production
in children with ineffective erythropoiesis[J]. Ann Clin Biochem, 1992,
29(Pt 4):397-399. https://doi.org/10.1177/000456329202900404 PMid:1642444
- Baynes R, Shih Y Cook J. Mechanism of production of the serum transferrin receptor[J]. Adv Exp Med Biol, 1994, 356:61-68. https://doi.org/10.1007/978-1-4615-2554-7_7 PMid:7534031
- Cazzola
M, Guarnone R, Cerani P, Centenara E, Rovati A Beguin Y. Red blood cell
precursor mass as an independent determinant of serum erythropoietin
level[J]. Blood, 1998, 91(6):2139-2145. https://doi.org/10.1182/blood.V91.6.2139 PMid:9490701
- Aysin
D, Nese Y, Tunc F, Feride D Abdurrahman K. Serum transferrin receptor
levels in beta-thalassemia trait[J]. J Trop Pediatr, 2004,
50(6):369-371. https://doi.org/10.1093/tropej/50.6.369 PMid:15537726
- Christophille
S, Ioannis P, Joanne T-S, Helene S, Vassilios L, Anna M-M, Alexandra S
Emmanuel K. Erythroid bone marrow activity and red cell
hemoglobinization in iron sufficient beta-thalassemia heterozygotes as
reflected by soluble transferrin receptor and reticulocyte hemoglobin
in content. Correlation with genotypes and Hb A(2) levels[J].
Haematologica, 2003, 88(6):631-636.
- Origa R. beta-Thalassemia[J]. Genet Med, 2017, 19(6):609-619. https://doi.org/10.1038/gim.2016.173 PMid:27811859
- Winichagoon
P, Fucharoen S, Chen P Wasi P. Genetic factors affecting clinical
severity in beta-thalassemia syndromes[J]. J Pediatr Hematol Oncol,
2000, 22(6):573-580. https://doi.org/10.1097/00043426-200011000-00026 PMid:11132233